Integrative Analysis to Manage Aquatic Resources Based on Fish Feeding Patterns in Neotropical Rivers
Abstract
1. Introduction
2. Materials and Methods
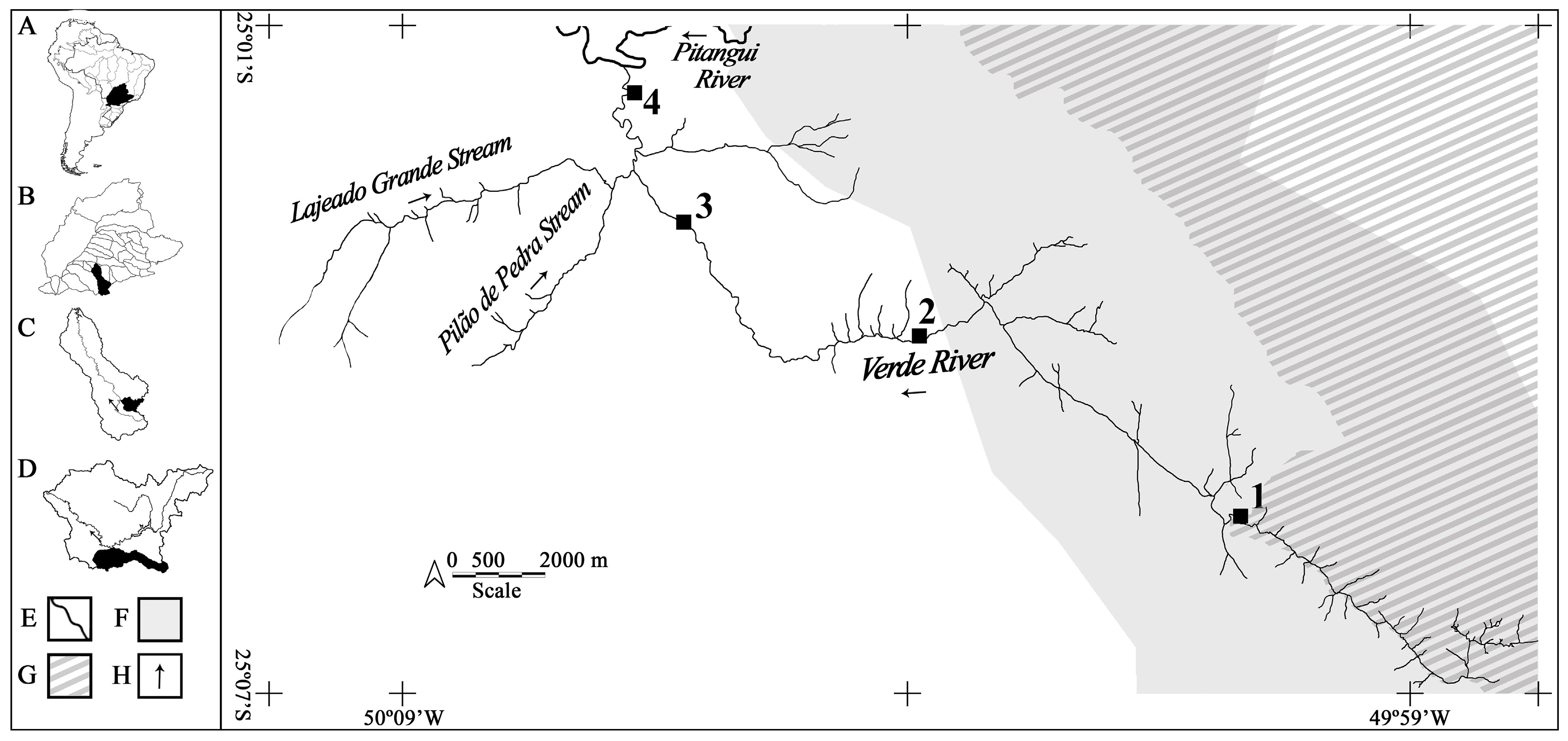
2.1. Sampling Area
2.2. Data Sampling
2.3. Data Analysis
3. Results
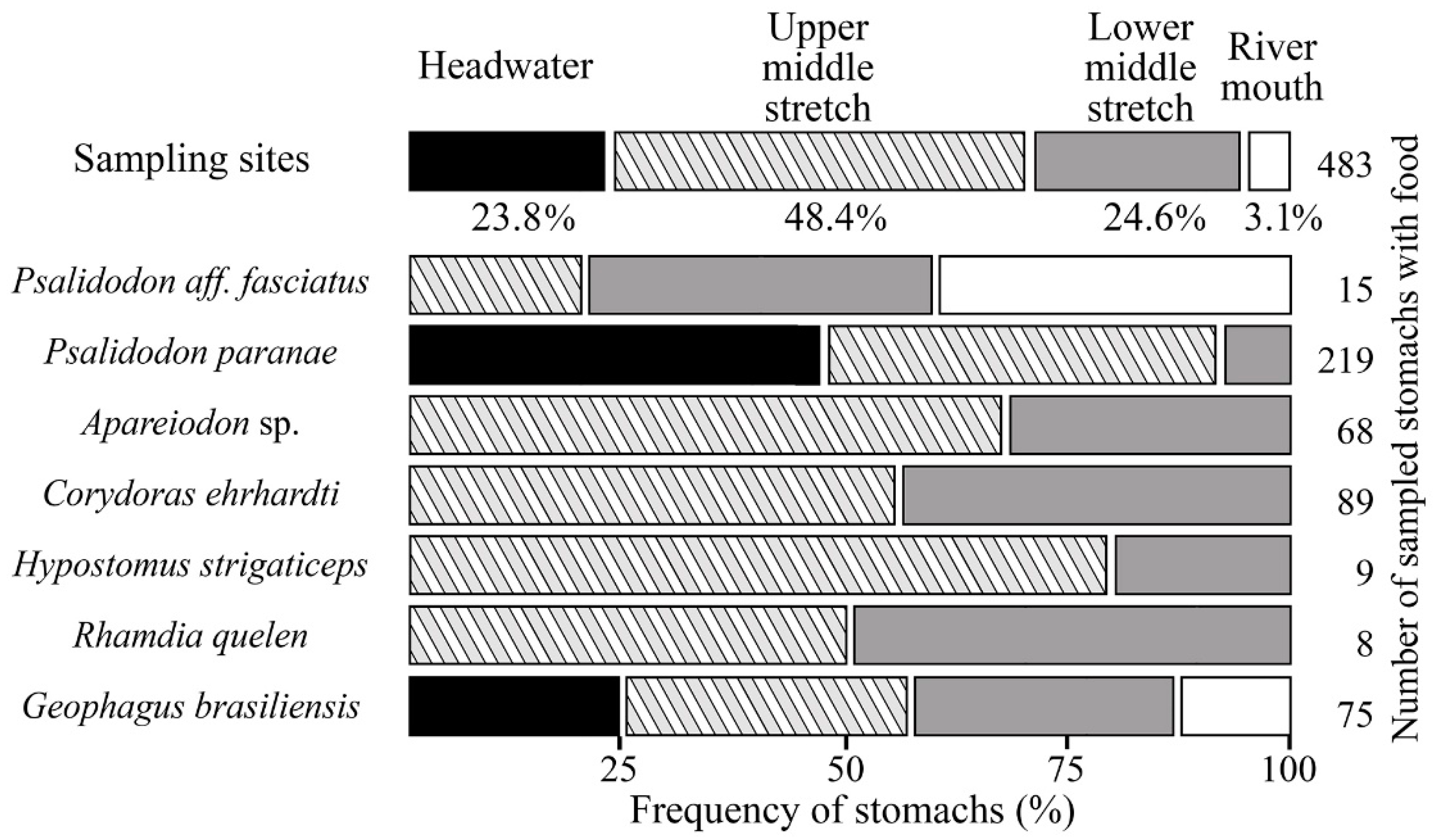
3.1. Spatial Distribution Analysis of Fish Species
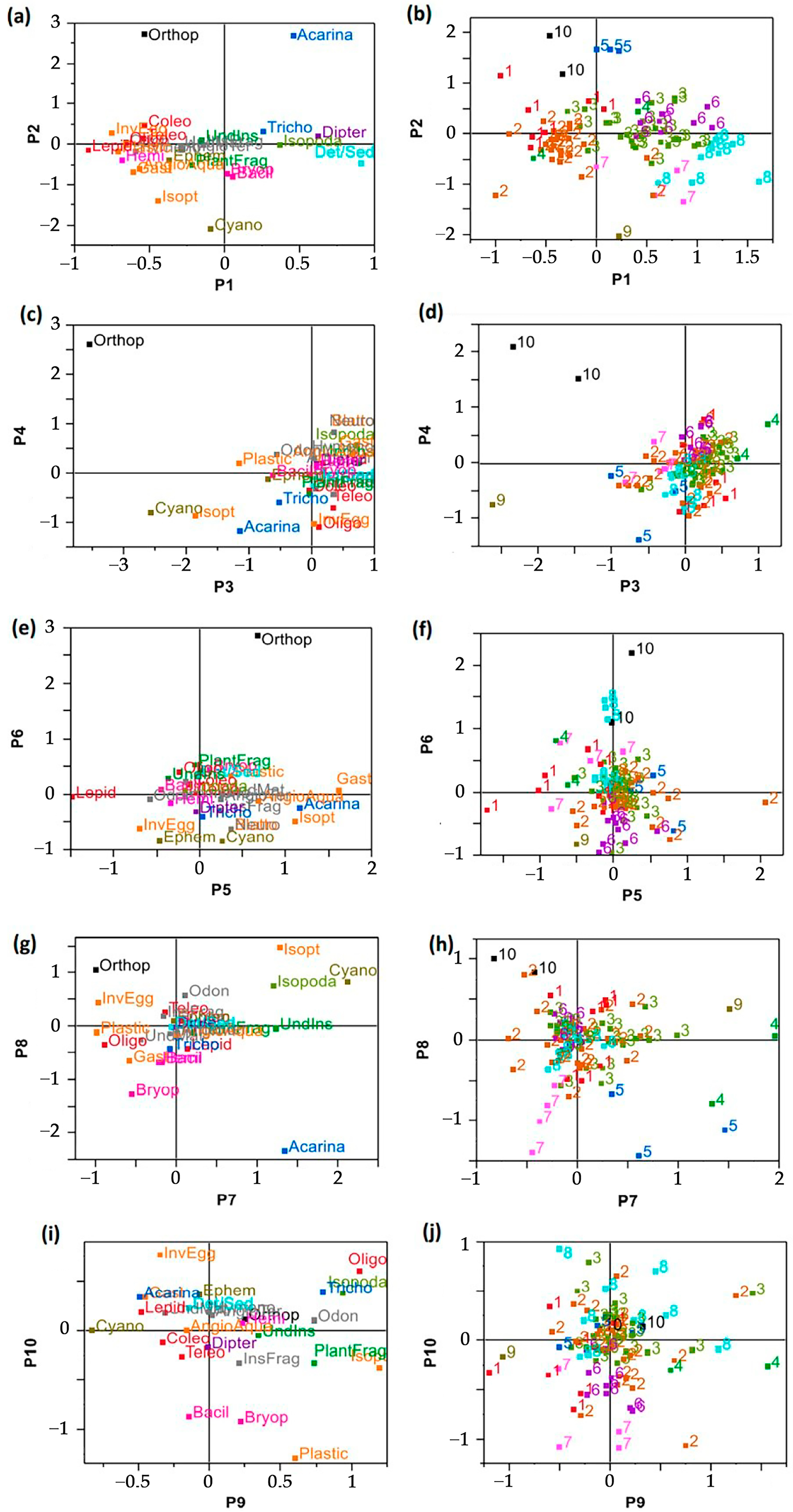
3.2. Highlighting of Feeding Trends
- Along P1, Diptera and sediments separated FTs 3, 6–8 from the rest of data with relatively high associations between FT6, FT8 and Diptera, Sediments, respectively (Figure 4a,b). However, FT2 showed opposite projection along P1 indicating relatively low consumption of Diptera and Sediments (Figure 4b). Moreover, on the negative side of P1, FTs 1 and 2 were distinguished by relatively high consumptions of Lepidoptera (absent elsewhere). This was indicative of different feeding behaviors combining targeting/preferences for some food categories and avoidances for other ones.
- Along P3, FTs 10, 5, 9 were further distinguished by relatively high consumptions of specific food categories (Orthoptera, Acarina, Cyanophyta, respectively) (quasi-absent elsewhere) (Figure 4d).
- Along P4, Orthoptera and Teleostean (were the most contributing food variables. Their opposition separated FT10 from FT1 with relatively high consumption of Orthoptera in FT10 (null in FT1) and Teleostean in FT1 (null in FT10) (Figure 4c,d).
- Along P5, FT1 and FT2 opposed because of higher relative consumption levels (preference) of Lepidoptera in FT1 (lower in FT2) vs. opposite pattern for Aquatic Angiosperm, Gastropoda and Isoptera showing high consumption in FT2 (and low in FT1) (Figure 4c,d).
