Abstract
The objective of the present study was to investigate the effects of different doses of dietary Eucommia ulmoides leaf extract (ELE) on juvenile red claw crayfish (Cherax quadricarinatus). A total number of 720 red claw crayfish (initial body weight of 0.24 ± 0.01 g) were randomly assigned to six groups and fed diets containing 0 (Diet 1), 0.5 (Diet 2), 1 (Diet 3), 2 (Diet 4), 4 (Diet 5) and 10 (Diet 6) g dry weight (dw) ELE kg (dw)−1 diets for eight weeks and challenged with microcystin-LR stress. The results indicated that dietary supplementation with 1–2 g dw ELE kg (dw)−1 diet could significantly improve the weight gain rate (WGR) and specific growth rate (SGR) of crayfish. Muscle crude protein contents of crayfish fed Diet 2, Diet 3, and Diet 4 were significantly higher than those of the control group. Compared with the control group, dietary ELE could increase total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GPx), acid phosphatase (ACP), alkaline phosphatase (AKP), and phenoloxidase (PO) activities and decrease malondialdehyde (MDA) level of crayfish. Dietary ELE significantly increased the relative expression levels of SOD, thioredoxin 1 (TRX1), GPx, selenium-dependent glutathione peroxidase (Se-GPx), cytochrome P450 (CYP450), anti-lipopolysaccharide factor (ALF) and C-type lysozyme (C-LZM) mRNA of crayfish compared with the control group during the feeding experiment. When subjected to MC-LR stress for 48 h, the mRNA expression levels of SOD, GPx, Se-GPx, glutathione-s-transferase 1 (GST1), ALF, hemocyanin (HEM), and C-LZM in the hepatopancreas could be improved to varying degrees compared with the Diet 1. Supplementation of 1–2 g dw ELE kg (dw)−1 diet could improve the survival rate (SR) of crayfish under MC-LR stress. These results indicated that dietary ELE (1–2 g dw ELE kg (dw)−1 diet) could improve the growth performance, muscle protein, and non-specific immune response and increase the SR of crayfish under MC-LR stress by regulating the mRNA expression levels of the immune- and antioxidant-related genes.
1. Introduction
The red claw crayfish (Cherax quadricarinatus) is suitable for intensive or semi-intensive culture in many countries, including Australia, America, Mexico, Argentina, Cuba, and China [1]. It is one of the most popular crayfish species widely farmed in Guangdong, Fujian, Hainan, and Jiangsu Provinces in China because of its high resistance, fast growth, large size, and ease of farming [2,3,4]. With the continuous development of the high-density intensive culture of aquaculture, the water environment has become a major concern for farmers [5]. Deterioration of the water environment, especially the eutrophication of aquaculture water, can affect aquatic species’ immunity, survival, and growth [6], leading to the occurrence of diseases. To avoid the outbreak of diseases, farmers frequently use chemicals and antimicrobials to prevent viral, fungal, and bacterial diseases. However, the abusive use of chemicals and antimicrobials can lead to the formation of drug residues, disruption of immune balance in aquatic animals, microbial resistance, immunosuppression, and environmental pollution, which affects crayfish culture [7]. The application of green plant additives to aquatic species is the key to the sustainable development of aquaculture [8]. Therefore, Chinese herbal extracts have become suitable alternatives to chemicals and antimicrobials for protecting aquaculture environments.
Cyanobacteria bloom can threaten water quality in many eutrophic ponds [9]. A cyanobacterial harmful algal bloom is one of the common microbial hazards in freshwater aquaculture and has become a serious global public health and environmental problem [10,11,12]. Toxins produced by cyanobacteria endanger the health of freshwater aquaculture animals and the safety of aquatic products [13]. The toxins of freshwater cyanobacteria, including cyclic-peptide microcystins and nodularin, are divided into neurotoxins and hepatotoxins. Microcystins (MCs) are a group of cyanobacterial hepatotoxins which have more than 279 kinds of different MC congeners, and microcystin-LR (MC-LR) is the most common and potently toxic MC [14,15,16,17]. MC-LR can damage the hepatopancreas, head kidney, spleen, etc. [18,19,20,21] and cause intrahepatic hemorrhage [22] and extensive centrilobular necrosis [23]. Besides, microcystins can suppress protein phosphatase expression, leading to increased protein phosphorylation, which is directly related to their cytotoxicity and immunotoxicity [23,24,25]. The immune system can be altered by MCs through several immune-related genes and pathways [26]. Several studies reported that MC-LR exposure significantly changed the glutathione (GSH) content and reactive oxygen species (ROS) formation [27]. MC-LR changes the activities of various antioxidant enzymes, including glutathione reductase (GR), glutathione-s-transferase (GST), superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) [18,25]. One study has shown a fast increase of SOD and CAT activities at the early stage and subsequent decrease at the later stage of MC-LR exposure, while GPx was activated by longer MC-LR exposure in Procambarus clarkii [18]. Exposed to the low dose of MC-LR, zebrafish exhibited a positive defensive response with an increase in the CAT, SOD, and GST activities [25]. On the other hand, high MC-LR dose in zebrafish resulted in a negative antioxidant response by inhibition in the CAT, GPx, and GST activities. GSH depletion in both dose group suggested the crucial role of GSH in cellular antioxidant protection and MC-LR detoxification [25].
