Localization of Acetylcholine, Alpha 7-NAChR and the Antimicrobial Peptide Piscidin 1 in the Macrophages of Fish Gut: Evidence for a Cholinergic System, Diverse Macrophage Populations and Polarization of Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals and Tissue Preparation
2.3. Immunofluorescence
2.4. Laser Confocal Immunofluorescence
2.5. Transmission Electron Microscopy
2.6. Statistical Analysis
3. Results
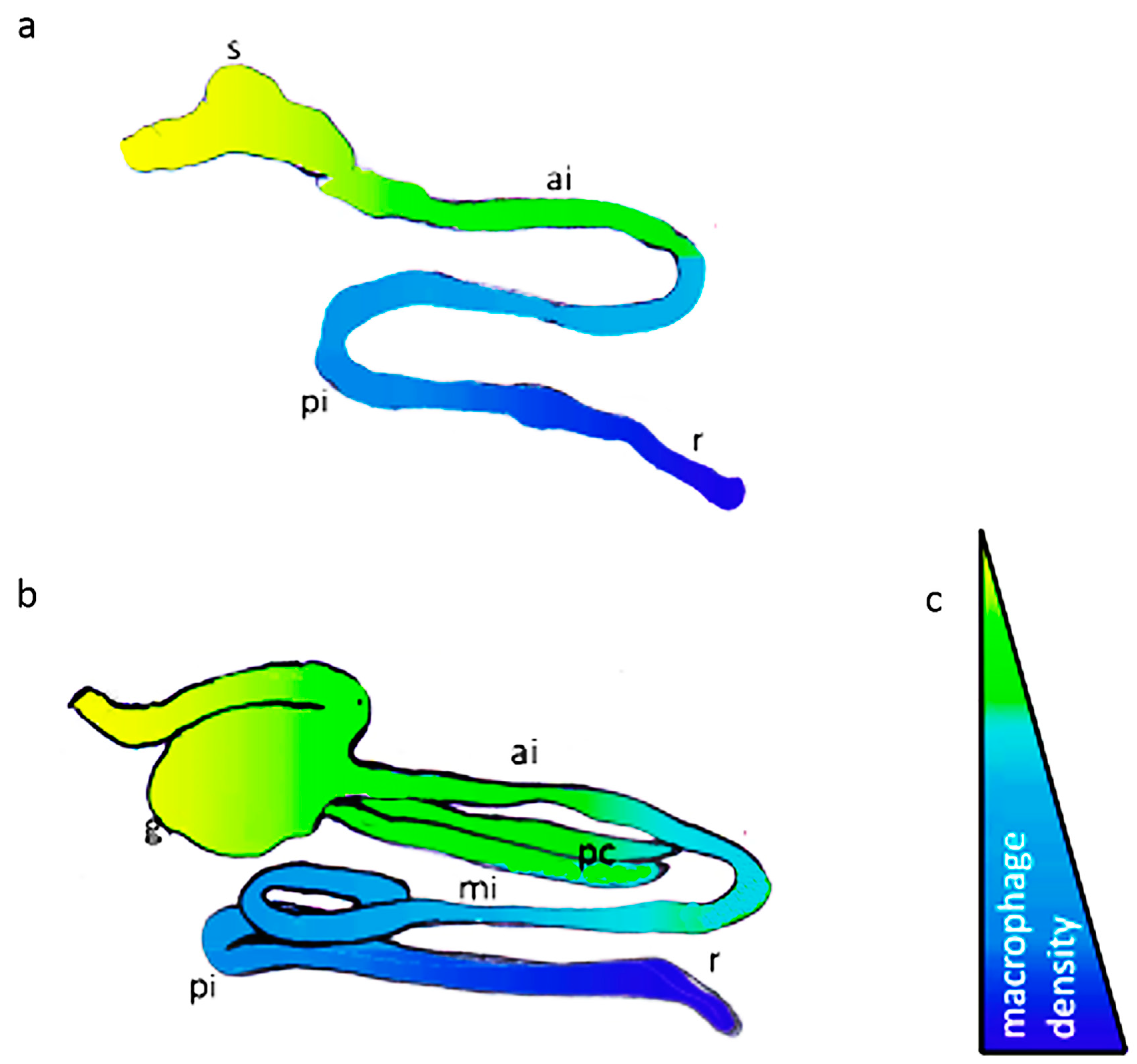
3.1. Intestinal Segmentation and Macrophage Density in the Anterior-Posterior Gut Axis of African Bonytongue and Indian Catfish
3.2. Double Labeling Methods
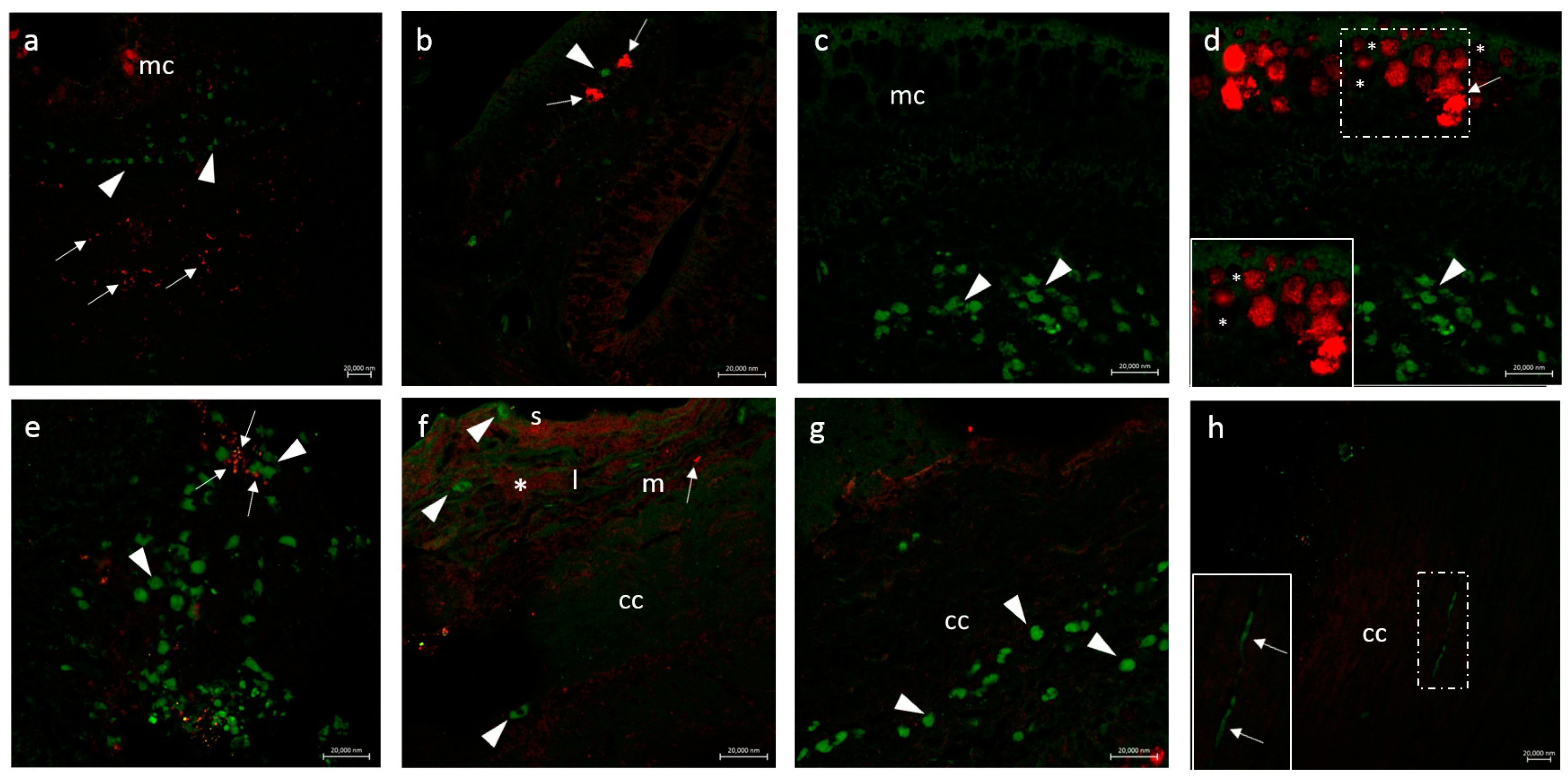
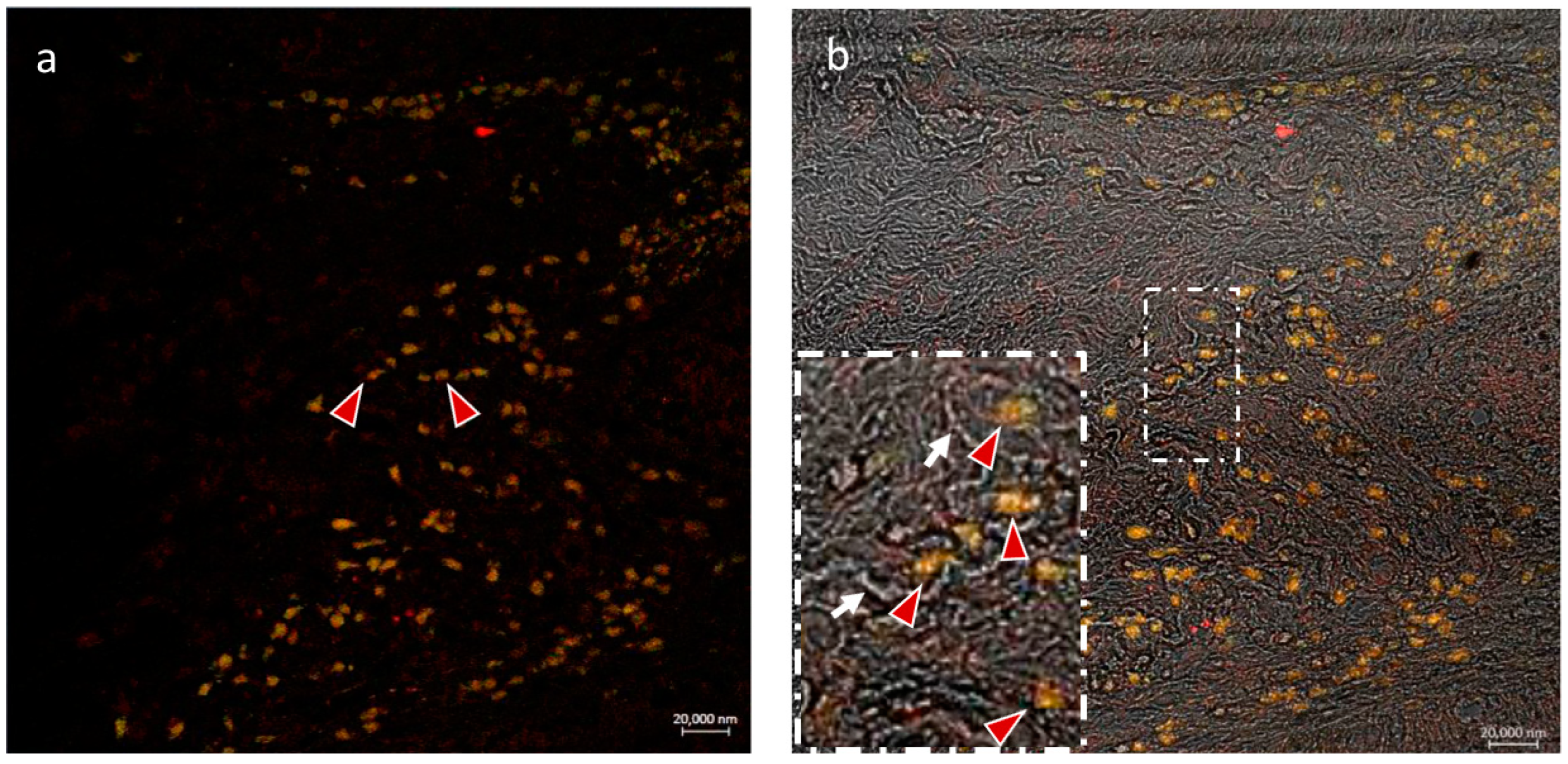
3.2.1. CD14 and VAChT
3.2.2. CD14 and nAChR Alpha 7 Subunit
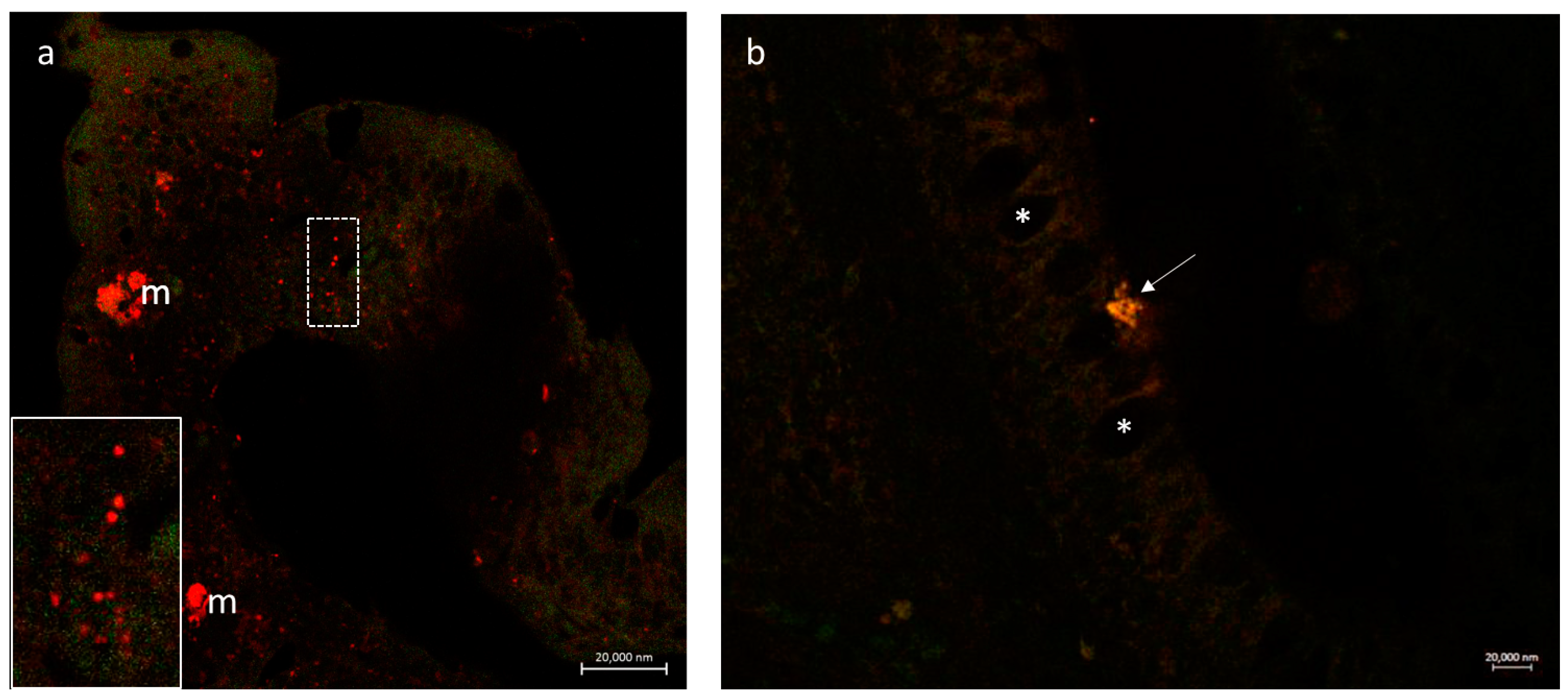
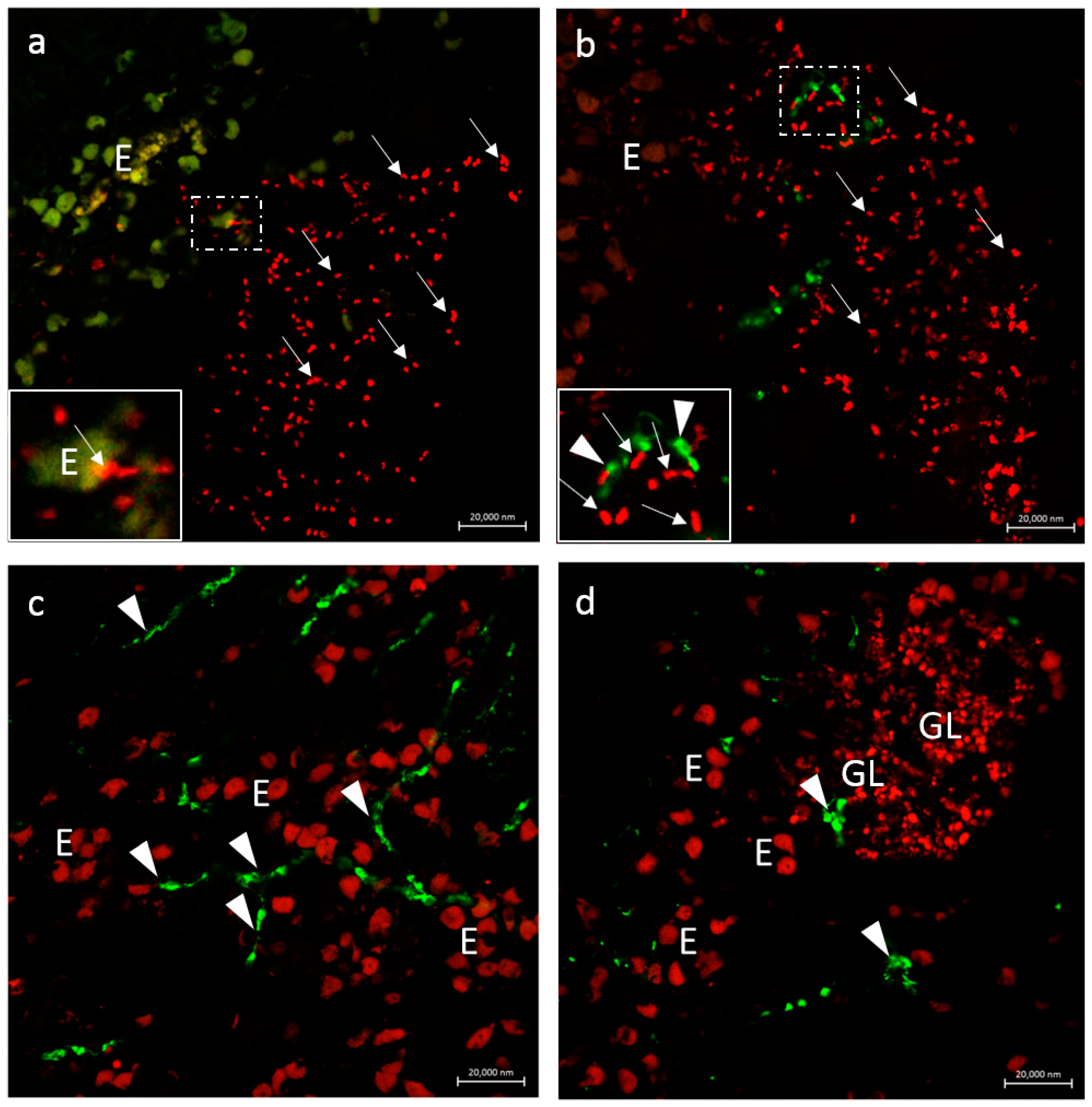
3.2.3. Secretion of the Antimicrobial Peptide Piscidin 1 by Intestinal Macrophages: CD14 and Piscidin 1 Double Immunolabeling
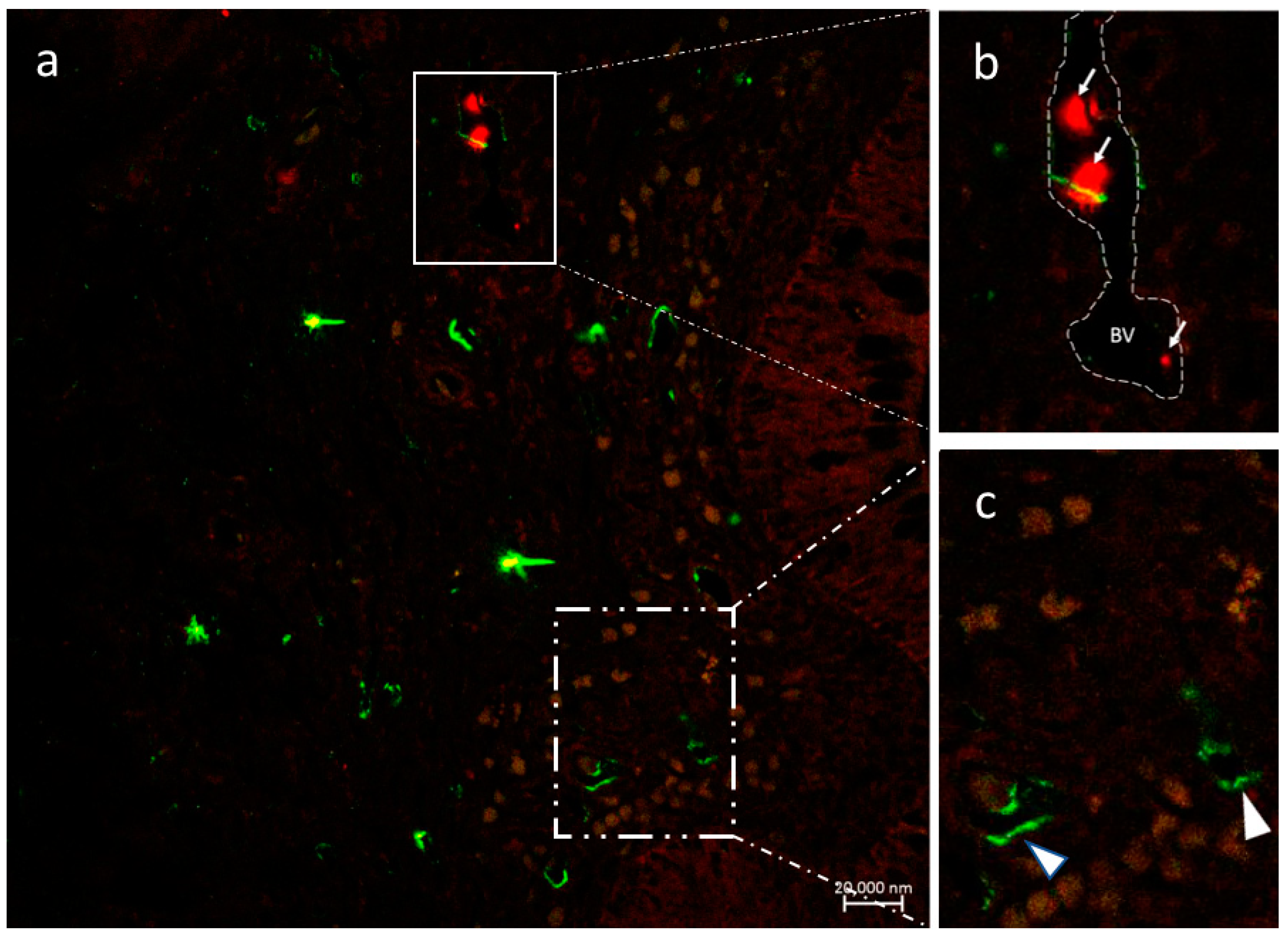
3.2.4. VAChT and iNOS
3.2.5. Immunolabeling with Antibodies against Calbindin, VAChT, and Tubulin
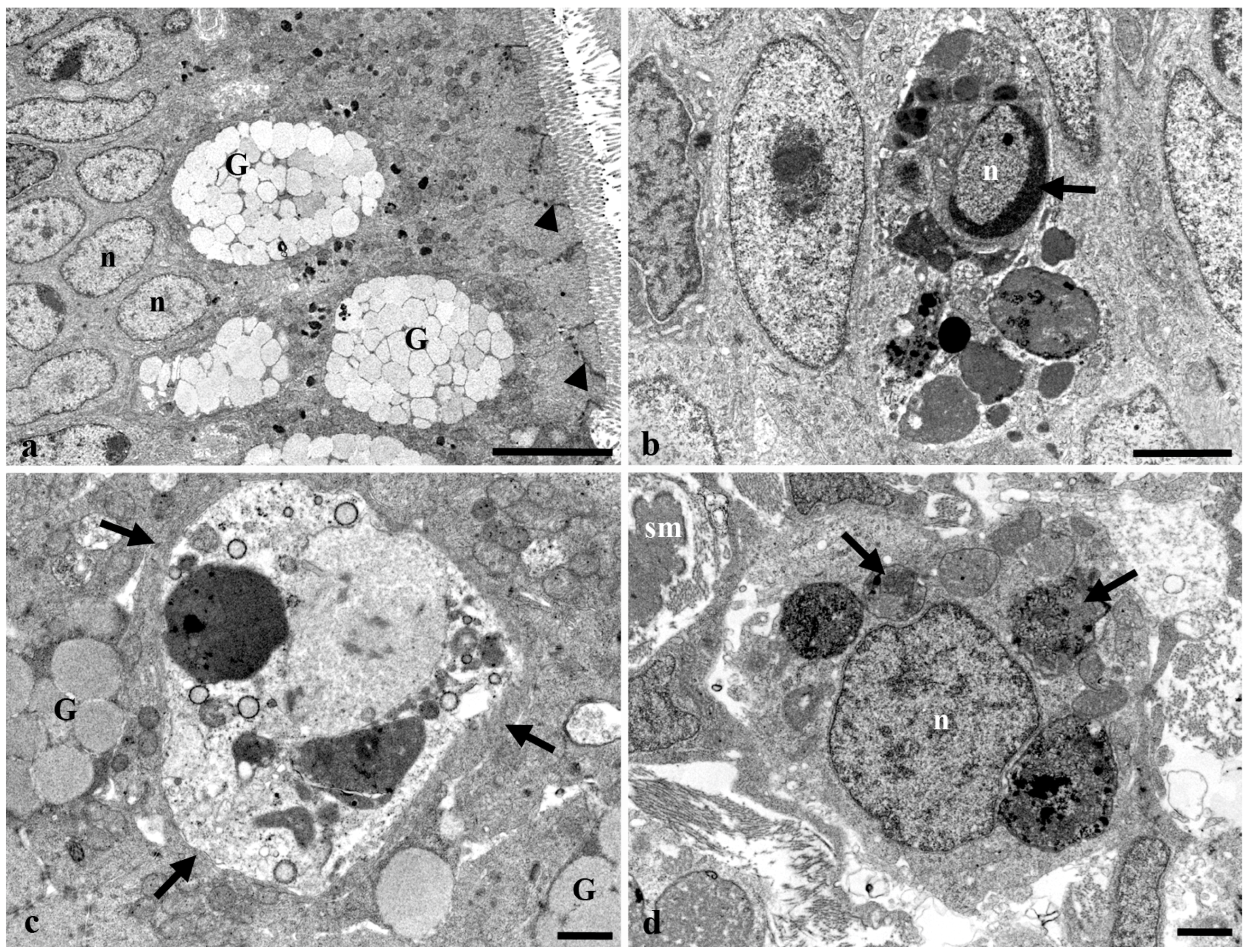
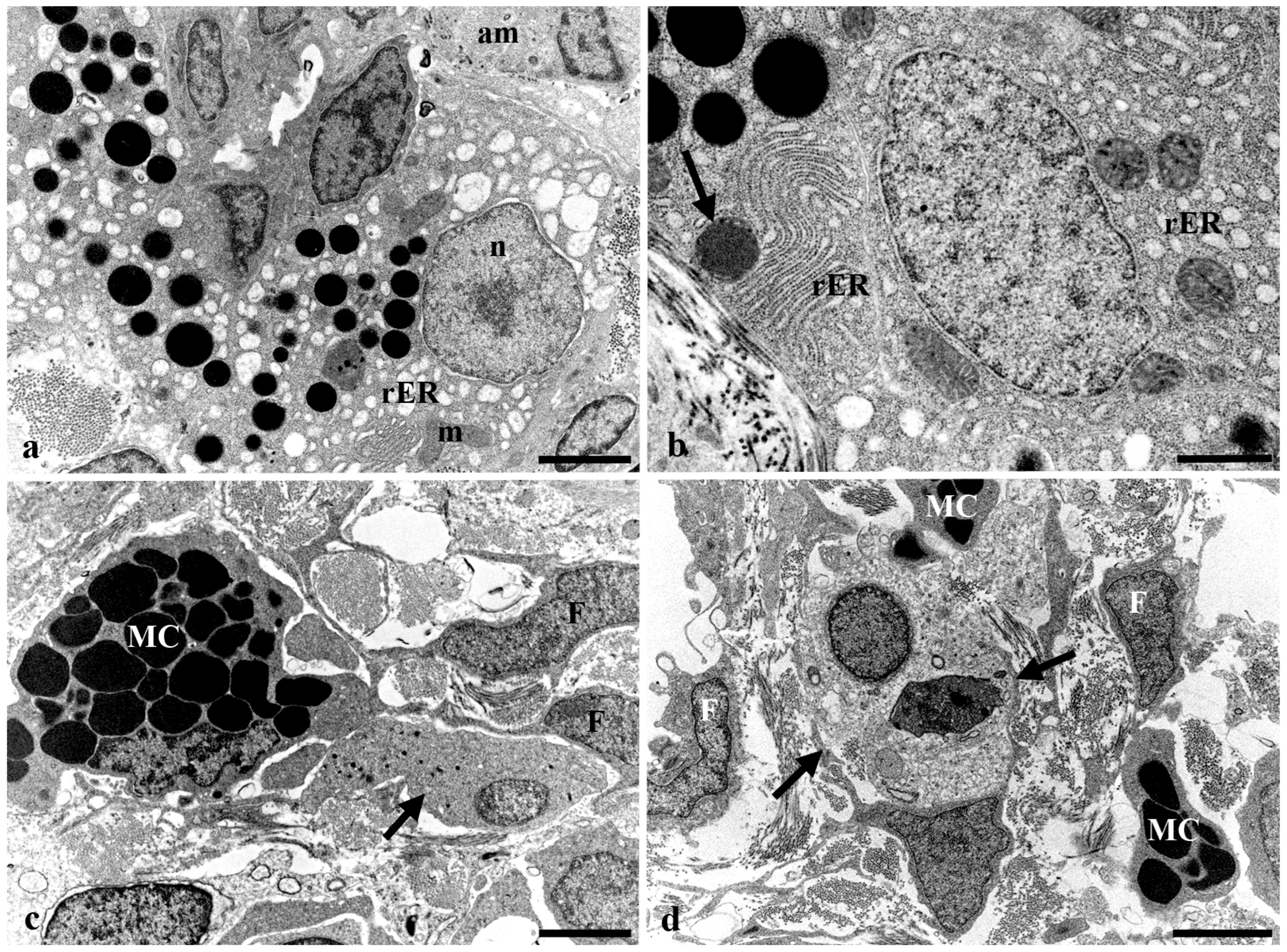
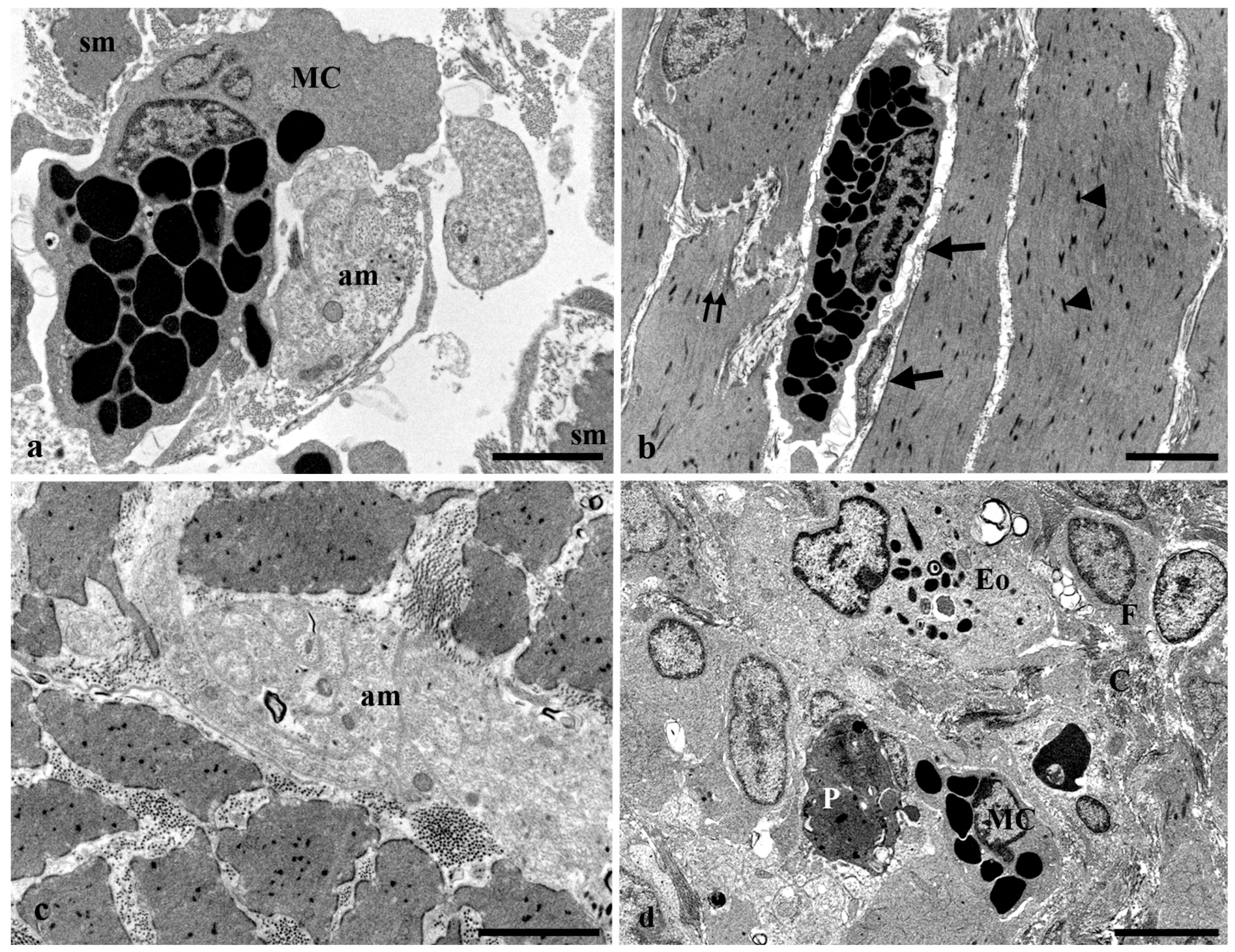
3.3. Transmission Electron Microscopy of the Long Gut of Heterotis Niloticus
4. Discussion
4.1. Macrophage Diversity and Their Interaction with Enteric Neurons in the African Bonytongue and Catfish Gut
4.2. Cholinergic System Components in the Intestinal Macrophages
4.3. The Piscidin 1 Is Expressed in Macrophages
4.4. iNOS and Macrophages
4.5. Neuro-Immune Axis in Fish Gut
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amici, S.A.; Dong, J.; Guerau-de-Arellano, M. Molecular Mechanisms Modulating the Phenotype of Macrophages and Microglia. Front. Immunol. 2017, 8, 1520. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Grayfer, L.; Kerimoglu, B.; Yaparla, A.; Hodgkinson, J.W.; Xie, J.; Belosevic, M. Mechanisms of Fish Macrophage Antimicrobial Immunity. Front. Immunol. 2018, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wiegertjes, G.F.; Wentzel, A.S.; Spaink, H.P.; Elks, P.M.; Fink, I.R. Polarization of Immune Responses in Fish: The ‘Macrophages First’Point of View. Mol. Immunol. 2016, 69, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Mischopoulou, M.; D’Ambrosio, M.; Bigagli, E.; Luceri, C.; Farrugia, G.; Cipriani, G. Role of Macrophages and Mast Cells as Key Players in the Maintenance of Gastrointestinal Smooth Muscle Homeostasis and Disease. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1849–1862. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Fumia, A.; Calabrò, C.; Lo Cascio, P.; Lauriano, E.R. Mast Cells in Goldfish (Carassius auratus) Gut: Immunohistochemical Characterization. Acta Zool. 2022, azo.12417. [Google Scholar] [CrossRef]