- Along P6, Ephemeroptera was the most contributing variable with relatively high consumptions in FT9 and some individuals of FTs 2 and 6 (Figure 4e,f). On the opposite side of P6, FT10 was marked by the absence of Ephemeroptera feeding.
- Along P7, FTs 3, 4, 5 were opposed to FTs 1, 2, 10 along P7 due to their relatively higher consumptions of Undetermined Insects (the most contributive variable to P7) (Figure 4g–h). Moreover, FTs 3, 5 were characterized by relatively high consumption of Isopoda and Acarina, respectively. Further, FT9 was revealed to be distinguished by Cyanophyta in addition to Undetermined insects. On the opposite side, Invertebrate Eggs characterized FT2.
- Along P8, Bacillariophyta and Bryophyta were the most contributing variables and characterized FT7 (Figure 4g–h). Bryophyta consumption was quasi-absent in the other FTs. By this way, FT7 was revealed to be specific feeding patterns of Bryophyta.
- Along P9, Plant Fragments, Coleoptera and Trichoptera were the most contributing variables (Figure 4i). Trichoptera consumption was shared by FTs 2, 3 and 8 (on the positive side of P9) (Figure 4j). Plant Fragments characterized more particularly FT4. On the opposite side, FT1 was characterized by Coleoptera in addition to Lepidoptera and Teleostean with absence of Trichoptera).
- Along P10, Bacillariophyta was the most contributing variable by characterizing FT7 (Figure 4i,j) by representing 20–50% a whole feeding profile.