Plant extracts can be effective alternatives to chemicals and antimicrobials in aquaculture [28,29,30]. Various plant extracts have been developed to improve immunity and protect tissues from a variety of environmental stress stimuli in aquaculture [31]. Among plants, E. ulmoides has been widely used in medicine and animal feed research [32,33,34,35]. E. ulmoides leaf extract (ELE) contains many biologically active compounds, including terpenoids, phenolics, lignans, steroids, iridoids, and flavonoids [36,37]. Chlorogenic acid is the principal bioactive component of ELE that scavenges free radicals and improves antioxidant ability and immunity [38,39]. E. ulmoides has several beneficial functions, including the antioxidant effect [40,41], immunity regulation [42,43], and antibacterial [44] and anti-inflammatory [32]. However, few published reports about the application of ELE in crayfish feed [45]. We studied the effect of ELE on growth performance, muscle composition, hepatopancreas histology, immune responses, gene expression, and disease resistance of juvenile red claw crayfish, which could promote the development of pond culture in red claw crayfish. This is the first time to apply ELE to crayfish feed research, laying the foundation for the screening of crayfish feed additives.
2. Materials and methods
2.1. Diet Preparation
The composition of the basal diet is shown in Table 1. E. ulmoides leaf extract (ELE) (alcoholic extract) was added to the diet at 0 (Diet 1), 0.5 (Diet 2), 1 (Diet 3), 2 (Diet 4), 4 (Diet 5), and 10 (Diet 6) g dry weight (dw) ELE kg (dw)−1 diets. ELE was provided by Shandong Longchang Biotechnology Co. Ltd. (Shandong, China). Preparation of ethanol extract of ELEs: Firstly, Eucommia ulmoides leaves are fully mixed with 70% ethanol at a ratio of 1:12, extracted twice by heating reflux under 60–70 °C, and then the ethanol extract is obtained by chromatographic purification. The main components of ELE are 5% chlorogenic acid, 8% flavonoids, and 20% polysaccharides. All the ingredients of the diet were ground, sifted through 80 mesh, and mixed with oil. Then deionized water (40%) was added, and the 1.5 mm diameter feed was wet-extruded by a pelletizer. Subsequently, all diets were air-dried to below 100 g moisture kg−1 diet. After drying, the diets were stored at −20 °C until used.

Table 1.
Composition and nutrient level of experimental diets (g dw matter kg (dw)−1 diet).
2.2. Experimental Crayfish and Sample Collection
The red claw crayfish purchased from a commercial farm in Hainan Province (Wenchang, China) were transported to the experimental condition in cylindrical tanks and were fed with the basal diet for two weeks to acclimate to the experimental diet and conditions. A total of 720 healthy crayfish with an average initial body weight of 0.24 ± 0.01 g were randomly divided into eighteen tanks (500 L; three tanks per group) with three replicates in each group and 40 crayfish in each replicate. To prevent crayfish cannibalism, 40 PVC pipes (Nominal diameter 150, length 10) were placed in each tank and glued together. The crayfish were fed diets two times each day at 8:00 and 18:00. The crayfish were fed every day until satiation. The feeding trial lasted for 8 weeks. During the experimental period, water temperature, pH, ammonia-N, and nitrite-N were 26–29 °C, 7.2–7.7, 0.05–0.11 mg L−1, and 0.05–0.10 mg L−1, respectively. Dissolved oxygen was maintained at 5.0–7.3 mg L−1.
After the culture experiment, crayfish were fasted for 24 h before sampling. The number, body length, and body weight of crayfish were measured. Hemolymph, hepatopancreas, and back muscle samples were collected as described previously [46].
2.3. Growth Performance
The weight gain rate (WGR), specific growth rate (SGR), and survival rate (SR) were calculated as follows [47]:
Survival rate (SR, %) = 100 × final number of crayfish/initial number of crayfish
Specific growth rate (SGR, % day−1) = 100 × (Ln final body weight − Ln initial body weight)/number of days
Weight gain rate (WGR, %) = 100 × (final body weight − initial body weight)/initial body weight
2.4. Proximate Composition Analysis
Muscle and diet compositions were analyzed according to the established methods of the Association of Official Analytical Chemists [48]. Crude protein content was determined by the Kjeldahl method using KjeltecTM 8400 (Kjeltec, Foss, Denmark) after digesting the samples with sulfuric acid. Crude lipid was determined by the Soxhlet extraction method using a lipid analyzer (SZF-06A, Hongji, Shanghai, China). Feed and muscle samples were placed in a muffle furnace (SX2-2.5-10, Yiheng, Shanghai, China) at 550 °C for 12 h to determine the ash content. Feed and muscle moisture contents were determined by oven drying at 105 °C.
2.5. Histopathological Analysis
Three hepatopancreas samples from each tank were collected for morphological analysis. All tissue samples were fixed and stored in 4% paraformaldehyde (Biosharp, Beijing, China) for 48 h. Then, all samples were dehydrated in graded ethanol concentration series and paraffin-embedded. Consequently, the pieces were stained with hematoxylin and eosin (H&E) for optical examination. Morphological changes in the hepatopancreas were observed using Nikon Eclipse E100 Microscope (Nikon, Tokyo, Japan).
2.6. Antioxidant and Immune Enzyme Assays
GPx, GSH, CAT, GST, SOD, alkaline phosphatase (AKP), and acid phosphatase (ACP) activities, and total antioxidant capacity (T-AOC) and malondialdehyde (MDA) contents were measured using their respective kits following the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). Phenoloxidase (PO) content was measured by the double antibody sandwich enzyme-linked immunosorbent assay (ELISA) method using commercial kits (Dongguan Enzyme-linked Biotechnology Co., Ltd., Dongguan, Guangdong, China).
