Effects of Fasting on Intermediary Metabolism Enzymes in the Liver and Muscle of Rainbow Trout
Abstract
1. Introduction
2. Materials and Methods
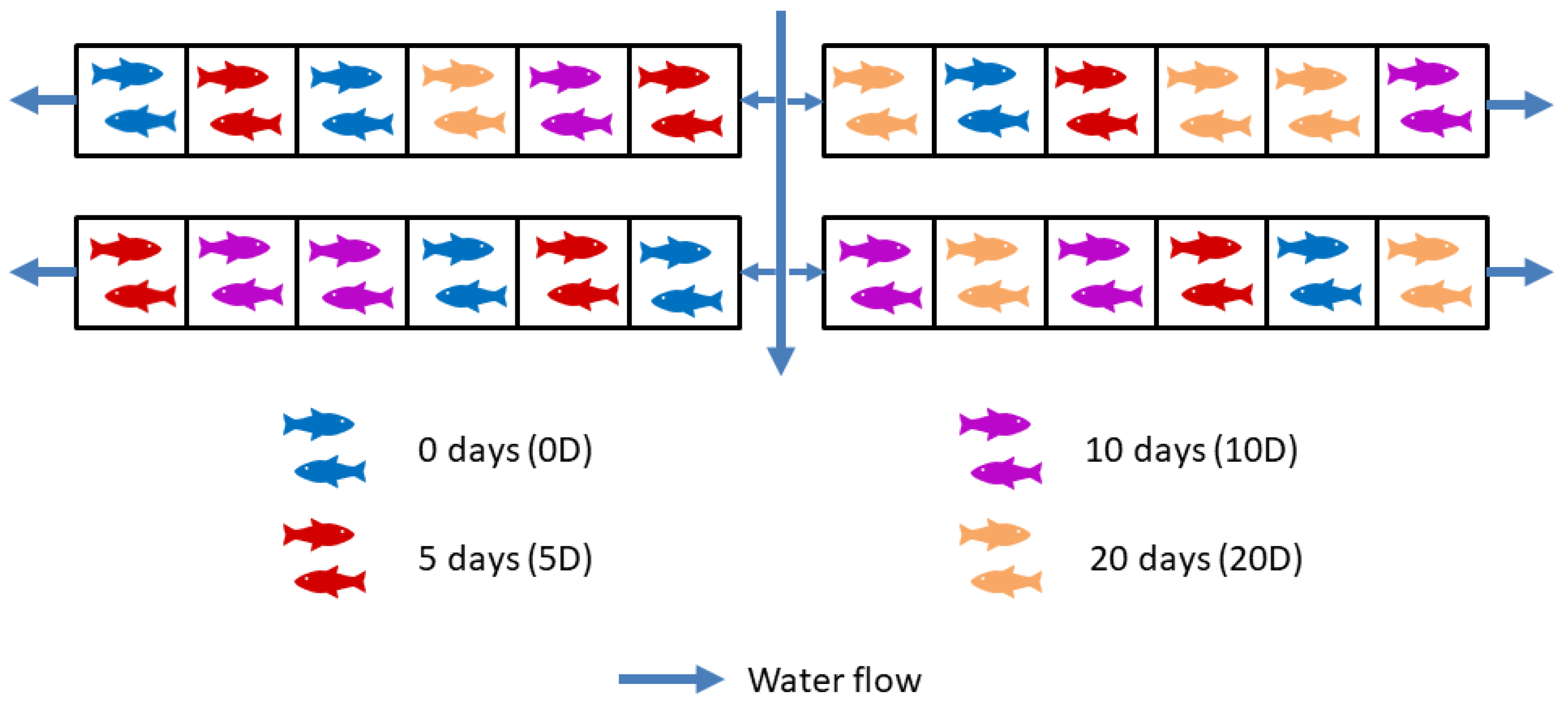
2.1. Experimental Design
2.2. Assay Procedures
2.3. Statistical Analysis
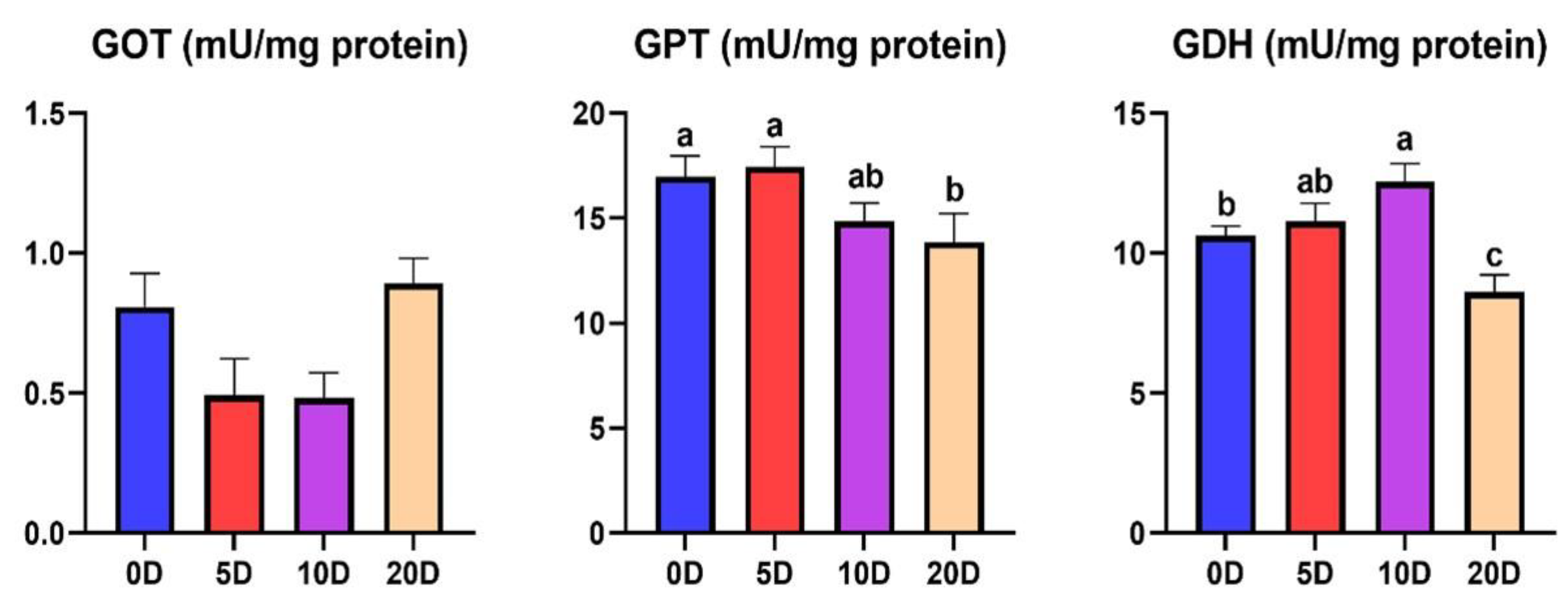
3. Results
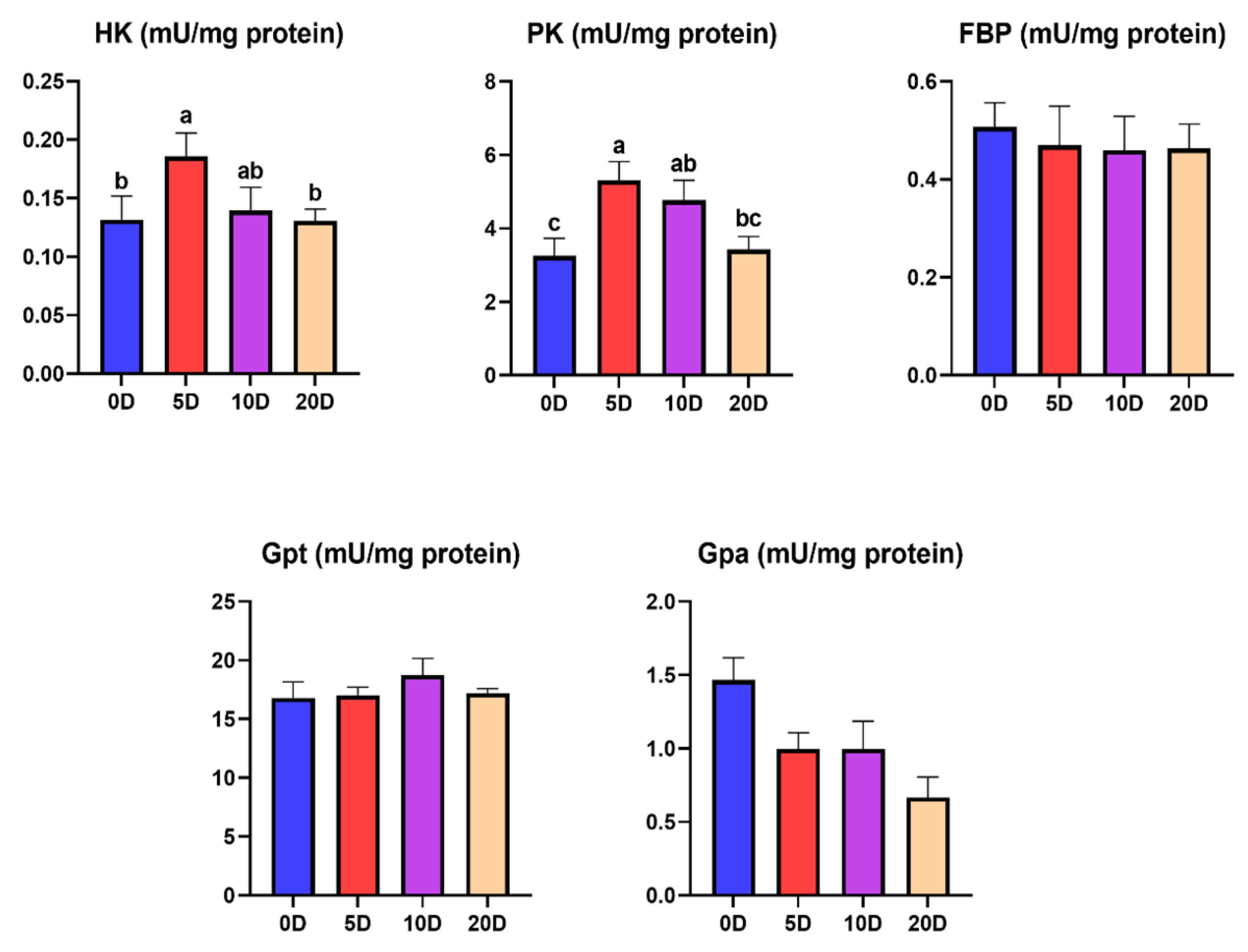
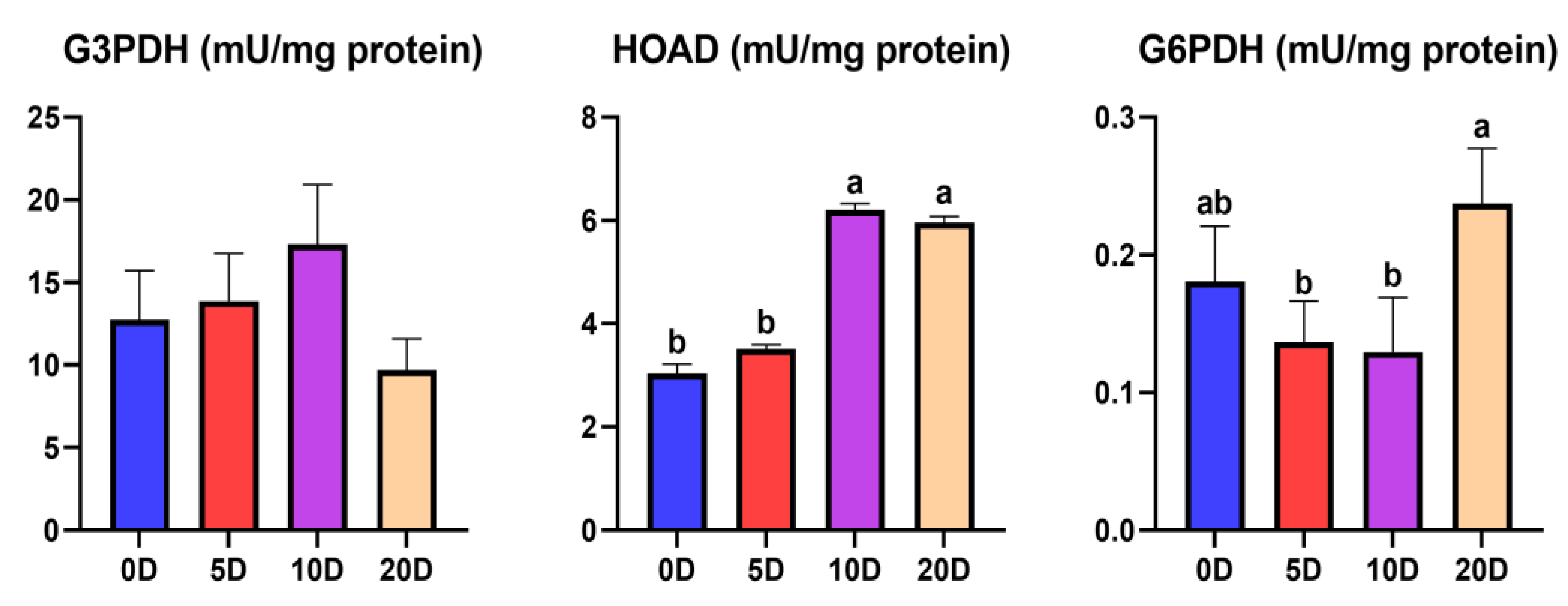
3.1. Enzymes of the Intermediate Metabolism in Muscle
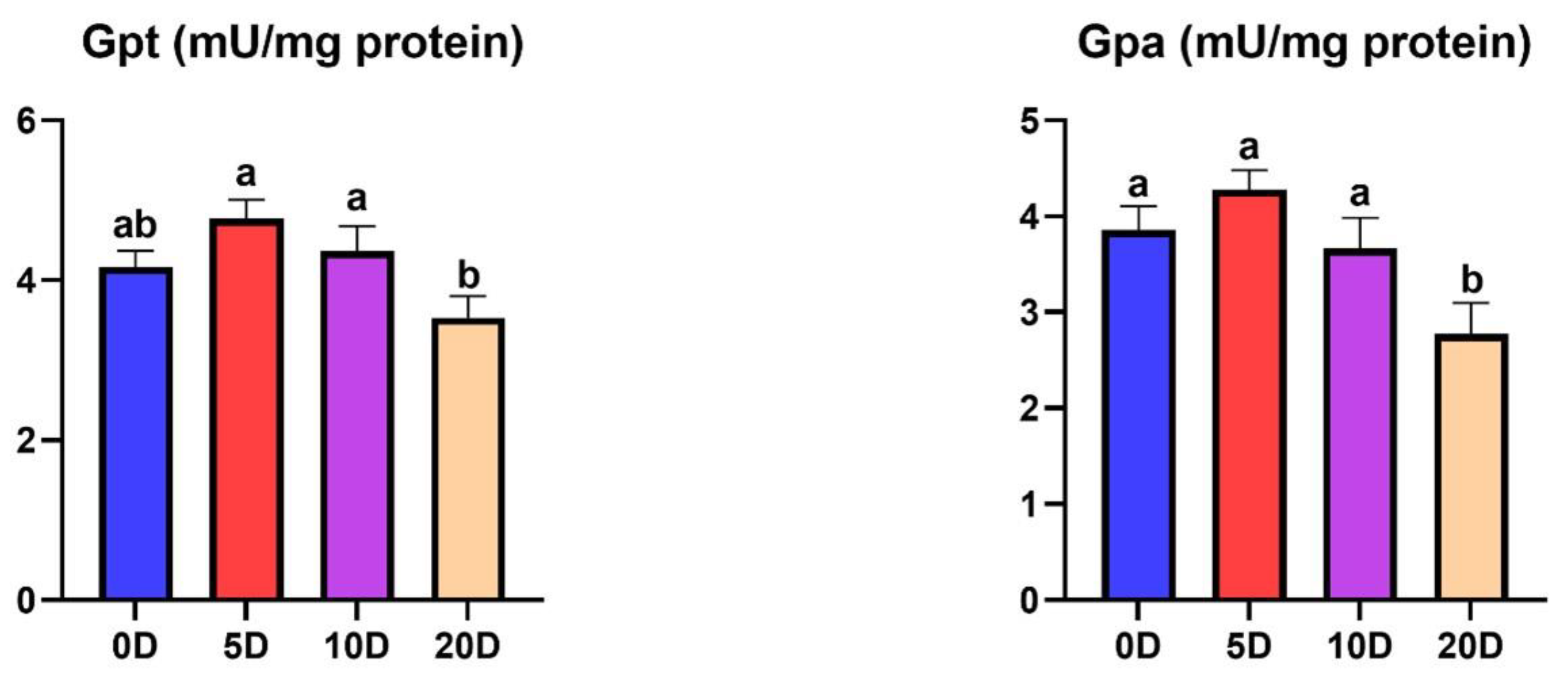
3.2. Enzymes of the Intermediate Metabolism in Liver
4. Discussion
4.1. Enzymes of the Intermediate Metabolism in Muscle
4.2. Enzymes of the Intermediate Metabolism in Liver
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, H.; Romsos, D.R.; Tack, P.I.; Leveille, G.A. Influence of dietary lipid on lipogenic enzyme activities in coho salmon, Oncorhynchus kisutch (Walbaum). J. Nutr. 1977, 107, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Karatas, T.; Onalan, S.; Yildirim, S. Effects of prolonged fasting on levels of metabolites, oxidative stress, immune-related gene expression, histopathology, and DNA damage in the liver and muscle tissues of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2021, 47, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- López-Luna, J.; Vásquez, L.; Torrent, F.; Villarroel, M. Short-term fasting and welfare prior to slaughter in rainbow trout, Oncorhynchus mykiss. Aquaculture 2013, 400, 142–147. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Ostenfeld, T.H.; Ronsholdt, B.; McLean, E. Manipulation of end-product quality of rainbow trout with finishing diets. Aquacult. Nutr. 2000, 6, 17–24. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Rand-Weaver, M.; Sumpter, J.P. Overwinter fasting and re-feeding in rainbow trout: Plasma growth hormone and cortisol levels in relation to energy mobilisation. Comp. Biochem. Phys. B 2003, 136, 403–417. [Google Scholar] [CrossRef]
- Navarro, I.; Gutierrez, J. Chapter 17 Fasting and starvation. In Biochemistry and Molecular Biology of Fishes, 1st ed.; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1995; Volume 4, pp. 393–434. [Google Scholar]
- Vigliano, F.A.; Quiroga, M.I.; Nieto, J.M. Metabolic adaptation to food deprivation and refeeding in fish. Rev. Ictiol. 2002, 10, 79–108. [Google Scholar]
- Barcellos, L.J.G.; Marqueze, A.; Trapp, M.; Quevedo, R.M.; Ferreira, D. The effects of fasting on cortisol, blood glucose and liver and muscle glycogen in adult jundiá Rhamdia quelen. Aquaculture 2010, 300, 231–236. [Google Scholar] [CrossRef]
- Cook, J.T.; McNiven, M.A.; Richardson, G.F.; Sutterlin, A.M. Growth rate, body composition and feed digestibility conversion of growth-enhanced transgenic Atlantic salmon, Salmo salar. Aquaculture 2000, 188, 15–32. [Google Scholar] [CrossRef]
- Moon, T.W. Adaptation, constraint, and the function of the gluconeogenesis pathway. Can. J. Zool. 1988, 66, 1059–1068. [Google Scholar] [CrossRef]
- Karatas, T. Effect of short-term starvation on serum metabolites, antioxidant enzymes and endogenous reserves of rainbow trout, Oncorhynchus mykiss. Pak. J. Zool. 2018, 50, 1723–1729. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Y.; Guo, J.; Yang, H.; Chen, F.; Zhang, W.; Wu, W.; Xu, X.; Li, J. Glycolysis and gluconeogenesis are involved of glucose metabolism adaptation during fasting and re-feeding in black carp (Mylopharyngodon piceus). Aquac. Fish. 2022; in press. [Google Scholar] [CrossRef]