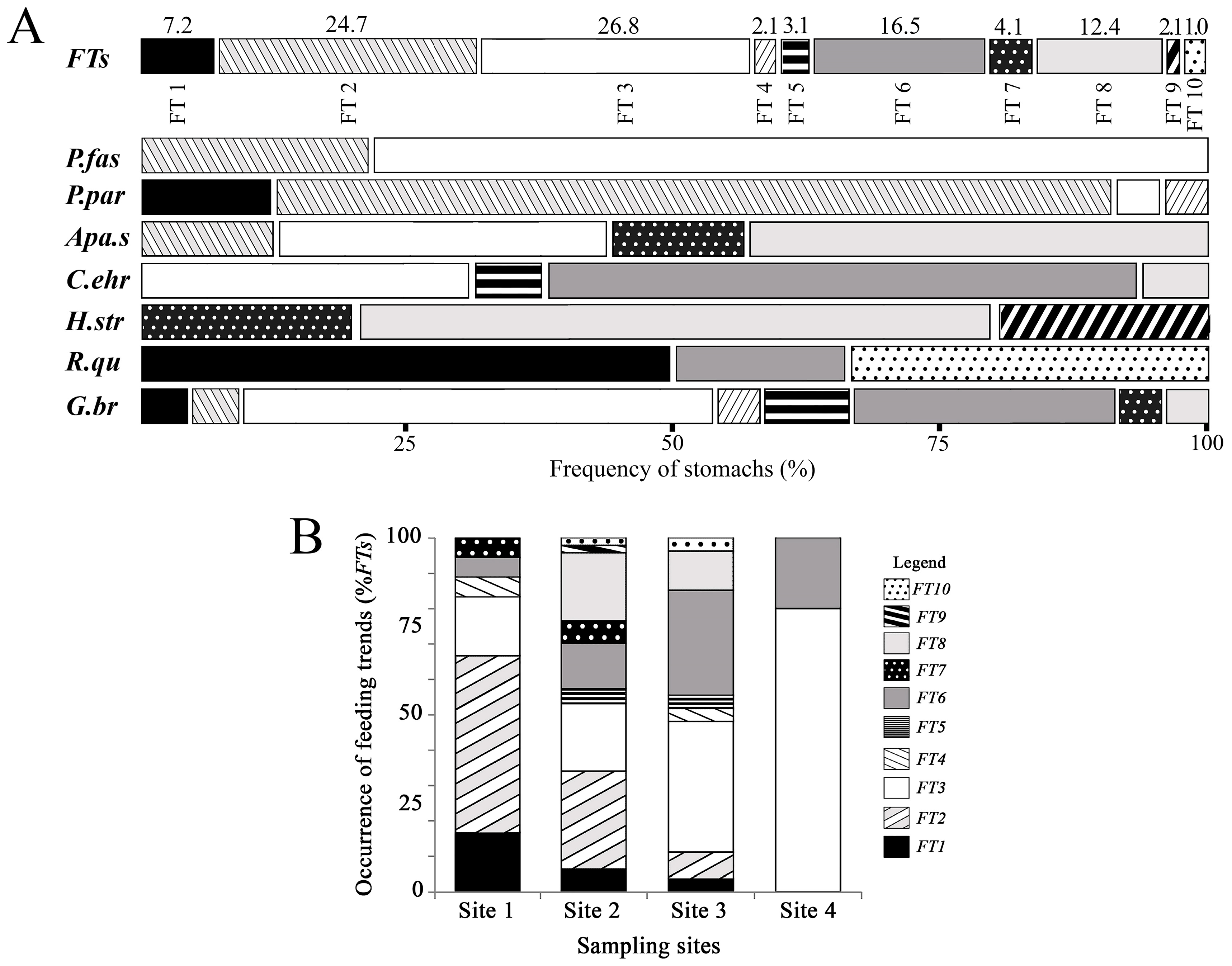
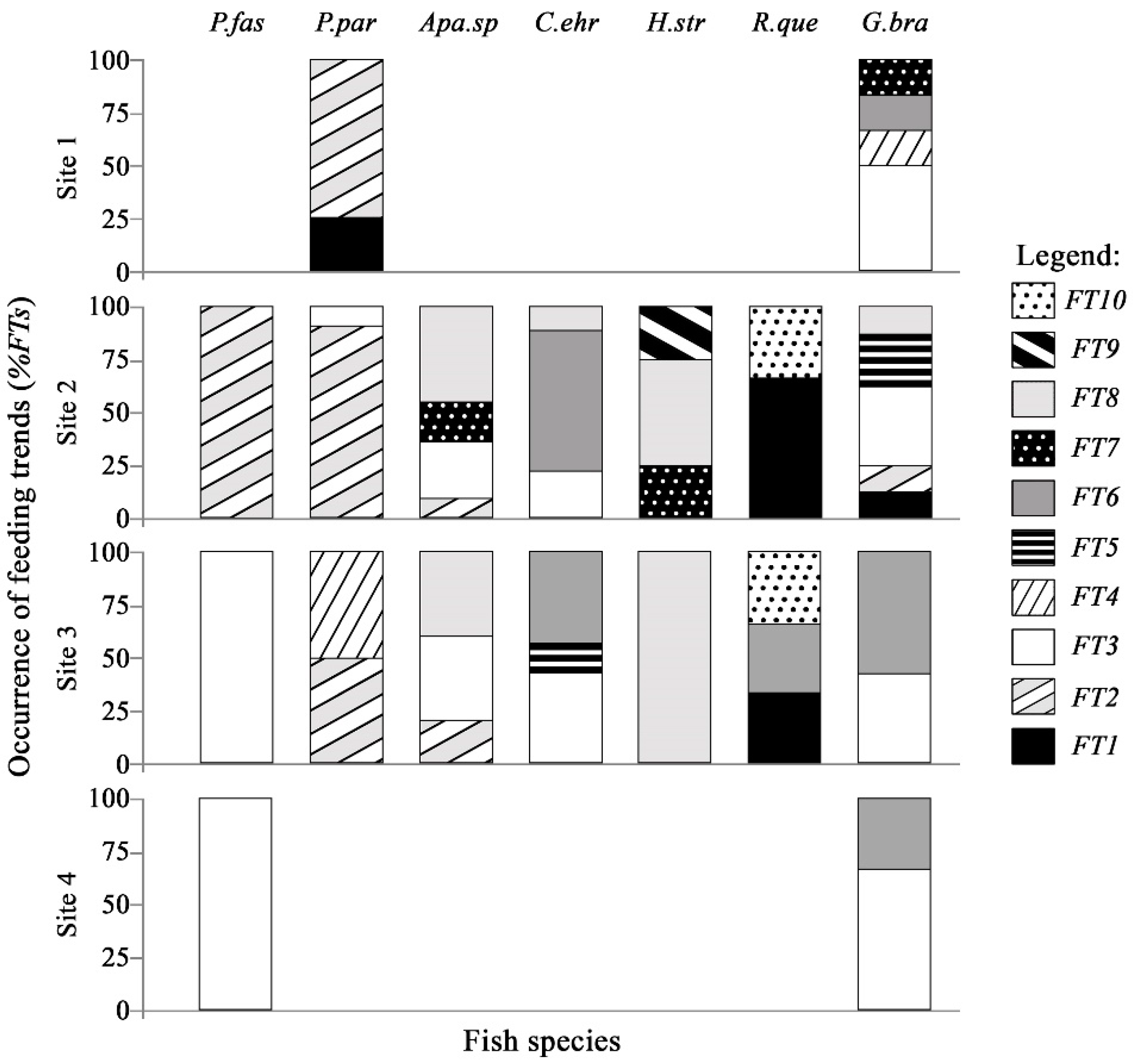
3.3. Association between Feeding Trends and Fish Species
3.4. Distribution Analysis of Feeding Trends in Sampling Sites
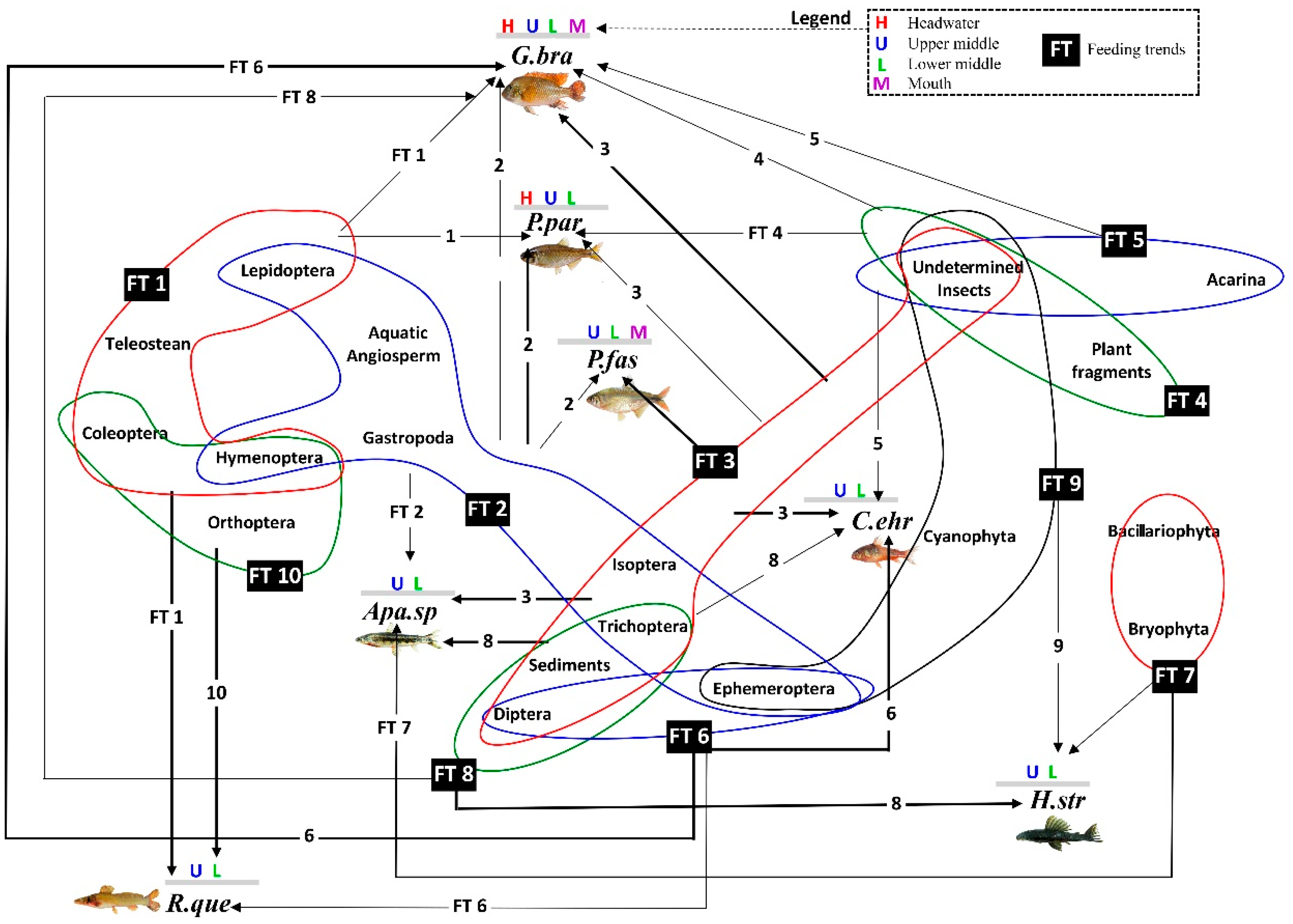
3.5. Link Analysis between Feeding Trends and Species–Sites Interaction
- For P. paranae, FT2 was the main feeding way and was completed by FTs 1, 3 and 4 at headwater, upper and lower middle stretches, respectively. Percentages of FT2 in P. paranae were higher at headwater and upper middle stretch than lower middle stretch, being positively associated with the occurrence levels of the species in these sites. This leads to a positive trend (attractive feeding process) between P. paranae and FT2. Plant and insect consumptions varied by opposite ways between headwater, upper (showing plant dominance; FTs 1, 2) and lower middle stretches (showing relatively more captured insects, FT4).
- In G. brasiliensis, diets showed differential variations alternating between FTs 3 and 6 according to the different sites. FT3 occurred in all the sites and was particularly dominant at headwater and river mouth, where the species consumed plants and aquatic and terrestrial insects. However, FT6 represented a main food source at headwater and lower middle stretch, highlighting insectivorous diets focused on dipterids.
- Apareiodon sp. showed heterogeneous diet based on FTs 2, 3 and 8 in both upper and lower middle stretches. Moreover, the fish species showed some occurrence of FT7 in the upper middle stretch.
- Corydoras ehrhardti (occurring in upper and lower middle stretch) showed diet mainly based on FTs 3 and 6. It was the fish species to be associated with FT6 at upper middle stretch, indicating a strong preference of Diptera.
- Hypostomus strigaticeps showed a diversified diet at upper middle stretch, combining FTs 8, 7 and 9 (including Bacillariophyta, Cyanophyta, Bryophyta, plant fragments). However, FT8-based exclusively diet (highlighting Sediments) occurred at lower middle stretch. This indicated a constrained feeding response to a loss of environmental quality. FT9 was particularly concerned with H. strigaticeps and manifested only at upper middle stretch.
- Rhamdia quelen showed a diet based on FTs 1 and 10 occurring at upper and lower middle stretches. FT10 was specific to R. quelen indicating feeding motivation for big insects. Further, the occurrence of FT1 highlighted the feeding motivation of R. quelen for big prey (Orthoptera, Coleoptera, Teleostei). However, this fish species manifested FT6 at lower middle stretch, highlighting a higher trend for insect consumption. Moreover, the occurrence of FT6 in site 3 (versus absence in site 2) could be indicative of feeding response to a loss of environmental quality.