2.7. RNA Extraction and Gene Expression Analysis
Hepatopancreas samples from three crayfish in each tank were used for total RNA isolation by TRIzol reagent (Invitrogen, Waltham, MA, USA). The quantity of each RNA was measured by NanoDrop One spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and RNA integrity was detected using agarose gel (1%) electrophoresis. Subsequently, the complementary DNA (cDNA) was synthesized from total RNA using a PrimeScript RT reagent Kit With gDNA Eraser (Takara, Dalian, China) according to the manufacturer’s instructions. The cDNA was stored at −20 °C for real-time quantitative PCR (qRT-PCR) analysis.
The appropriate primers of each gene (Table 2) were designed based on our transcriptome unigenes and references [49] using Primer Premier v5 (PREMIER Biosoft International (Palo Alto, CA, USA). The real-time PCR reactions were carried out using 2× SYBR® Green qPCR Mix buffer (Takara, Dalian, China) on a Stratagene Mx3005P real-time PCR machine (Agilent, Santa Clara, CA, USA). The cycling parameters were as follows: 94 °C for 3 min, followed by 40 cycles of 94 °C for 15 s, 58 °C for 15 s, and 72 °C for 20 s. The expression results were calculated by the 2−ΔΔCt method [50].

Table 2.
Primers used in this study.
2.8. MC-LR LD50 Determination
The MC-LR (Purity ≥ 95%) was obtained from Algal Science (Algal Science Co. Ltd., Taoyuan, Taiwan). MC-LR was suspended in 0.85% NaCl solution. The concentration of the pre-experiment was 0, 30.2, 42.66, 60.26, 85.11, 120.11, 169.82, and 239.88 μg kg−1. The lethal dose 50 (LD50) LD50 was determined using the Statistical Package for the Social Sciences (SPSS) 18.0 software (SPSS, Chicago, IL, USA) with the regression (Probit analysis method). The mortality rates of each dose of the pre-experiment are shown in Table 3. The LD50s are shown in Table 4.

Table 3.
The mortality rates of each dose of the pre-experiment at 24, 48, 72, and 96 h.

Table 4.
The LD50s of MC-LR to Cherax quadricarinatus.
2.9. MC-LR Challenge
At the end of the breeding trial, ten crayfish per tank were randomly selected for MC-LR challenge experiments, and the muscular injection dose was 108 μg MC-LR kg−1 BW (body weight). The dose of MC-LR used in the study was based on the result from the 48-h lethal doses 50 (LD50) of MC-LR in the pre-experiment of red claw crayfish (Table 4). After MC-LR injection, crayfish mortality was recorded at 48 h, and SR was then determined. The surviving crayfish hepatopancreas was dissected and stored at −80 °C.
2.10. Statistical Analysis
The results were expressed as means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to analyze the data. SPSS 18.0 software (SPSS, Chicago, IL, USA) was used to analyze the data. The significant differences among means were detected using Tukey’s multiple range tests at a significance level of 0.05.
3. Results
3.1. Growth Performance
Dietary ELE levels affected the WGR and SGR of crayfish after eight weeks of feeding (p ˂ 0.05) (Table 5). Due to the increase of ELE levels, WGR and SGR first increased and then decreased (p ˂ 0.05). The highest WGR and SGR were obtained in crayfish fed Diet 3 (p ˂ 0.05). There were no significant differences in the WGR and SGR values of crayfish fed Diet 3, Diet 4, and Diet 5 (p ˃ 0.05). The SRs of the crayfish ranged from 78.33% to 90.00%, showing no significant differences among the treatments (p ˃ 0.05).

Table 5.
Effects of ELE on growth performance of Cherax quadricarinatus.
3.2. Muscle Composition
Effects of dietary ELE levels on the proximate composition of the muscle of crayfish are shown in Table 6. Significantly higher muscle crude protein content was observed in crayfish fed ELE-supplemented feed, and muscle crude protein contents in Diet 3 and Diet 4 groups were significantly higher than those in the other groups (p < 0.05). The muscle lipid content first decreased and then increased with the increase of dietary ELE levels (p < 0.05), and the muscle lipid content of the Diet 3 group was significantly lower than that of the control group (p < 0.05). Compared with the control group, the muscle ash content from the Diet 6 group increased significantly. However, no significant change was observed in muscle moisture content among the different dietary treatments (p ˃ 0.05).

Table 6.
Effects of ELE on proximate muscle composition of Cherax quadricarinatus.