- Viola, M.F.; Boeckxstaens, G. Intestinal Resident Macrophages: Multitaskers of the Gut. Neurogastroenterol. Motil. 2020, 32, e13843. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Grayfer, L.; Belosevic, M. Biology of Bony Fish Macrophages. Biology 2015, 4, 881–906. [Google Scholar] [CrossRef]
- Pastorelli, L.; De Salvo, C.; Mercado, J.R.; Vecchi, M.; Pizarro, T.T. Central Role of the Gut Epithelial Barrier in the Pathogenesis of Chronic Intestinal Inflammation: Lessons Learned from Animal Models and Human Genetics. Front. Immunol. 2013, 4, 280. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Bording-Jorgensen, M.; Armstrong, H.; Wickenberg, M.; LaPointe, P.; Wine, E. Macrophages and Epithelial Cells Mutually Interact through NLRP3 to Clear Infection and Enhance the Gastrointestinal Barrier. Immuno 2021, 2, 13–25. [Google Scholar] [CrossRef]
- Rieger, A.M.; Hall, B.E.; Barreda, D.R. Macrophage Activation Differentially Modulates Particle Binding, Phagocytosis and Downstream Antimicrobial Mechanisms. Dev. Comp. Immunol. 2010, 34, 1144–1159. [Google Scholar] [CrossRef]
- Barreda, D. Flow Cytometric Analysis of PKH26-Labeled Goldfish Kidney-Derived Macrophages. Dev. Comp. Immunol. 2000, 24, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.F.; Stafford, J.L.; Belosevic, M. Biochemical and Functional Characterisation of Macrophage Stimulating Factors Secreted by Mitogen-Induced Goldfish Kidney Leucocytes. Fish Shellfish Immunol. 2000, 10, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A. Approaching the Asymptote? Evolution and Revolution in Immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Taniguchi, Y.; Yoshida, A.; Nakata, K.; Nishizawa, T.; Inagawa, H.; Kohchi, C.; Soma, G.-I. Intracellular Localization of CD14 Protein in Intestinal Macrophages. Anticancer Res. 2009, 29, 865–869. [Google Scholar]
- Zanoni, I.; Granucci, F. Role of CD14 in Host Protection against Infections and in Metabolism Regulation. Front. Cell. Infect. Microbiol. 2013, 3, 32. [Google Scholar] [CrossRef]
- Smith, P.D.; Smythies, L.E.; Shen, R.; Greenwell-Wild, T.; Gliozzi, M.; Wahl, S.M. Intestinal Macrophages and Response to Microbial Encroachment. Mucosal Immunol. 2011, 4, 31–42. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Fumia, A.; Lauriano, E.R. Neuronal Regeneration: Vertebrates Comparative Overview and New Perspectives for Neurodegenerative Diseases. Acta Zool. 2021, 103, 129–140. [Google Scholar] [CrossRef]
- Bi, D.; Wang, Y.; Gao, Y.; Li, X.; Chu, Q.; Cui, J.; Xu, T. Recognition of Lipopolysaccharide and Activation of NF-ΚB by Cytosolic Sensor NOD1 in Teleost Fish. Front. Immunol. 2018, 9, 1413. [Google Scholar] [CrossRef]
- Bernardo, D.; Marin, A.C.; Fernández-Tomé, S.; Montalban-Arques, A.; Carrasco, A.; Tristán, E.; Ortega-Moreno, L.; Mora-Gutiérrez, I.; Díaz-Guerra, A.; Caminero-Fernández, R. Human Intestinal Pro-Inflammatory CD11chighCCR2+ CX3CR1+ Macrophages, but Not Their Tolerogenic CD11c- CCR2- CX3CR1- Counterparts, Are Expanded in Inflammatory Bowel Disease. Mucosal Immunol. 2018, 11, 1114–1126. [Google Scholar] [CrossRef]