4. Discussion
4.1. Phenotypic and Behavioral Dietary Adaptations
4.2. Spatial-Interspecific Diet Trends
- The diet of G. brasiliensis was focused on benthic prey items and complemented by terrestrial items, such as Hymenoptera and seeds. This trend is common in freshwater fishes inhabiting areas with good status of riverbank conservation that tend to be energetically dependent on allochthonous resources [61,62,99].
- In contrast, C ehrhardti, G. brasiliensis and R. quelen adopted highly selective diets. The former and the second species mainly consumed Diptera larvae. The latter mainly exploited big terrestrial insects (Coleoptera and Orthoptera), highlighting the relevance of the riparian vegetation in providing energetic resources for the aquatic systems [41,97]. These focused diets reflect the intrinsic features of each species, considering the effectiveness of their sensory and mechanical apparatus for seeking and selecting nutritious foods [69,80,82,84,92,104].
- Finally, Apareiodon sp. and H. strigaticeps consumed aquatic resources, focusing on Diptera larvae and Bacillariophyta with recurrent ingestion of sediments. Both species are morphologically well adapted to inhabit stable riverbeds [43,48,78], commonly ingesting large amounts of sediments and detritus associated with benthic invertebrates [75,78].
- Psalidodon aff. fasciatus mainly consumed Diptera larvae, in addition to terrestrial plants and invertebrates, whilst P. paranae showed a generalist diet, consuming aquatic plants and invertebrates. The environmental conditions of this area reduced the influx of terrestrial energetic resources into the river, leading fishes to exploit foods from aquatic origin [41,55,95].
- Apareiodon sp., C. ehrhardti, G. brasiliensis and H. strigaticeps mainly consumed Diptera larvae and sediments, while R. quelen recurrently consumed Diptera larvae and big terrestrial insects (Coleoptera and Orthoptera). An increase in the ingestion of sediments is a consequence of the environmental changes noticed in the lower middle stretch, changing the availability of potential foods for the ichthyofauna [64,95,96,97].
- Psalidodon aff. fasciatus and G. brasiliensis were highly dependent on Diptera chironomids larvae and sediments. Although chironomids represent a highly diversified, abundant, and nutritive insect group distributed in worldwide freshwater environments [72,91,108], their strongest predominance in the diet of P. aff. fasciatus and G. brasiliensis indicate a decrease in the diversity and abundance of other aquatic macroinvertebrates [104,108,109,110].