3.3. Hepatopancreas Histology
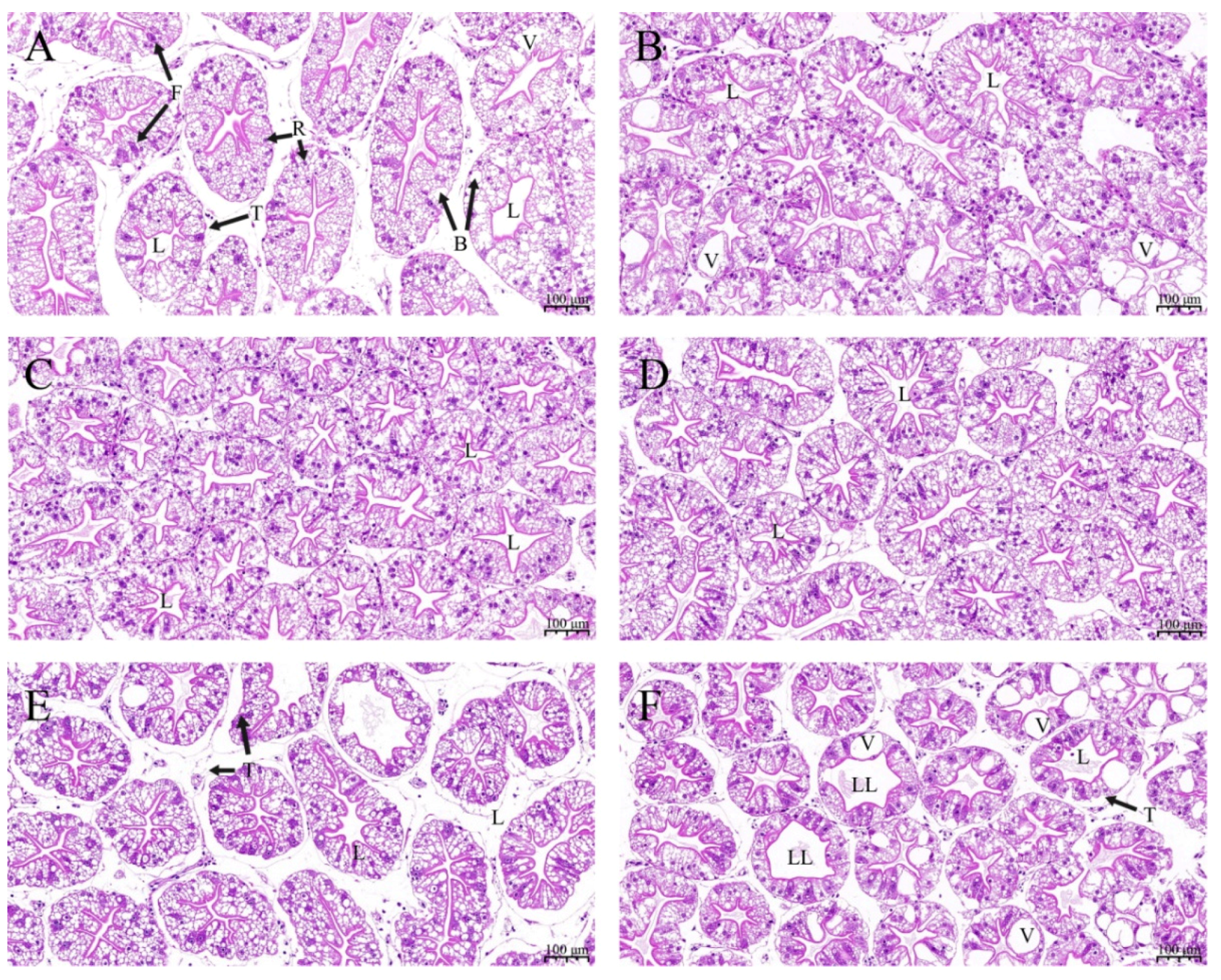
Morphological characteristics of hepatopancreas tissue were examined to evaluate the effects of ELE on crayfish (Figure 1). Compared with the control group (Figure 1A), crayfish fed Diet 2 (Figure 1B), Diet 3 (Figure 1C), Diet 4 (Figure 1D), and Diet 5 (Figure 1F) showed no significant changes. A higher incidence of loss of the typical star-shaped tubular lumen, hepatocyte swelling and vacuolization was observed in the hepatopancreas from crayfish fed Diet 6 (Figure 1F).
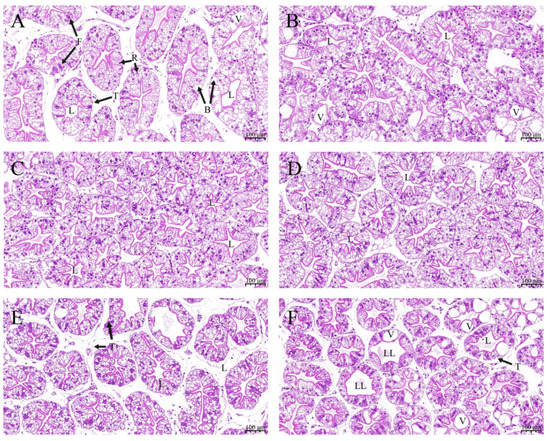
Figure 1.
Hepatopancreas sections of test crayfish fed diet supplementation with different ELE levels (H&E staining 100×). (A) Hepatopancreas from crayfish fed Diet 1 (0 g dw ELE kg (dw)−1 diet); (B) Hepatopancreas from crayfish fed Diet 2 (0.50 g dw ELE kg (dw)−1 diet); (C) Hepatopancreas from crayfish fed Diet 3 (1.00 g dw ELE kg (dw)−1 diet); (D) Hepatopancreas from crayfish fed Diet 4 (2.00 g dw ELE kg (dw)−1 diet); (E) Hepatopancreas from crayfish fed Diet 5 (4.00 g dw ELE kg (dw)−1 diet); (F) Hepatopancreas from crayfish fed Diet 6 (10 g dw ELE kg (dw)−1 diet). T: tubule, L: lumen, B: B-cell, F: F-cell, R: R-cell, V: vacuolation, LL: loss of the typical star-shaped tubular lumen.
3.4. Antioxidant Enzymes in Hepatopancreas and Hemolymph
Antioxidant activities of crayfish hepatopancreas are shown in Table 7. Diet 3 and Diet 4 groups exhibited higher T-AOC and lower MDA levels than the control group (Diet 1) (p < 0.05). The SOD and GPx activities in hepatopancreases of the Diet 4 group were significantly higher than those of the control group (p < 0.05). There was no significant increase in T-AOC, SOD, and GPx activities, and no decrease in MDA level was observed in the hepatopancreas of crayfish fed Diet 5 and Diet 6 (p ˃ 0.05). There were no significant differences in the CAT and GST activities and GSH content of crayfish hepatopancreas among all treatments (p ˃ 0.05).

Table 7.
Effects of ELE on antioxidant abilities of the hepatopancreas in Cherax quadricarinatus.