- Rueda Ruzafa, L.; Cedillo, J.L.; Hone, A.J. Nicotinic Acetylcholine Receptor Involvement in Inflammatory Bowel Disease and Interactions with Gut Microbiota. Int. J. Environ. Res. Public Health 2021, 18, 1189. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Yasuda, R.P.; Fan, H.; Wolfe, B.B.; Kellar, K.J. Heterogeneity of Nicotinic Cholinergic Receptors in Rat Superior Cervical and Nodose Ganglia. Mol. Pharmacol. 2006, 70, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Vazquez, G. Evidence for a Prosurvival Role of Alpha-7 Nicotinic Acetylcholine Receptor in Alternatively (M 2)-Activated Macrophages. Physiol. Rep. 2013, 1, e00189. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Alves, I.; Coimbra-Campos, L.; Sancho, M.; Da Silva, R.F.; Cortes, S.F.; Lemos, V.S. Role of the A7 Nicotinic Acetylcholine Receptor in the Pathophysiology of Atherosclerosis. Front. Physiol. 2020, 1719. [Google Scholar] [CrossRef]
- Morris, S.J.; Kunzek, S.; Shore, A.C. The Effect of Acetylcholine on Finger Capillary Pressure and Capillary Flow in Healthy Volunteers. J. Physiol. 1996, 494, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Reardon, C.; Duncan, G.S.; Brüstle, A.; Brenner, D.; Tusche, M.W.; Olofsson, P.S.; Rosas-Ballina, M.; Tracey, K.J.; Mak, T.W. Lymphocyte-Derived ACh Regulates Local Innate but Not Adaptive Immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 1410–1415. [Google Scholar] [CrossRef]
- Bankova, L.G.; Dwyer, D.F.; Yoshimoto, E.; Ualiyeva, S.; McGinty, J.W.; Raff, H.; von Moltke, J.; Kanaoka, Y.; Frank Austen, K.; Barrett, N.A. The Cysteinyl Leukotriene 3 Receptor Regulates Expansion of IL-25–Producing Airway Brush Cells Leading to Type 2 Inflammation. Sci. Immunol. 2018, 3, eaat9453. [Google Scholar] [CrossRef]
- Schütz, B.; Ruppert, A.-L.; Strobel, O.; Lazarus, M.; Urade, Y.; Büchler, M.W.; Weihe, E. Distribution Pattern and Molecular Signature of Cholinergic Tuft Cells in Human Gastro-Intestinal and Pancreatic-Biliary Tract. Sci. Rep. 2019, 9, 17466. [Google Scholar] [CrossRef]
- Schneider, C.; O’Leary, C.E.; Locksley, R.M. Regulation of Immune Responses by Tuft Cells. Nat. Rev. Immunol. 2019, 19, 584–593. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; Shao, X.; Huang, J. Acetylcholine from Tuft Cells: The Updated Insights beyond Its Immune and Chemosensory Functions. Front. Cell Dev. Biol. 2020, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Capillo, G.; Zaccone, G.; Cupello, C.; Fernandes, J.M.O.; Viswanath, K.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Icardo, J.M. Expression of Acetylcholine, Its Contribution to Regulation of Immune Function and O2 Sensing and Phylogenetic Interpretations of the African Butterfly Fish Pantodon buchholzi (Osteoglossiformes, Pantodontidae). Fish Shellfish Immunol. 2021, 111, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Fantozzi, R.; Blandina, P.; Brunelleschi, S.; Mannaioni, P.F. Muscarinic Cholinergic Receptor Binding in Rat Mast Cells. Agents Actions 1983, 13, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kageyama-Yahara, N.; Suehiro, Y.; Yamamoto, T.; Kadowaki, M. IgE-Induced Degranulation of Mucosal Mast Cells Is Negatively Regulated via Nicotinic Acetylcholine Receptors. Biochem. Biophys. Res. Commun. 2008, 377, 321–325. [Google Scholar] [CrossRef]