4.3. Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gotelli, N.J.; Ellison, A.M. A Primer of Ecological Statistics, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2013. [Google Scholar]
- Begon, M.L.; Townsend, C.R. Ecology: From Individuals to Ecosystems, 5th ed.; John Wiley & Sons: Oxford, UK, 2021. [Google Scholar]
- MacArthur, R.H.; Pianka, E.R. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. [Google Scholar] [CrossRef]
- Emlen, J.M. The role of time and energy in food preference. Am. Nat. 1966, 100, 611–617. [Google Scholar] [CrossRef]
- Toscano, B.J.; Gownaris, N.J.; Heerhartz, S.M.; Monaco, C.J. Personality, foraging behavior and specialization: Integrating behavioral and food web ecology at the individual level. Oecologia 2016, 182, 55–69. [Google Scholar] [CrossRef]
- Gilmour, M.E.; Castillo-Guerrero, J.A.; Fleishman, A.B.; Hernández-Vázquez, S.; Young, H.S.; Shaffer, S.A. Plasticity of foraging behaviors in response to diverse environmental conditions. Ecosphere 2018, 9, e02301. [Google Scholar] [CrossRef]
- Cozzoli, F.; Ligetta, G.; Vignes, F.; Basset, A. Revisiting GUD: An empirical test on size-dependencies of patch exploitation behaviour. PLoS ONE 2018, 13, e0204448. [Google Scholar] [CrossRef]
- Roman, L.; Bell, E.; Wilcox, C.; Hardesty, B.D.; Hindell, M. Ecological drivers of marine debris ingestion in Procellariiform Seabirds. Sci. Rep. 2019, 9, 916. [Google Scholar] [CrossRef]
- Cozzoli, F.; Shokri, M.; Boulamail, S.; Marrocco, V.; Vignes, F.; Basset, A. The size dependency of foraging behaviour: An empirical test performed on aquatic amphipods. Oecologia 2022, 199, 377–386. [Google Scholar] [CrossRef]
- Gerking, S.D. Feeding Ecology of Fish; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Helfman, G.S.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution, and Ecology, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2009. [Google Scholar]
- Lowe-McConnell, R.H. Ecological Studies in Tropical Fish Communities; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Froese, R.; Pauly, D. FishBase, Version 08/2022; Creative Commons Attribution-Noncommercial 3.0: Stockholm, Sweden, 2022. Available online: https://www.fishbase.org (accessed on 6 February 2023).
- Wootton, R.J. Ecology of Teleost Fishes, 2nd ed.; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar]
- Townsend, C.R.; Winfield, I.J. The application of Optimal Foraging Theory to feeding behaviour in fish. In Fish Energetics: New Perspectives; Tytler, P., Calow, P., Eds.; Croom Helm, Kent: London, UK, 1985; pp. 67–98. [Google Scholar]
- Silveira, E.L.; Semmar, N.; Cartes, J.E.; Tuset, V.M.; Lombarte, A.; Ballester, E.L.C.; Vaz-dos-Santos, A.M. Methods for Trophic Ecology Assessment in Fishes: A Critical Review of Stomach Analyses. Rev. Fish. Sci. Aquac. 2020, 28, 71–106. [Google Scholar] [CrossRef]
- Clarke, K.R.; Green, R.H. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Gatz, A.J. Community Organization in Fishes as Indicated by Morphological Features. Ecology 1979, 60, 711–718. [Google Scholar] [CrossRef]
- Werner, E.E.; Gilliam, J.F.; Hall, D.J.; Mittelbach, G.G. An Experimental Test of the Effects of Predation Risk on Habitat Use in Fish. Ecology 1983, 64, 1540–1548. [Google Scholar] [CrossRef]
- Delariva, R.L.; Agostinho, A.A. Relationship between morphology and diets of six neotropical loricariids. J. Fish. Biol. 2001, 58, 832–847. [Google Scholar] [CrossRef]
- Hahn, N.S.; Pavanelli, C.S.; Okada, E.K. Dental development and ontogenetic diet shifts of Roeboides paranensis Pignalberi (Osteichthyes, Characinae) in pools of the Upper Rio Paraná floodplain (State of Paraná, Brazil). Rev. Bras. Biol. 2000, 60, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.F.; Huntingford, F.A. Feeding and Avoiding Predation Hazard: The Behavioral Response of the Prey. Ethology 2010, 73, 56–68. [Google Scholar] [CrossRef]
- Bennemann, S.T.; Gealh, A.M.; Orsi, M.L.; Souza, L.M. Ocorrência e ecologia trófica de quatro espécies de Astyanax (Characidae) em diferentes rios da bacia do rio Tibagi, Paraná, Brasil. Iheringia Sér. Zool. 2005, 95, 247–254. [Google Scholar] [CrossRef]
- Mise, F.T.; Fugi, R.; Pagotto, J.P.A.; Goulart, E. The coexistence of endemic species of Astyanax (Teleostei: Characidae) is propitiated by ecomorphological and trophic variations. Biota Neotrop. 2013, 13, 21–28. [Google Scholar] [CrossRef]
- Manna, L.R.; Villéger, S.; Rezende, C.F.; Mazzoni, R. High intraspecific variability in morphology and diet in tropical stream fish communities. Ecol. Freshw. Fish 2019, 28, 41–52. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J.; Nunn, A.D.; Adams, C.E.; Amundsen, P.A. Causes and consequences of ontogenetic dietary shifts: A global synthesis using fish models. Biol. Rev. 2019, 94, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.E.; Albert, J.S.; Di Dario, F.; Mincarone, M.M.; Petry, P.; Rocha, L.A. Fish biodiversity and conservation in South America. J. Fish Biol. 2016, 89, 12–47. [Google Scholar] [CrossRef]
- Albert, J.S.; Tagliacollo, V.A.; Dagosta, F. Diversification of Neotropical Freshwater Fishes. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 27–53. [Google Scholar] [CrossRef]
- Nabil, S.; Roux, M. A new simplex approach to highlight multi-scale feeding behaviors in forager species from stomach contents: Application to insectivore lizard population. Biosystems 2014, 118, 60–75. [Google Scholar] [CrossRef]
- Esteves, K.E.; Aranha, J.M.R. Ecologia trófica de peixes de riacho. Oecol. Bras. 1999, 6, 157–182. [Google Scholar] [CrossRef]
- Esteves, K.E.; Aranha, J.M.R.; Albrecht, M.P. Ecologia trófica de peixes de riacho: Uma releitura 20 anos depois. Oecol. Bras. 2021, 25, 266–282. [Google Scholar] [CrossRef]
- Ahlbeck, I.; Hansson, S.; Hjerne, O. Evaluating fish diet analysis methods by individual-based modelling. Can. J. Fish. Aquat. Sci. 2012, 69, 1184–1201. [Google Scholar] [CrossRef]
- Greenacre, M.J. Theory and Applications of Correspondence Analysis; Academic Press: London, UK, 1984. [Google Scholar]
- Gordon, A.D. Classification; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Moro, R.S.; Carmo, M.R.B. A vegetação campestre nos Campos Gerais. In Patrimônio Natural dos Campos Gerais; Melo, M.S., Moro, R.S., Guimarães, G.B., Eds.; Editora UEPG: Ponta Grossa, Brazil, 2007; pp. 93–98. [Google Scholar]
- Gealh, A.M.; Silveira, E.L. Conhecendo os Peixes do Rio São João—Carambeí-Pr. In Rio São João, Carambeí, PR: Fonte de Vida, Cuidados Devidos; Gealh, A.M., Melo, M.S., Eds.; Editora UEPG: Ponta Grossa, Brazil, 2014; pp. 182–203. [Google Scholar]
- Guimarães, G.B.; Godoy, L.C.; Melo, M.S.; Flügel Filho, J.C. Geodiversidade. In Rio São João, Carambeí, PR: Fonte de Vida, Cuidados Devidos; Gealh, A.M., Melo, M.S., Eds.; Editora UEPG: Ponta Grossa, Brazil, 2014; pp. 15–37. [Google Scholar]
- Silveira, E.L.; Ballester, E.L.C.; Costa, K.A.; Scheffer, E.W.O.; Vaz-dos-Santos, A.M. Fish community response to environmental variations in an impacted Neotropical basin. Ecol. Freshw. Fish 2018, 27, 1126–1139. [Google Scholar] [CrossRef]
- Langeani, F.; Castro, R.M.C.; Oyakawa, O.T.; Shibatta, O.A.; Pavanelli, C.S.; Casatti, L. Diversidade da ictiofauna do Alto Rio Paraná: Composição atual e perspectivas futuras. Biota Neotrop. 2007, 7, 181–197. [Google Scholar] [CrossRef]
- Rocha, C.H.; Weirich Neto, P.H. Padrões de uso das terras e implicações ambientais. In Pitangui, rio de Contrastes: Seus Lugares, Seus Peixes, Sua Gente; Gealh, A.M., Melo, M.S., Moro, R.S., Eds.; Editora UEPG: Ponta Grossa, Brazil, 2010; pp. 23–41. [Google Scholar]
- Rautenberg, K.A.; Silveira, E.L.; Vaz-dos-Santos, A.M. Feeding trends of Psalidodon paranae in an impacted Neotropical basin: A multifactor and integrative approach. Environ. Biol. Fish. 2021, 104, 89–105. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.