Antioxidant activities of crayfish hemolymph are shown in Table 8. Diet 4 group exhibited higher T-AOC and SOD activities than the control group (p < 0.05). Diet 3 group exhibited higher GPx activity than the control group (p < 0.05). The hemolymph MDA content of the Diet 3 and Diet 4 groups was significantly lower than that of the control group (p < 0.05). There was no significant increase in T-AOC, SOD, and GPx activities, and no decrease in MDA level was observed in hemolymphs of crayfish fed Diet 5 and Diet 6 (p ˃ 0.05). There were no significant differences in the CAT and GST activities and GSH content of crayfish hemolymph among all treatments (p ˃ 0.05).

Table 8.
Effects of ELE on antioxidant abilities of the hemolymph in Cherax quadricarinatus.
3.5. ACP, AKP, and PO in Hepatopancreas and Hemolymph
Immune parameters in the hepatopancreas and hemolymph of crayfish are shown in Table 9. ACP activity in the hepatopancreas of the Diet 3 group was significantly higher than that of the control group (p < 0.05). AKP activity in the hemolymph of the Diet 3 and Diet 4 groups was significantly higher than that of the control group (p < 0.05). PO content was significantly increased in the hepatopancreas and hemolymph of the Diet 3 and Diet 4 groups compared with the control group (p < 0.05). There were no significant differences in hepatopancreas AKP and hemolymph ACP activities (p ˃ 0.05).

Table 9.
Effects of ELE on immune parameters in hepatopancreas and hemolymph of Cherax quadricarinatus.
3.6. Antioxidant, Glutathione Metabolism, and Immunity Gene Expression
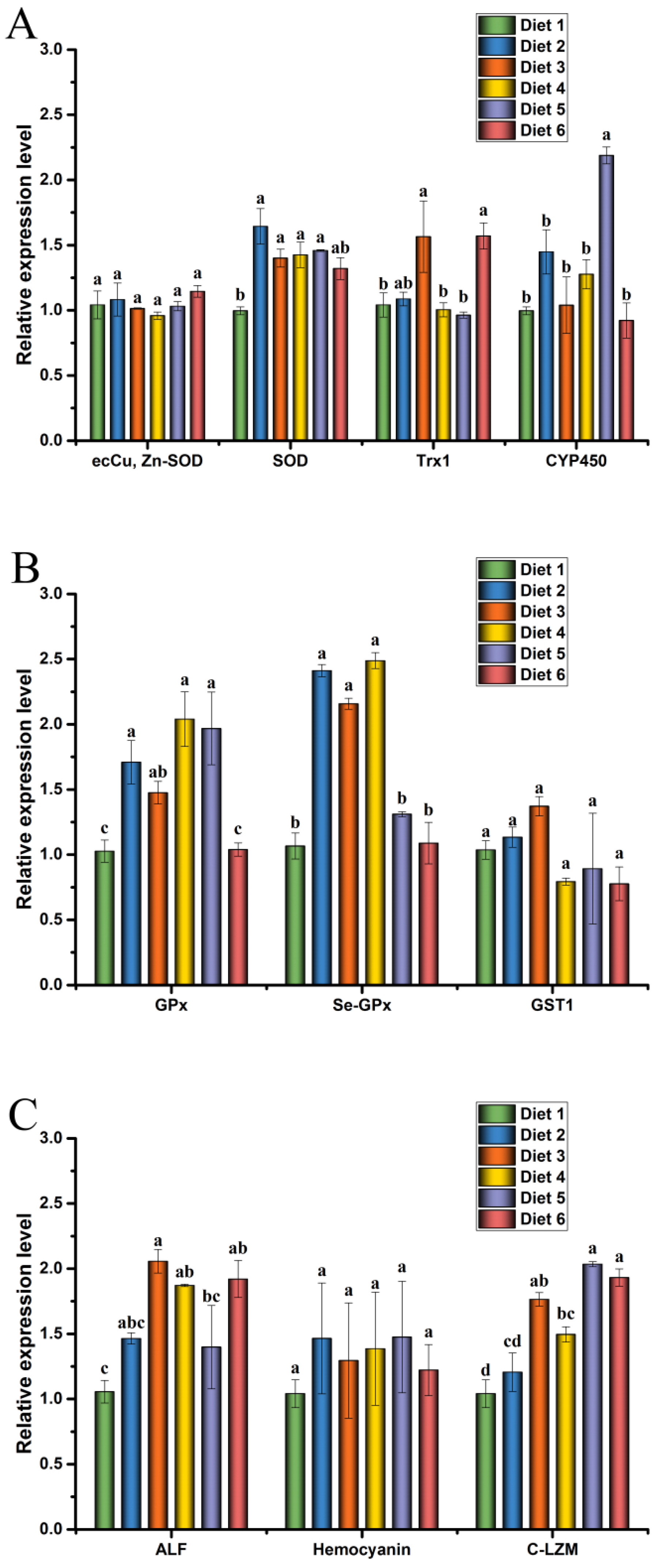
The expression levels of antioxidant, glutathione metabolism, and immunity gene in the hepatopancreas of crayfish are presented in Figure 2.
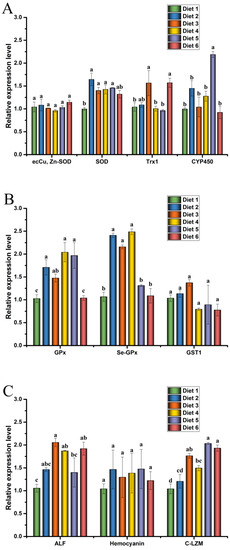
Figure 2.