- Bosmans, G.; Shimizu Bassi, G.; Florens, M.; Gonzalez-Dominguez, E.; Matteoli, G.; Boeckxstaens, G.E. Cholinergic Modulation of Type 2 Immune Responses. Front. Immunol. 2017, 8, 1873. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.B.; Mazmanian, S.K. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef]
- Rahman, Z.; Dandekar, M.P. Crosstalk between Gut Microbiome and Immunology in the Management of Ischemic Brain Injury. J. Neuroimmunol. 2021, 353, 577498. [Google Scholar] [CrossRef]
- Hirota, C.L.; McKay, D.M. Cholinergic Regulation of Epithelial Ion Transport in the Mammalian Intestine: Cholinergically Mediated Intestinal Ion Transport. Br. J. Pharmacol. 2006, 149, 463–479. [Google Scholar] [CrossRef]
- Zaccone, G.; Capillo, G.; Aragona, M.; Alesci, A.; Cupello, C.; Lauriano, E.R.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; Germana, A. Gill Structure and Neurochemical Markers in the African Bonytongue (Heterotis niloticus): A Preliminary Study. Acta Histochem. 2022, 124, 151954. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Capillo, G.; Lauriano, E.R. Localization of Vasoactive Intestinal Peptide and Toll-like Receptor 2 Immunoreactive Cells in Endostyle of Urochordate Styela plicata (Lesueur, 1823). Microsc. Res. Tech. 2022, jemt.24119. [Google Scholar] [CrossRef]
- Alesci, A.; Cicero, N.; Fumia, A.; Petrarca, C.; Mangifesta, R.; Nava, V.; Lo Cascio, P.; Gangemi, S.; Di Gioacchino, M.; Lauriano, E.R. Histological and Chemical Analysis of Heavy Metals in Kidney and Gills of Boops Boops: Melanomacrophages Centers and Rodlet Cells as Environmental Biomarkers. Toxics 2022, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, S.; Rizzo, G.; Favaloro, A.; Alesci, A.; Pallio, S.; Melita, G.; Cutroneo, G.; Lauriano, E.R. Expression of VAChT and 5-HT in Ulcerative Colitis Dendritic Cells. Acta Histochem. 2021, 123, 151715. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Capillo, G.; Mokhtar, D.M.; Fumia, A.; D’Angelo, R.; Lo Cascio, P.; Albano, M.; Guerrera, M.C.; Sayed, R.K.A.; Spanò, N.; et al. Expression of Antimicrobic Peptide Piscidin1 in Gills Mast Cells of Giant Mudskipper Periophthalmodon Schlosseri (Pallas, 1770). Int. J. Mol. Sci. 2022, 23, 13707. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Savoca, S.; Fumia, A.; Mangano, A.; Albano, M.; Messina, E.; Aragona, M.; Lo Cascio, P.; Capillo, G.; et al. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus Cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology 2022, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Albano, M.; Savoca, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lo Cascio, P.; Hussein, M.M.; Capillo, G.; Pergolizzi, S.; et al. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology 2022, 11, 1653. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Capillo, G.; Lo Cascio, P.; Lauriano, E.R. Rodlet Cells in Kidney of Goldfish (Carassius auratus, Linnaeus 1758): A Light and Confocal Microscopy Study. Acta Histochem. 2022, 124, 151876. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Guerrera, M.C.; Laurà, R.; Capillo, G.; Pergolizzi, S.; Aragona, M.; Abbate, F.; Germanà, A. Effect of Light on the Calretinin and Calbindin Expression in Skin Club Cells of Adult Zebrafish. Histochem. Cell Biol. 2020, 154, 495–505. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Fumia, A.; Messina, E.; Albano, M.; Aragona, M.; Lo Cascio, P.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Confocal Characterization of Intestinal Dendritic Cells from Myxines to Teleosts. Biology 2022, 11, 1045. [Google Scholar] [CrossRef]
- Zaccone, G.; Capillo, G.; Fernandes, J.M.O.; Kiron, V.; Lauriano, E.R.; Alesci, A.; Lo Cascio, P.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; et al. Expression of the Antimicrobial Peptide Piscidin 1 and Neuropeptides in Fish Gill and Skin: A Potential Participation in Neuro-Immune Interaction. Mar. Drugs 2022, 20, 145. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Pergolizzi, S.; Lo Cascio, P.; Kuciel, M.; Zizzo, N.; Guerrera, M.C.; Aragona, M.; Capillo, G. Expression of Langerin/CD207 in Airways, Lung and Associated Lymph Nodes of a Stranded Striped Dolphin (Stenella coeruleoalba). Acta Histochem. 2020, 122, 151471. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Capillo, G.; Icardo, J.M.; Fernandes, J.M.O.; Kiron, V.; Kuciel, M.; Zuwala, K.; Guerrera, M.C.; Aragona, M.; Zaccone, G. Neuroepithelial Cells (NECs) and Mucous Cells Express a Variety of Neurotransmitters and Neurotransmitter Receptors in the Gill and Respiratory Air-Sac of the Catfish Heteropneustes fossilis (Siluriformes, Heteropneustidae): A Possible Role in Local Immune Defence. Zoology 2021, 148, 125958. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Graves, C.L.; Chen, A.; Kwon, V.; Shiau, C.E. Zebrafish Harbor Diverse Intestinal Macrophage Populations Including a Subset Intimately Associated with Enteric Neural Processes. Iscience 2021, 24, 102496. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, M.C.; Aragona, M.; Briglia, M.; Porcino, C.; Mhalhel, K.; Cometa, M.; Abbate, F.; Montalbano, G.; Laurà, R.; Levanti, M. The Alimentary Tract of African Bony-Tongue, Heterotis niloticus (Cuvier, 1829): Morphology Study. Animals 2022, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.N.; Akhter, S.; Smith, E.M.; Lorent, K.; Pack, M. Intestinal Growth and Differentiation in Zebrafish. Mech. Dev. 2005, 122, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cabezudo, M.J.; George, J.A.; Bashir, G.; Mohamed, Y.A.; Al-Mansori, A.; Qureshi, M.M.; Lorke, D.E.; Petroianu, G.; al-Ramadi, B.K. Involvement of Acetylcholine Receptors in Cholinergic Pathway-Mediated Protection Against Autoimmune Diabetes. Front. Immunol. 2019, 10, 1038. [Google Scholar] [CrossRef]