- Casatti, L.; Castro, R.M.C. Testing the ecomorphological hypothesis in a headwater riffles fish assemblage of the Rio São Francisco, southeastern Brazil. Neotrop. Ichthyol. 2006, 4, 203–2014. [Google Scholar] [CrossRef]
- Semmar, N. Computational Metabolomics; Nova Science Publishers: New York, NY, USA, 2011. [Google Scholar]
- Thioulouse, J.; Chessel, D.; Dolédec, S.; Olivier, J.M. ADE-4: A multivariate analysis and graphical display software. Stat. Comp. 1997, 7, 75–83. [Google Scholar] [CrossRef]
- Semmar, N. Native Statistics for Natural Sciences; Nova Science Publishers: New York, NY, USA, 2013. [Google Scholar]
- Ferreira, K.M. Biology and ecomorphology of stream fishes from the Rio Mogi-Guaçu basin, Southeastern Brazil. Neotrop. Ichthyol. 2007, 5, 311–326. [Google Scholar] [CrossRef]
- Oliveira, E.F.; Goulart, E.; Breda, L.; Minte-Vera, C.V.; Paiva, L.R.S.; Vismara, M.R. Ecomorphological patterns of the fish assemblage in a tropical floodplain: Effects of trophic, spatial and phylogenetic structures. Neotrop. Ichthyol. 2010, 8, 569–586. [Google Scholar] [CrossRef]
- Portella, T.; Lobón-Cerviá, J.; Manna, L.R.; Bergallo, H.G.; Mazzoni, R. Eco-morphological attributes and feeding habits in coexisting characins. J. Fish Biol. 2017, 90, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Baldasso, M.C.; Wolff, L.L.; Neves, M.P.; Delariva, R.L. Ecomorphological variations and food supply drive trophic relationships in the fish fauna of a pristine neotropical stream. Environ. Biol. Fish. 2019, 102, 783–800. [Google Scholar] [CrossRef]
- Esteves, K.E. Feeding ecology of three Astyanax species (Characidae, Tetragonopterinae) from a floodplain lake of Mogi-Guaçú River, Paraná River Basin, Brazil. Environ. Biol. Fish. 1996, 46, 83–101. [Google Scholar] [CrossRef]
- Vilella, F.S.; Becker, F.G.; Hartz, S.M. Diet of Astyanax species (Teleostei, Characidae) in an Atlantic Forest River in Southern Brazil. Braz. Arch. Biol. Technol. 2002, 45, 223–232. [Google Scholar] [CrossRef]
- Abelha, M.C.F.; Goulart, E.; Kashiwaqui, E.A.L.; Silva, M.R. Astyanax paranae Eigenmann, 1914 (Characiformes: Characidae) in the Alagados Reservoir, Paraná, Brazil: Diet composition and variation. Neotrop. Ichthyol. 2006, 4, 349–356. [Google Scholar] [CrossRef]
- Wolff, L.L.; Abilhoa, V.; Rios, F.S.; Donatti, L. Spatial, seasonal and ontogenetic variation in the diet of Astyanax aff. fasciatus (Ostariophysi: Characidae) in an Atlantic Forest river, southern Brazil. Neotrop. Ichthyol. 2009, 7, 257–266. [Google Scholar] [CrossRef]
- Ferreira, A.; Paula, F.R.; Ferraz, S.F.B.; Gerhard, P.; Kashiwaqui, E.A.L.; Cyrino, J.E.P.; Martinelli, L.A. Riparian coverage affects diets of characids in neotropical streams. Ecol. Freshw. Fish 2012, 21, 12–22. [Google Scholar] [CrossRef]
- Hahn, N.S.; Fugi, R. Alimentação de peixes em reservatórios brasileiros: Alterações e conseqüências nos estágios iniciais do represamento. Oecol. Bras. 2007, 11, 469–480. [Google Scholar] [CrossRef]
- Loureiro, V.E.; Hahn, N.S. Dieta e atividade alimentar da traíra, Hoplias malabaricus (Bloch, 1794) (Osteichthyes, Erythrinidae), nos primeiros anos de formação do reservatório de Segredo—PR. Acta Limnol. Bras. 1996, 8, 195–205. [Google Scholar]
- Kütter, M.T.; Bemvenuti, M.A.; Moresco, A. Feeding strategy of the jundiá Rhamdia quelen (Siluriformes, Heptapteridae) in costal lagoons of southern Brazil. Acta Sci. Biol. Sci. 2009, 31, 41–47. [Google Scholar] [CrossRef]
- Hendry, A.P. Key Questions on the Role of Phenotypic Plasticity in Eco-Evolutionary Dynamics. J. Hered. 2016, 107, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Wimberger, P.H. Plasticity of fish body shape. The effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae). Biol. J. Linn. Soc. 1992, 45, 197–218. [Google Scholar] [CrossRef]
- Abelha, M.C.F.; Goulart, E. Oportunismo trófico de Geophagus brasiliensis (Quoy & Gaimard, 1824) (Osteichthyes, Cichlidae) no reservatório de Capivari, Estado do Paraná, Brasil. Acta Sci. Biol. Sci. 2004, 26, 37–45. [Google Scholar] [CrossRef]
- Moraes, M.F.P.G.; Barbola, I.F.; Duboc, L.F. Feeding habits and morphometry of digestive tracts of Geophagus brasiliensis (Osteichthyes, Cichlidae), in a lagoon of High Tibagi river, Paraná state, Brazil. Publ. UEPG Ciênc. Biol. Saúde 2004, 10, 37–45. [Google Scholar] [CrossRef]
- Souza, G.R.S.; Sabino, J.; Garrone-Neto, D. The surprising “B-side”: Description of a new foraging tactic for the pearl cichlid, Geophagus brasiliensis, in a coastal stream of the Atlantic Forest. Biota Neotrop. 2019, 19, e20180702. [Google Scholar] [CrossRef]
- Casatti, L.; Teresa, F.B.; Gonçalves-Souza, T.; Bessa, E.; Manzotti, A.R.; Gonçalves, C.S.; Zeni, J.O. From forests to cattail: How does the riparian zone influence stream fish? Neotrop. Ichthyol. 2012, 10, 205–214. [Google Scholar] [CrossRef]
- Furlan, N.; Esteves, K.E.; Quináglia, G.A. Environmental factors associated with fish distribution in an urban neotropical river (Upper Tietê River Basin, São Paulo, Brazil). Environ. Biol. Fish. 2013, 96, 77–92. [Google Scholar] [CrossRef]
- Santos, A.B.I.; Camilo, F.L.; Albieri, R.J.; Araújo, F.G. Morphological patterns of five fish species (four characiforms, one perciform) in relation to feeding habits in a tropical reservoir in south-eastern Brazil. J. Appl. Ichthyol. 2011, 27, 1360–1364. [Google Scholar] [CrossRef]
- Montaña, C.G.; Winemiller, K.O. Evolutionary convergence in Neotropical cichlids and Nearctic centrarchids: Evidence from morphology, diet, and stable isotope analysis. Biol. J. Linn. Soc. 2013, 109, 146–164. [Google Scholar] [CrossRef]
- Casatti, L.; Teresa, F.B.; Zeni, J.O.; Ribeiro, M.D.; Brejão, G.L.; Ceneviva-Bastos, M. More of the Same: High Functional Redundancy in Stream Fish Assemblages from Tropical Agroecosystems. Environ. Manag. 2015, 55, 1300–1314. [Google Scholar] [CrossRef]
- Sabino, J.; Castro, R.M.C. Alimentação, período de atividade e distribuição espacial dos peixes de um riacho da floresta atlântica (sudeste do Brasil). Rev. Bras. Biol. 1990, 50, 23–36. [Google Scholar]
- Bastos, R.F.; Condini, M.V.; Varela Junior, A.S.; Garcia, A.M. Diet and food consumption of the pearl cichlid Geophagus brasiliensis (Teleostei: Cichlidae): Relationships with gender and sexual maturity. Neotrop. Ichthyol. 2011, 9, 825–830. [Google Scholar] [CrossRef]
- Nunes, M.V.; Rocha, O.; Verani, J.R. Trophic interactions between the fish Geophagus brasiliensis (Cichlidae) and the benthic macroinvertebrate community. Stud. Neotrop. 2014, 49, 11–17. [Google Scholar] [CrossRef]