Effects of dietary ELE on antioxidant (A), glutathione metabolism (B) and immunity (C) gene expression levels in hepatopancreas of Cherax quadricarinatus. Different letters above the error line indicate significant differences (p < 0.05).
Relative expression levels of copper-zinc superoxide dismutase (ecCu, Zn-SOD), SOD, thioredoxin 1 (TRX1), and cytochrome P450 (CYP450) in crayfish are presented in Figure 2A. The relative expression levels of SOD mRNA in the hepatopancreas were significantly increased in Diet 2, Diet 3, Diet 4, and Diet 5 groups compared with the control group (p < 0.05). The mRNA levels of TRX1 were significantly up-regulated in the hepatopancreas of Diet 3 and Diet 6 groups than those of the control group (p < 0.05). The relative mRNA expression levels of CYP450 in the hepatopancreas were significantly increased in the Diet 5 group compared with the control group (p < 0.05). There was no significant difference in the expression of ecCu, Zn-SOD among the groups (p ˃ 0.05).
Relative expression levels of GPx, selenium-dependent glutathione peroxidase (Se-GPx), and glutathione-s-transferase 1 (GST1) in crayfish are presented in Figure 2B. The relative expression levels of GPx mRNA in the hepatopancreas were significantly increased in Diet 2, Diet 3, Diet 4, and Diet 5 groups compared with the control group (p < 0.05). The mRNA levels of Se-GPx were significantly up-regulated in the hepatopancreas of Diet 2, Diet 3, and Diet 4 groups than that of the control group (p < 0.05). However, there was no significant difference in the expression of GST1 among the groups (p ˃ 0.05).
Relative expression levels of anti-lipopolysaccharide factor (ALF), hemocyanin (HEM), and C-type lysozyme (C-LZM) in crayfish are presented in Figure 2C. The relative expression levels of ALF mRNA in the hepatopancreas were significantly increased in Diet 3, Diet 4, and Diet 6 groups compared with the control group (p < 0.05). The mRNA levels of C-LZM were significantly up-regulated in the Diet 3, Diet 4, Diet 5, and Diet 6 groups than those of the control group (p < 0.05). However, there was no significant difference in the expression of HEM among the groups (p ˃ 0.05).
3.7. MC-LR Challenge
3.7.1. SR of Crayfish Fed ELE Supplemented Diets under MC-LR Stress
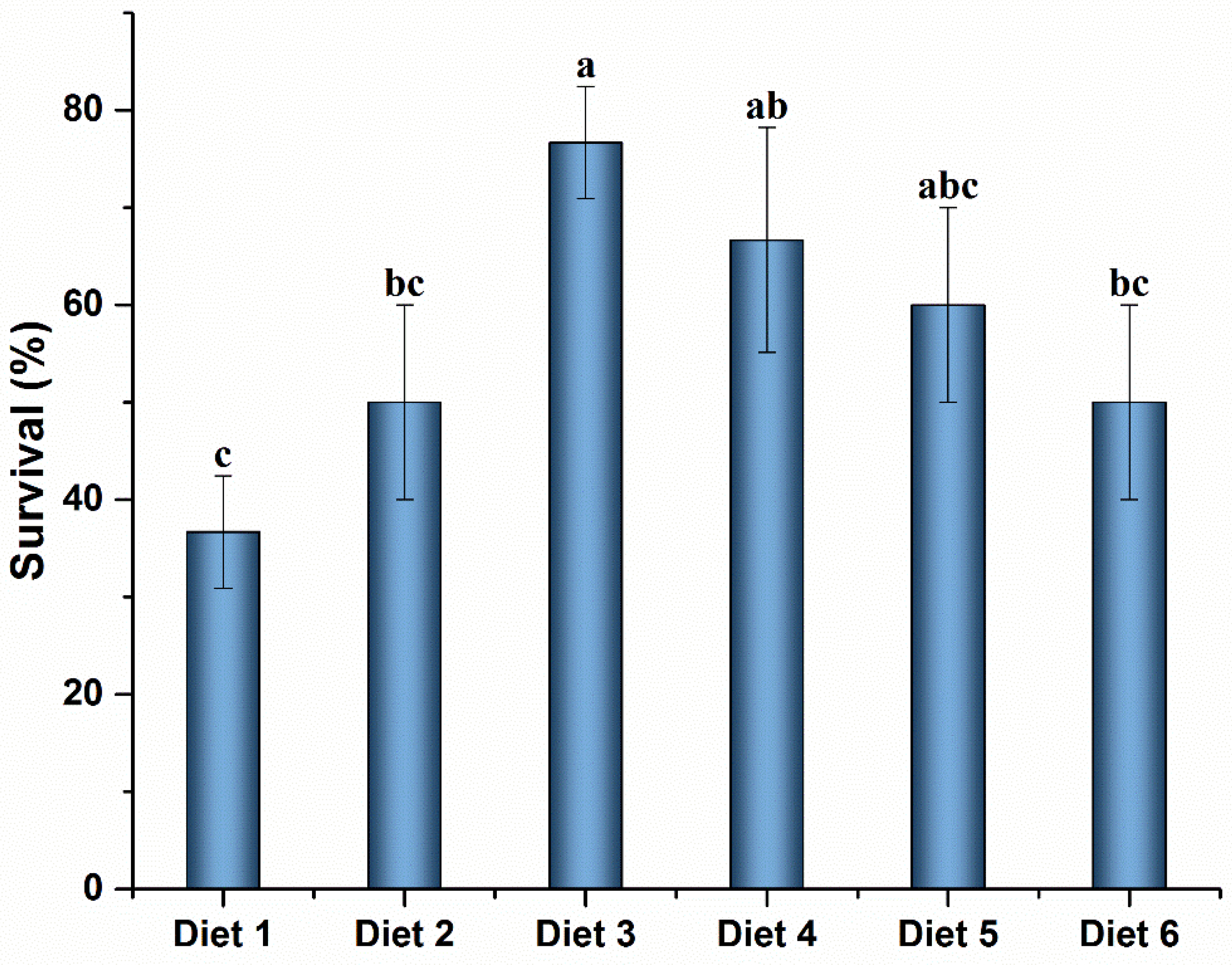
The SR of crayfish after the MC-LR challenge is shown in Figure 3. The SR was significantly higher in Diet 3 and Diet 4 groups than that in the control group (p < 0.05). No significant differences were observed among Diet 3, Diet 4, and Diet 5 groups (p ˃ 0.05).