- Wang, T.; Hung, C.C.; Randall, D.J. The comparative physiology of food deprivation: From feast to famine. Annu. Rev. Physiol. 2006, 68, 223–251. [Google Scholar] [CrossRef]
- Furné, M.; Morales, A.E.; Trenzado, C.E.; García-Gallego, M.; Carmen Hidalgo, M.; Domezain, A.; Sanz Rus, A. The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J. Comp. Physiol. B 2012, 182, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Laiz-carrión, R.; Martín Del Río, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Influence of cortisol on osmoregulation and energy metabolism in gilthead seabream Sparus aurata. J. Exp. Zool. Part A 2003, 298, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Polakof, S.; Arjona, F.J.; Kleszczynska, A.; Del Río, M.P.M.; Míguez, J.M.; Soengas, J.L.; Mancera, J.M. Osmoregulatory and metabolic changes in the gilthead sea bream Sparus auratus after arginine vasotocin (AVT) treatment. Gen. Comp. Endocrinol. 2006, 148, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Arjona, F.J.; Sangiao-Alvarellos, S.; Martín del Río, M.P.; Mancera, J.M.; Soengas, J.L. Food deprivation alters osmoregulatory and metabolic responses to salinity acclimation in gilthead sea bream Sparus auratus. J. Comp. Physiol. B 2006, 176, 441–452. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Arjona, F.J.; Laiz-Carrión, R.; Flik, G.; Klaren, P.H.; Mancera, J.M. Energy metabolism of hyperthyroid gilthead sea bream Sparus aurata L. Comp. Biochem. Phys. A 2016, 191, 25–34. [Google Scholar] [CrossRef]