- Novakowski, G.C.; Hahn, N.S.; Fugi, R. Diet seasonality and food overlap of the fish assemblage in a Pantanal pond. Neotrop. Ichthyol. 2008, 6, 567–576. [Google Scholar] [CrossRef]
- Cardone, I.B.; Lima, S.E.; Goitein, R. Diet and capture of Hypostomus strigaticeps (Siluriformes, Loricariidae) in a small Brazilian stream: Relationship with limnological aspects. Braz. J. Biol. 2006, 66, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Villares-Junior, G.A.; Cardone, I.B.; Goitein, R. Comparative feeding ecology of four syntopic Hypostomus species in a Brazilian southeastern river. Braz. J. Biol. 2016, 76, 692–699. [Google Scholar] [CrossRef]
- Celestino, L.F.; Sanz-Ronda, F.J.; Kashiwaqui, E.A.L.; Celestino, E.F.; Makrakis, M.C.; Makrakis, S. Daily movement behavior of two Neotropical armored catfish species (Ancistrus aff. cirrhosus Valenciennes, 1836 and Hypostomus ancistroides Ihering, 1911) at a road-stream crossing culvert. J. Appl. Ichthyol. 2017, 33, 1092–1099. [Google Scholar] [CrossRef]
- Keenleyside, M.H.A. Diversity and Adaptation in Fish Behaviour; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Bellafronte, E.; Mariguela, T.C.; Pereira, L.H.G.; Oliveira, C.; Moreira-Filho, O. DNA barcode of Parodontidae species from the La Plata river basin—Applying new data to clarify taxonomic problems. Neotrop. Ichthyol. 2013, 11, 497–506. [Google Scholar] [CrossRef]
- Sazima, I. Behavior of two Brazilian species of Parodontid fishes, Apareiodon piracicabae and A. ibitiensis. Copeia 1980, 1980, 166–169. [Google Scholar] [CrossRef]
- Santin, M.; Bialetzki, A.; Nakatani, K. Mudanças ontogênicas no trato digestório e dieta de Apareiodon affinis (Steindachner, 1879) (Osteichthyes, Parodontidae). Acta Sci. Biol. Sci. 2004, 26, 291–298. [Google Scholar] [CrossRef]
- Aranha, J.M.R.; Caramaschi, É.P.; Caramaschi, U. Ocupação espacial, alimentação e época reprodutiva de duas espécies de Corydoras Lacépède (Siluroidei, Callichthyidae) coexistentes no rio Alambari (Botucatu, São Paulo). Rev. Bras. Zool. 1993, 10, 453–466. [Google Scholar] [CrossRef]
- Nijssen, H. Revision of the Surinam Catfishes of the genus Corydoras Lacépède, 1803 (Pisces, Siluriformes, Callichthyidae). Beaufortia 1970, 18, 1–75. [Google Scholar]
- Sazima, I. Similarities in feeding behaviour between some marine and freshwater fishes in two tropical communities. J. Fish Biol. 1986, 29, 53–65. [Google Scholar] [CrossRef]
- Ovalle, W.K.; Shinn, S.L. Surface morphology of taste buds in catfish barbels. Cell Tissue Res. 1977, 178, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Yamamoto, K. Electron microscopy of terminal buds on the barbels of the silurid fish, Corydoras paleatus. Anat. Rec. 1980, 197, 133–141. [Google Scholar] [CrossRef]
- Kirchheim, P.D.; Goulart, E. Ecomorfologia de predação e antipredação em Siluriformes (Osteichthyes). Oecol. Aust. 2010, 14, 550–568. [Google Scholar] [CrossRef]
- Bosher, B.T.; Newton, S.H.; Fine, M.L. The spines of the channel catfish, Ictalurus punctatus, as an anti-predator adaptation: An experimental study. J. Ethol. 2006, 112, 188–195. [Google Scholar] [CrossRef]
- Sands, D.D. Crypsis in Corydoras (Pisces: Siluriformes, Callichthyidae). Aquat. Int. J. Ichthyol. 1994, 1, 13–20. [Google Scholar]
- Riley, R.J.; Gillie, E.R.; Savage, J.L.; Boogert, N.J.; Manica, A.; Jungwirth, A. The role of tactile interactions in flight responses in the Bronze Cory catfish (Corydoras aeneus). J. Ethol. 2019, 125, 810–820. [Google Scholar] [CrossRef]
- Bistoni, M.A.; Haro, J.G.; Gutiérrez, M. Feeding of Hoplias malabaricus in the wetlands of Dulce river (Córdoba, Argentina). Hydrobiologia 1995, 316, 103–107. [Google Scholar] [CrossRef]
- Dill, L.M. Adaptive Flexibility in the Foraging Behavior of Fishes. Can. J. Fish. Aquat. Sci. 1983, 40, 398–408. [Google Scholar] [CrossRef]
- Neves, M.P.; Delariva, R.L.; Wolff, L.L. Diet and ecomorphological relationships of an endemic, species-poor fish assemblage in a stream in the Iguaçu National Park. Neotrop. Ichthyol. 2015, 13, 245–254. [Google Scholar] [CrossRef]
- Schneider, M.; Aquino, P.P.U.; Silva, M.J.M.; Fonseca, C.P. Trophic structure of a fish community in Bananal stream subbasin in Brasília National Park, Cerrado biome (Brazilian Savanna), DF. Neotrop. Ichthyol. 2011, 9, 579–592. [Google Scholar] [CrossRef]
- Weber, P.; Vogel, C.; Lang, C.; Baldisserotto, B. Antipredator and alarm reaction responses of silver catfish (Rhamdia quelen) juveniles exposed to waterborne ammonia. Neotrop. Ichthyol. 2012, 10, 445–450. [Google Scholar] [CrossRef]
- Miserendino, M.L.; Casaux, R.; Archangelsky, M.; Di Prinzio, C.Y.; Brand, C.; Kutschker, A.M. Assessing land-use effects on water quality, in-stream habitat, riparian ecosystems and biodiversity in Patagonian northwest streams. Sci. Total Environ. 2011, 409, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Zeni, J.O.; Casatti, L. The influence of habitat homogenization on the trophic structure of fish fauna in tropical streams. Hydrobiologia 2014, 726, 259–270. [Google Scholar] [CrossRef]
- Lobón-Cerviá, J.; Mazzoni, R.; Rezende, C.F. Effects of riparian forest removal on the trophic dynamics of a Neotropical stream fish assemblage. J. Fish Biol. 2016, 89, 50–64. [Google Scholar] [CrossRef]
- Pusey, B.J.; Arthington, A.H. Importance of the riparian zone to the conservation and management of freshwater fish: A review. Mar. Freshw. Res. 2003, 54, 1–16. [Google Scholar] [CrossRef]
- Mazzoni, R.; Iglesias-Rios, R. Environmentally related life history variations in Geophagus brasiliensis. J. Fish Biol. 2002, 61, 1606–1618. [Google Scholar] [CrossRef]
- Ferreira, A.; Gerhard, P.; Cyrino, J.E.P. Diet of Astyanax paranae (Characidae) in streams with different riparian land covers in the Passa-Cinco River basin, southeastern Brazil. Iheringia Sér. Zool. 2012, 102, 80–87. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Riis, T. Macrophyte diversity and composition in relation to substratum characteristics in regulated and unregulated Danish streams. Freshw. Biol. 1999, 42, 375–385. [Google Scholar] [CrossRef]
- Mello, A.S.; Tavares, A.S.; Trevisan, R. Podostemaceae in Southern Brazil. Rodriguésia 2011, 62, 867–885. [Google Scholar] [CrossRef]
- Mor, J.R.; Dolédec, S.; Acuña, V.; Sabater, S.; Muñoz, I. Invertebrate community responses to urban wastewater effluent pollution under different hydro-morphological conditions. Environ. Pollut. 2019, 252, 483–492. [Google Scholar] [CrossRef]