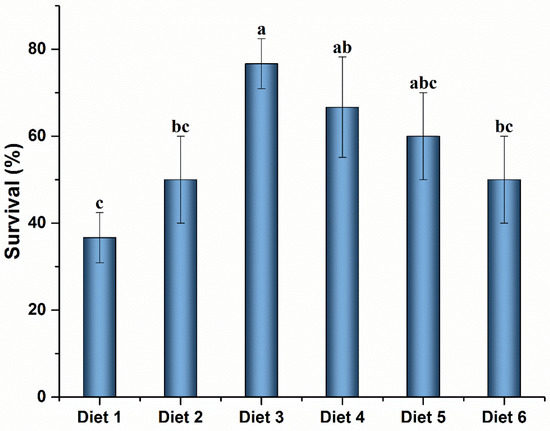
Figure 3.
Survival rate of Cherax quadricarinatus fed ELE-supplemented diets under MC-LR stress. Different letters above the error line indicate significant differences (p < 0.05).
3.7.2. Antioxidant, Glutathione Metabolism, and Immunity Gene Expression after MC-LR Challenge
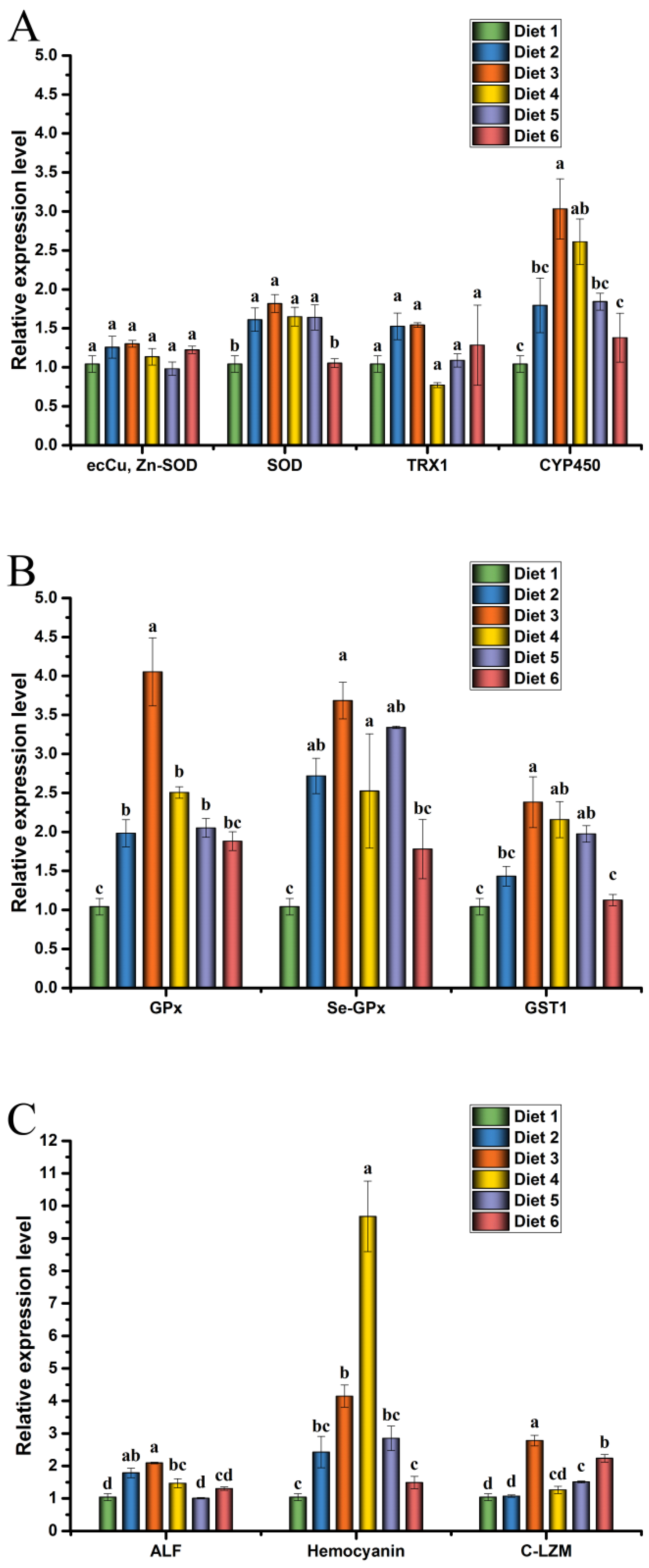
The expression levels of antioxidant, glutathione metabolism and immunity gene in the hepatopancreas of crayfish are presented in Figure 4.
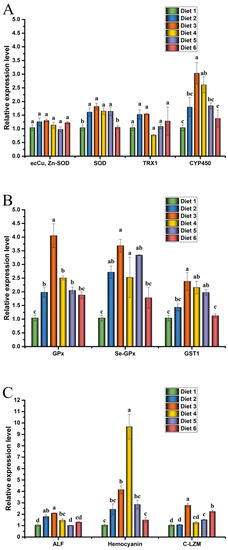
Figure 4.