- Soengas, J.L.; Strong, E.F.; Fuentes, J.; Veira, J.A.; Andrés, M.D. Food deprivation and refeeding in Atlantic salmon, Salmo salar: Effects on brain and liver carbohydrate and ketone bodies metabolism. Fish Physiol. Biochem. 1996, 15, 491–511. [Google Scholar] [CrossRef]
- Soengas, J.L.; Strong, E.F.; Andres, M.D. Glucose, lactate, and b-hydroxybutyrate utilization by rainbow trout brain: Changes during food deprivation. Physiol. Zool. 1998, 71, 285–293. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Suarez, R.K.; Mommsen, T.P. Gluconeogenesis in teleost fishes. Can. J. Zool. 1987, 65, 1869–1882. [Google Scholar] [CrossRef]
- Bermejo-Poza, R.; Fernández-Muela, M.; De la Fuente, J.; Pérez, C.; de Chavarri, E.G.; Díaz, M.T.; Torrent, F.; Villarroel, M. Physio-Metabolic response of rainbow trout during prolonged food deprivation before slaughter. Fish Physiol. Biochem. 2019, 45, 253–265. [Google Scholar] [CrossRef]
- Costas, B.; Aragão, C.; Ruiz-Jarabo, I.; Vargas-Chacoff, L.; Arjona, F.J.; Dinis, M.T.; Mancera, J.M.; Conceição, L.E. Feed deprivation in Senegalese sole (Solea senegalensis Kaup, 1858) juveniles: Effects on blood plasma metabolites and free amino acid levels. Fish Physiol. Biochem. 2011, 37, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.; Eilertson, C.; Sheridan, M.; Plisetskaya, E. Insulin suppression is associated with hypersomatostatinemia and hyperglucagonemia in glucose-injected trout. Am. J. Physiol. 1991, 261, R609–R613. [Google Scholar] [CrossRef]
- Metón, I.; Fernández, F.; Baanante, I.V. Short- and long-term effects of refeeding on key enzyme activities in glycolysis–gluconeogenesis in the liver of gilthead seabream (Sparus aurata). Aquaculture 2003, 225, 99–107. [Google Scholar] [CrossRef]