- Liu, E.Y.; Xia, Y.; Kong, X.; Guo, M.S.; Anna, X.D.; Zheng, B.Z.; Mak, S.; Xu, M.L.; Tsim, K.W. Interacting with A7 NAChR Is a New Mechanism for AChE to Enhance the Inflammatory Response in Macrophages. Acta Pharm. Sin. B 2020, 10, 1926–1942. [Google Scholar] [CrossRef]
- Roach, S.K.; Lee, S.-B.; Schorey, J.S. Differential Activation of the Transcription Factor Cyclic AMP Response Element Binding Protein (CREB) in Macrophages Following Infection with Pathogenic and Nonpathogenic Mycobacteria and Role for CREB in Tumor Necrosis Factor Alpha Production. Infect. Immun. 2005, 73, 514–522. [Google Scholar] [CrossRef]
- Valledor, A.F.; Borras, F.E.; Cullell-Young, M.; Celada, A. Transcription Factors That Regulate Monocyte/Macrophage Differentiation. J. Leukoc. Biol. 1998, 63, 405–417. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus Nerve Stimulation Attenuates the Systemic Inflammatory Response to Endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, D.; Saika, F.; Matsuzaki, S.; Kishioka, S. Inhibition of Peripheral Macrophages by Nicotinic Acetylcholine Receptor Agonists Suppresses Spinal Microglial Activation and Neuropathic Pain in Mice with Peripheral Nerve Injury. J. Neuroinflamm. 2018, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kolter, J.; Kierdorf, K.; Henneke, P. Origin and Differentiation of Nerve-Associated Macrophages. J. Immunol. 2020, 204, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Specian, R.D.; Neutra, M.R. Mechanism of Rapid Mucus Secretion in Goblet Cells Stimulated by Acetylcholine. J. Cell Biol. 1980, 85, 626–640. [Google Scholar] [CrossRef]
- En-Nosse, M.; Hartmann, S.; Trinkaus, K.; Alt, V.; Stigler, B.; Heiss, C.; Kilian, O.; Schnettler, R.; Lips, K.S. Expression of Non-Neuronal Cholinergic System in Osteoblast-like Cells and Its Involvement in Osteogenesis. Cell Tissue Res. 2009, 338, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, S.; Reichrath, J.; Moussa, A.-T.; Meier, C.; Tschernig, T. Targeting the Non-Neuronal Cholinergic System in Macrophages for the Management of Infectious Diseases and Cancer: Challenge and Promise. Cell Death Discov. 2016, 2, 1–4. [Google Scholar] [CrossRef]
- Selsted, M.E.; Brown, D.M.; DeLange, R.J.; Lehrer, R.I. Primary Structures of MCP-1 and MCP-2, Natural Peptide Antibiotics of Rabbit Lung Macrophages. J. Biol. Chem. 1983, 258, 14485–14489. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Selsted, M.E.; Szklarek, D.; Fleischmann, J. Antibacterial Activity of Microbicidal Cationic Proteins 1 and 2, Natural Peptide Antibiotics of Rabbit Lung Macrophages. Infect. Immun. 1983, 42, 10–14. [Google Scholar] [CrossRef]