- Casatti, L.; Langeani, F.; Silva, A.M.; Castro, R.M.C. Stream fish, water and habitat quality in a pasture dominated basin, southeastern Brazil. Braz. J. Biol. 2006, 66, 681–696. [Google Scholar] [CrossRef]
- Conrado, A.L.; Neyrão, I.M.; Iunes, R.S.; Bruno, C.E.M.; Lopes, P.R.S. Microscopy and classification of barbels of silver catfish Rhamdia quelen, Siluriformes, Pimelodidae. Investigação 2019, 18, 50–55. [Google Scholar] [CrossRef]
- Silva, C.P.; Silveira, E.L.; Seremeta, D.C.H.; Matos, D.G.C.; Vaz-dos-Santos, A.M.; Campos, S.X. Bioaccumulation and bioconcentration of metals in Characidae from a Neotropical river basin under anthropic activities. Environ. Sci. Pollut. Res. 2021, 28, 38434–38447. [Google Scholar] [CrossRef]
- Schulz, U.H.; Martins-Junior, H. Astyanax fasciatus as bioindicator of water pollution of Rio dos Sinos, RS, Brazil. Braz. J. Biol. 2001, 61, 615–622. [Google Scholar] [CrossRef]
- Alberto, A.; Camargo, A.F.M.; Verani, J.R.; Costa, O.F.T.; Fernandes, M.N. Health variables and gill morphology in the tropical fish Astyanax fasciatus from a sewage-contaminated river. Ecotoxicol. Environ. Saf. 2005, 61, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.D.; Cranston, O.S.; Pinder, L.C.V. The Chironomidae. Biology and Ecology of Non-Biting Midges; Springer-Science+Business Media: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Batista, H.U.; Barbola, I.F.; Kloth, A.E.G.; Milléo, J. Structure and composition of the macroinvertebrate community as a way of evaluating the quality of the water from rio Verde, Ponta Grossa, state of Paraná, Brazil. Terra Plur. 2010, 4, 241–256. [Google Scholar] [CrossRef]
- Baptista, D.F.; Henriques-Oliveira, A.L.; Oliveira, R.B.S.; Mugnai, R.; Nessimian, J.L.; Buss, D.F. Development of a benthic multimetric index for the Serra da Bocaina bioregion in Southeast Brazil. Braz. J. Biol. 2013, 73, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.A.; Peres-Neto, P.R.; Olden, J.D. What controls who is where in freshwater fish communities—The roles of biotic, abiotic, and spatial factors. Can. J. Fish. Aquat. Sci. 2001, 58, 157–170. [Google Scholar] [CrossRef]
- Thorp, J.H.; Thoms, M.C.; Delong, M.D. The riverine ecosystem synthesis: Biocomplexity in river networks across space and time. River Res. Appl. 2006, 22, 123–147. [Google Scholar] [CrossRef]
- Azevedo-Santos, V.M.; Frederico, R.G.; Fagundes, C.K.; Pompeu, P.S.; Pelicice, F.M.; Padial, A.A.; Nogueira, M.G.; Fearnside, P.M.; Lima, L.B.; Daga, V.S.; et al. Protected areas: A focus on Brazilian freshwater biodiversity. Divers Distrib. 2019, 25, 442–448. [Google Scholar] [CrossRef]
- Rohr, J.M.; Meiners, S.J.; Thomas, T.D.; Colombo, R.E. Recovery of riverine fish assemblages after anthropogenic disturbances. Ecosphere 2021, 12, e03459. [Google Scholar] [CrossRef]
- Frederico, R.G.; Reis, V.C.S.; Polaz, C.N.M. Conservação de peixes de riacho: Planejamento e políticas públicas. Oecol. Aust. 2021, 25, 546–564. [Google Scholar] [CrossRef]

| Feeding Trend | Sample Units | Feeding Trend Description |
|---|---|---|
| FT1 | 7 | Characterized by high consumption of Teleostei (up to 50% of total diet, TD) (Figure 2aSM) and Lepidoptera (up to 35% TD; Figure 2bSM). Some sample units showed high consumption of Coleoptera (50–100% TD; Figure 2cSM) and Hymenoptera (20–35% TD; Figure 2dSM), demonstrating ability to exploit terrestrial resources. |
| FT2 | 24 | Characterized by high consumption of Aquatic angiosperms (up to 25% TD; Figure 2eSM) and Ephemeroptera (up to 18% TD; Figure 2fSM). FT2 showed the most diversified diet profile, presenting consumed food categories that were absent in other FTs, such as Blattodea, Gastropoda, Isoptera, Invertebrate Eggs, Neuroptera and Plastic debris (Figure 2g-lSM). |
| FT3 | 26 | Also presented heterogeneous diet pattern, consuming Terrestrial angiosperms, Sediments and Diptera (up to 50% TD for each one; Figure 2m-oSM), in addition to Undetermined invertebrates, Undetermined matter, Invertebrate fragments, Trichoptera and Isopoda (Figure 1p-tSM). |
| FT4 | 2 | Characterized by relatively high consumption of Undetermined invertebrates (35–50% TD; Figure 2pSM), in addition to Diptera (Figure 2oSM), Hemiptera and Plant fragments (Figure 1u-vSM). |
| FT5 | 3 | Characterized by relative high consumption of Acarina (10–20% TD; Figure 2wSM), Trichoptera (up to 20% TD; Figure 2sSM) and Coleoptera (up to 35% TD, Figure 2cSM). |
| FT6 | 16 | Characterized by relative high consumption of Diptera (up to 50% TD; Figure 2oSM), in addition to Invertebrate fragments (Figure 2rSM). |
| FT7 | 4 | Characterized by relative high consumption of Bacillariophyta (20–50% TD; Figure 2xSM) and Sediments (up to 50% TD; Figure 2nSM), in addition to Bryophyta (Figure 2ySM). |
| FT8 | 12 | Characterized by relative high consumption of Sediments (40–100% TD; Figure 2nSM) associated with Plant fragments (Figure 2vSM) and Trichoptera (Figure 2sSM). |
| FT9 | 1 | Characterized by consumption peaks of Cyanophyta, Bacillariophyta and Ephemeroptera (each one up to 25% TD; Figure 1zSM, 1xSM and 1fSM) in addition to relative high levels of Sediments (25% TD; Figure 2nSM). |
| FT10 | 2 | Characterized by relative high consumption of Hymenoptera, Orthoptera and Coleoptera (15–25% TD for each one; Figure 1dSM, 1aSM and 1SMc). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silveira, E.L.; Semmar, N.; Ballester, E.L.C.; Vaz-dos-Santos, A.M. Integrative Analysis to Manage Aquatic Resources Based on Fish Feeding Patterns in Neotropical Rivers. Fishes 2023, 8, 157. https://doi.org/10.3390/fishes8030157
da Silveira EL, Semmar N, Ballester ELC, Vaz-dos-Santos AM. Integrative Analysis to Manage Aquatic Resources Based on Fish Feeding Patterns in Neotropical Rivers. Fishes. 2023; 8(3):157. https://doi.org/10.3390/fishes8030157
Chicago/Turabian Styleda Silveira, Estevan Luiz, Nabil Semmar, Eduardo Luis Cupertino Ballester, and André Martins Vaz-dos-Santos. 2023. "Integrative Analysis to Manage Aquatic Resources Based on Fish Feeding Patterns in Neotropical Rivers" Fishes 8, no. 3: 157. https://doi.org/10.3390/fishes8030157
APA Styleda Silveira, E. L., Semmar, N., Ballester, E. L. C., & Vaz-dos-Santos, A. M. (2023). Integrative Analysis to Manage Aquatic Resources Based on Fish Feeding Patterns in Neotropical Rivers. Fishes, 8(3), 157. https://doi.org/10.3390/fishes8030157