- Lu, D.L.; Ma, Q.; Wang, J.; Li, L.Y.; Han, S.L.; Limbu, S.M.; Li, D.L.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. Fasting enhances cold resistance in fish through stimulating lipid catabolism and autophagy. J. Physiol. 2019, 597, 1585–1603. [Google Scholar] [CrossRef]
- De la Roche, M.; Tessier, S.N.; Storey, K.B. Structural and Functional Properties of Glycerol-3-Phosphate Dehydrogenase from a Mammalian Hibernator. Protein J. 2012, 31, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.R.; Garofalo, M.A.R.; Roselino, J.E.S.; Kettelhut, I.C.; Migliorini, R.H. Effects of starvation, refeeding, and insulin on energy-linked metabolic processes in catfish (Rhamdia hilarii) adapted to a carbohydrate-rich diet. Gen. Comp. Endocrinol. 1988, 71, 429–437. [Google Scholar] [CrossRef]
- Frick, N.T.; Bystriansky, J.S.; Ip, Y.K.; Chew, S.F.; Ballantyne, J.S. Carbohydrate and amino acid metabolism in fasting and aestivating African lungfish (Protopterus dolloi). Comp. Biochem. Physiol. B 2008, 151, 85–92. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Cardenete, G.; Hidalgo, M.C.; García-Alcázar, A.; Abellán, E.; Morales, A.E. Metabolic adjustments of Dentex dentex to prolonged starvation and refeeding. Fish Physiol. Biochem. 2012, 38, 1145–1157. [Google Scholar] [CrossRef]