- Poltavets, A.S.; Vishnyakova, P.A.; Elchaninov, A.V.; Sukhikh, G.T.; Fatkhudinov, T.K. Macrophage Modification Strategies for Efficient Cell Therapy. Cells 2020, 9, 1535. [Google Scholar] [CrossRef]
- Specht, H.; Emmott, E.; Petelski, A.A.; Huffman, R.G.; Perlman, D.H.; Serra, M.; Kharchenko, P.; Koller, A.; Slavov, N. Single-Cell Proteomic and Transcriptomic Analysis of Macrophage Heterogeneity; Immunology: Singapore, 2019. [Google Scholar]
- Mantovani, A.; Sica, A.; Locati, M. Macrophage Polarization Comes of Age. Immunity 2005, 23, 344–346. [Google Scholar] [CrossRef]
- Harwani, S.C. Macrophages under Pressure: The Role of Macrophage Polarization in Hypertension. Transl. Res. 2018, 191, 45–63. [Google Scholar] [CrossRef]
- Chokshi, N.K.; Guner, Y.S.; Hunter, C.J.; Upperman, J.S.; Grishin, A.; Ford, H.R. The Role of Nitric Oxide in Intestinal Epithelial Injury and Restitution in Neonatal Necrotizing Enterocolitis. In Proceedings of the Seminars in Perinatology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 32, pp. 92–99. [Google Scholar]
- Godinho-Silva, C.; Cardoso, F.; Veiga-Fernandes, H. Neuro–Immune Cell Units: A New Paradigm in Physiology. Annu. Rev. Immunol. 2019, 37, 19–46. [Google Scholar] [CrossRef]
- Chu, C.; Artis, D.; Chiu, I.M. Neuro-Immune Interactions in the Tissues. Immunity 2020, 52, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Rühl, A. Glial Cells in the Gut. Neurogastroenterol. Motil. 2005, 17, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, G.; Gomez-Pinilla, P.J.; Nemethova, A.; Di Giovangiulio, M.; Cailotto, C.; van Bree, S.H.; Michel, K.; Tracey, K.J.; Schemann, M.; Boesmans, W. A Distinct Vagal Anti-Inflammatory Pathway Modulates Intestinal Muscularis Resident Macrophages Independent of the Spleen. Gut 2014, 63, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Kurapati, S.; Bogunovic, M. Neuro-Innate Immune Interactions in Gut Mucosal Immunity. Curr. Opin. Immunol. 2021, 68, 64–71. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Supplier | Dilution | Animal Source |
|---|---|---|---|
| CD14 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:200 | Mouse |
| VAChT | Sigma-Aldrich, Inc., St. Louis, MO, USA | 1:300 | Rabbit |
| nAChR-alpha7 | Alomone Labs, Ltd., Jerusalem, Israel | 1:200 | Rabbit |
| iNOS | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:200 | Mouse |
| Tubulin | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:500 | Mouse |
| Calbindin | Sigma-Aldrich, Inc., St. Louis, MO, USA | 1:100 | Mouse |
| Alexa Fluor 488 Donkey anti-Mouse IgG FITC conjugated | Molecular Probes, Invitrogen | 1:300 | Donkey |
| Alexa Fluor 594 Donkey anti-Rabbit IgG TRITC conjugated | Molecular Probes, Invitrogen | 1:300 | Donkey |
| Anterior Intestine | Middle Intestine | Distal Intestine | |
|---|---|---|---|
| Piscidin1 | 112.45 ± 21.72 * | 163.56 ± 13.89 ** | / |
| CD14 | 102.66 ± 8.37 ** | 154.96 ± 14.75 * | 294.74 ± 45.16 * |
| nAChR-alpha7 | 99.54 ± 8.93 ** | / | / |
| iNOS | 105.33 ± 16.04 * | / | 217.98 ± 34.47 ** |