- Viegas, I.; Caballero-Solares, A.; Rito, J.; Giralt, M.; Pardal, M.A.; Metón, I.; Jones, J.G.; Baanante, I.V. Expressional regulation of key hepatic enzymes of intermediary metabolism in European seabass (Dicentrarchus labrax) during food deprivation and refeeding. Comp. Biochem. Physiol. A 2014, 174, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Caseras, A.; Metón, I.; Vives, C.; Egea, M.; Fernández, F.; Baanante, A.I. Nutritional regulation of glucose-6-phosphatase gene expression in liver of the gilthead sea bream (Sparus aurata). Brit. J. Nutr. 2002, 88, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.D.; Mahadevappa, V.G.; Ballantyne, J.S. Aspects of the energy metabolism of lake sturgeon, Acipenser fulvescens, with special emphasis on lipid and ketone body metabolism. Can. J. Fish. Aquat. Sci. 1990, 47, 873–881. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Moon, T.W. The metabolic potential of hepatocytes and kidney tissue in the little skate, Raja erinacea. J. Exp. Zool. 1987, 244, 1–8. [Google Scholar] [CrossRef]
- Singer, T.D.; Ballantyne, J.S. Absence of extrahepatic lipid oxidation in a freshwater elasmobranch, the dwarf stingray Potamotrygon magdalenae: Evidence from enzyme activities. J. Exp. Zool. 1989, 251, 355–360. [Google Scholar] [CrossRef]
- Kirchner, S.; Seixas, P.; Kaushik, S.; Panserat, S. Effects of low protein intake on extra-hepatic gluconeogenic enzyme expression and peripheral glucose phosphorylation in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B 2005, 140, 333–340. [Google Scholar] [CrossRef]
- Collins, A.L.; Anderson, T.A. The influence of changes in food availability on the activities of key degradative and metabolic enzymes in the liver and epaxial muscle of the golden perch. J. Fish Biol. 1997, 50, 1158–1165. [Google Scholar] [CrossRef]
- Shimeno, S.; Shikata, T.; Hosokawa, H.; Masumoto, T.; Kheyyali, D. Metabolic response to feeding rates in common carp, Cyprinus carpio. Aquaculture 1997, 151, 371–377. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Guedes, M.J.; Morales, A.E.; Oliva-Teles, A. Metabolic responses to short starvation and refeeding in Dicentrarchus labrax. Effect of dietary composition. Aquaculture 2007, 265, 325–335. [Google Scholar] [CrossRef]
- Polakof, S.; Ceinos, R.; Fernández-Duran, B.; Míguez, J.; Soengas, J. Daily changes in parameters of energy metabolism in brain of rainbow trout: Dependence on feeding. Comp. Biochem. Physiol. A 2007, 146, 265–273. [Google Scholar] [CrossRef]
- Bermejo-Poza, R.; De la Fuente, J.; Pérez, C.; González de Chavarri, E.; Diaz, M.T.; Torrent, F.; Villarroel, M. Determination of optimal degree days of fasting before slaughter in rainbow trout (Oncorhynchus mykiss). Aquaculture 2017, 473, 272–277. [Google Scholar] [CrossRef]
- Aster, P.L.; Moon, T.W. Influence of the fasting and diet on lipogenic enzymes in the American eel, Anguilla rostrata (LeSueur). J. Nutr. 1981, 111, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, F.; Ohshima, H.; Umezawa, K. Distribution of glucose-6-phosphate metabolizing enzymes in fish. Bull. Jpn. Sot. Sci. Fish 1972, 38, 589–593. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Rodríguez-Sabarís, R.; Aldegunde, M.; Soengas, J.L. Effects of food deprivation on 24 h-changes in brain and liver carbohydrate and ketone body metabolism of rainbow trout. J. Fish Biol. 2000, 57, 631–646. [Google Scholar] [CrossRef]
- Favero, G.C.; Gimbo, R.Y.; Franco Montoya, L.N.; Zanuzzo, F.S.; Urbinati, E.C. Fasting and refeeding lead to more efficient growth in lean pacu (Piaractus mesopotamicus). Aquac. Res. 2018, 49, 359–366. [Google Scholar] [CrossRef]