| Tubulin | / | / | 267.46 ± 23.01 * |
| Calbindin | / | / | 253.92 ± 35.27 ** |
| VAChT | 115.18 ± 14.92 ** | 165.75 ± 16,83 * | 206.67 ± 24.98 * |
| Piscidin1 + CD14 | 76.23 ± 9.18 * | 104.65 ± 7.15 ** | / |
| nAChR-alpha7 + CD14 | 43.76 ± 6.99 ** | / | / |
| VAChT + CD14 | / | 107.63 ± 7.82 ** | 113.79 ± 21.14 ** |
| VAChT + iNOS | 93.67 ± 15.17 * | / | 196.04 ± 29.65 * |
| VAChT + Tubulin | / | / | 203.19 ± 19.41 * |
| VAChT + Calbindin | / | / | 199.02 ± 16.81 ** |
| Anterior Intestine | Middle Intestine | Distal Intestine | |
|---|---|---|---|
| Piscidin1 | / | 174.35 ± 17.84 * | / |
| CD14 | 116.73 ± 21.34 * | 146.92 ± 15.44 ** | 315.16 ± 18.65 ** |
| nAChR-alpha7 | 101.58 ± 16.62 ** | / | / |
| iNOS | / | / | 237.84 ± 26.43 ** |
| VAChT | / | / | 248.36 ± 25.79 * |
| Piscidin1 + CD14 | / | 112.78 ± 16.77 * | / |
| nAChRalpha7 + CD14 | 36.85 ± 3.95 * | / | / |
| VAChT + CD14 | / | / | 121.87 ± 13.46 * |
| VAChT + iNOS | / | / | 221.06 ± 24.93 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaccone, G.; Alesci, A.; Mokhtar, D.M.; Aragona, M.; Guerrera, M.C.; Capillo, G.; Albano, M.; de Oliveira Fernandes, J.; Kiron, V.; Sayed, R.K.A.; et al. Localization of Acetylcholine, Alpha 7-NAChR and the Antimicrobial Peptide Piscidin 1 in the Macrophages of Fish Gut: Evidence for a Cholinergic System, Diverse Macrophage Populations and Polarization of Immune Responses. Fishes 2023, 8, 43. https://doi.org/10.3390/fishes8010043
Zaccone G, Alesci A, Mokhtar DM, Aragona M, Guerrera MC, Capillo G, Albano M, de Oliveira Fernandes J, Kiron V, Sayed RKA, et al. Localization of Acetylcholine, Alpha 7-NAChR and the Antimicrobial Peptide Piscidin 1 in the Macrophages of Fish Gut: Evidence for a Cholinergic System, Diverse Macrophage Populations and Polarization of Immune Responses. Fishes. 2023; 8(1):43. https://doi.org/10.3390/fishes8010043
Chicago/Turabian StyleZaccone, Giacomo, Alessio Alesci, Doaa M. Mokhtar, Marialuisa Aragona, Maria Cristina Guerrera, Gioele Capillo, Marco Albano, Jorge de Oliveira Fernandes, Viswanath Kiron, Ramy K. A. Sayed, and et al. 2023. "Localization of Acetylcholine, Alpha 7-NAChR and the Antimicrobial Peptide Piscidin 1 in the Macrophages of Fish Gut: Evidence for a Cholinergic System, Diverse Macrophage Populations and Polarization of Immune Responses" Fishes 8, no. 1: 43. https://doi.org/10.3390/fishes8010043
APA StyleZaccone, G., Alesci, A., Mokhtar, D. M., Aragona, M., Guerrera, M. C., Capillo, G., Albano, M., de Oliveira Fernandes, J., Kiron, V., Sayed, R. K. A., Hussein, M. M., Lo Cascio, P., Kuciel, M., Zuwala, K., Germanà, A., Icardo, J. M., & Lauriano, E. R. (2023). Localization of Acetylcholine, Alpha 7-NAChR and the Antimicrobial Peptide Piscidin 1 in the Macrophages of Fish Gut: Evidence for a Cholinergic System, Diverse Macrophage Populations and Polarization of Immune Responses. Fishes, 8(1), 43. https://doi.org/10.3390/fishes8010043