- Favero, G.; Gimbo, R.Y.; Montoya, L.N.F.; Carneiro, D.J.; Urbinati, E.C. A fasting period during grow-out make juvenile pacu (Piaractus mesopotamicus) leaner but does not impair growth. Aquaculture 2020, 524, 735242. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Muela, M.; Bermejo-Poza, R.; Cabezas, A.; Pérez, C.; González de Chavarri, E.; Díaz, M.T.; Torrent, F.; Villarroel, M.; De la Fuente, J. Effects of Fasting on Intermediary Metabolism Enzymes in the Liver and Muscle of Rainbow Trout. Fishes 2023, 8, 53. https://doi.org/10.3390/fishes8010053
Fernández-Muela M, Bermejo-Poza R, Cabezas A, Pérez C, González de Chavarri E, Díaz MT, Torrent F, Villarroel M, De la Fuente J. Effects of Fasting on Intermediary Metabolism Enzymes in the Liver and Muscle of Rainbow Trout. Fishes. 2023; 8(1):53. https://doi.org/10.3390/fishes8010053
Chicago/Turabian StyleFernández-Muela, Montserrat, Rubén Bermejo-Poza, Almudena Cabezas, Concepción Pérez, Elisabet González de Chavarri, María Teresa Díaz, Fernando Torrent, Morris Villarroel, and Jesús De la Fuente. 2023. "Effects of Fasting on Intermediary Metabolism Enzymes in the Liver and Muscle of Rainbow Trout" Fishes 8, no. 1: 53. https://doi.org/10.3390/fishes8010053
APA StyleFernández-Muela, M., Bermejo-Poza, R., Cabezas, A., Pérez, C., González de Chavarri, E., Díaz, M. T., Torrent, F., Villarroel, M., & De la Fuente, J. (2023). Effects of Fasting on Intermediary Metabolism Enzymes in the Liver and Muscle of Rainbow Trout. Fishes, 8(1), 53. https://doi.org/10.3390/fishes8010053