Antioxidant (A), glutathione metabolism (B), and immunity (C) gene expression levels in hepatopancreas of Cherax quadricarinatus fed ELE-supplemented diets under MC-LR stress. Different letters above the error line indicate significant differences (p < 0.05).
Relative expression levels of ecCu, Zn-SOD, SOD, TRX1, and CYP450 in crayfish are presented in Figure 4A. The mRNA expression levels of SOD and CYP450 in the hepatopancreas of Diet 3 and Diet 4 groups were significantly higher than those of the Diet 1 (p < 0.05).
Relative expression levels of GPx, Se-GPx and GST1 in crayfish are presented in Figure 4B. The mRNA expression levels of GPx and Se-GPx in the hepatopancreas of Diet 3, Diet 4, and Diet 5 groups were significantly higher than those of the Diet 1 (p < 0.05). The mRNA expression levels of GST1 in the hepatopancreas of Diet 3 and Diet 4 groups were significantly higher than those of the Diet 1 (p < 0.05).
Relative expression levels of ALF, HEM, and C-LZM in crayfish are presented in Figure 4C. The mRNA levels of ALF were significantly up-regulated in the hepatopancreas of the Diet 2, Diet 3, and Diet 4 groups than those of the Diet 1 (p < 0.05). The relative expression level of HEM mRNA in the hepatopancreas was significantly increased in the Diet 3 and Diet 4 groups compared with the Diet 1 (p < 0.05). Compared with the Diet 1, the Diet 3, Diet 4, and Diet 6 groups up-regulated the mRNA levels of C-LZM (p < 0.05).
4. Discussion
The potential efficacy of plant extracts for abiotic stress and disease prevention has received widespread attention in aquaculture. Few studies have been reported on the application of ELE in aquatic animal feeds. We reported the effects of dietary ELE on growth, muscle composition, non-specific immunity, and disease resistance of juvenile red claw crayfish. In the present study, a diet supplemented with 1–2 g dw ELE kg (dw)−1 diet significantly increased the WGR and SGR of crayfish, which was similar to previous studies in weaned piglets [51] and broilers [39]. Supplementation with a 4–10 g dw ELE kg (dw)−1 diet did not significantly improve the growth performance of crayfish, which indicates that the beneficial effects of plant supplementation on crayfish growth are dose-dependent. If optimal doses are exceeded, the benefits might be lost [52,53]. The supplementation of excessive ELE in the feed could affect the metabolism of crayfish, leading to high energy consumption, stress, and toxic effects on crayfish [54]. Compared with the control group, dietary treatment of 1 g dw ELE kg (dw)−1 diet increased crude protein content and decreased crude lipid content in crayfish muscle. The results are consistent with previous studies on plant extracts [54,55]. Sufficient nutrient utilization improves the physiological metabolism of crayfish, affecting the metabolism of amino acids, lipids, and glycogen in the tissue. Crayfish fed diets with proper ELE levels alter protein and lipid utilization, causing lipid consumption and protein deposition. Our results indicated that dietary ELE could optimize the utilization of nutrients and increase protein synthesis in crayfish.
The hepatopancreas is an important organ in crustaceans and plays an important role in digestion, immune defense, nutrient absorption, and metabolism [56,57]. The nutritional status can be assessed by observing changes in the morphology of the hepatopancreas in crustaceans [58,59]. Previous studies reported that dietary ELE could significantly reduce an increase in lipid accumulation and preserve liver function in chickens and rats [60,61]. In this study, crayfish fed Diet 3 and Diet 4 showed regular hepatocyte morphology. However, hepatocytes of crayfish in the Diet 6 group showed cell swelling and mild vacuolation. The increased vacuolation of the crayfish hepatopancreas may be caused by the complex composition of ELE, suggesting that extra ELE may damage the crayfish hepatopancreas, which may be the reason for not improving the growth performance of crayfish fed Diet 6 [62]. Inadequate crayfish feed formulation may cause mild vacuolation of the hepatopancreas in the Diet 1 and Diet 2 groups. These results indicated that dietary supplementation with 1–2 g dw ELE kg (dw)−1 diet could improve the morphology of the hepatopancreas with the improvement of absorptive function for various nutrients.
The antioxidant system plays a crucial part in protecting crayfish from oxidative stress [18]. Antioxidant enzymes are important components of the antioxidant system in crustaceans. The T-AOC refers to the antioxidant capacity of the enzymatic and non-enzymatic antioxidants of crustaceans [63]. The SOD, GPx, and CAT are important antioxidant enzymes of aquatic animals and can eliminate unnecessary free superoxide anion radicals [34,64]. MDA can induce tissue damage, reflecting the degree of lipid peroxidation and cell damage [65]. The results showed that T-AOC, GPx and SOD activities significantly increased, and the MDA content decreased in the hemolymph and the hepatopancreas of crayfish fed the optimal ELE level, which is similar to the findings in grass carp [66]. Yang et al. reported that the supplementation of ELE in diets significantly increased the activities of SOD and GPx in grass carp [66]. Previous studies reported that plant extract supplements could improve the antioxidant capacity of red swamp crayfish (Procambarus clarkii) [67,68]. The trends in the change expression levels of antioxidant genes were consistent with the enzyme activity analysis in this study. Our results showed that dietary ELE significantly up-regulated the expression levels of SOD, GPx, and Se-GPx in the hepatopancreas of crayfish. Tan et al. reported that dietary Ginkgo (Ginkgo biloba) leaf extract could improve antioxidant ability in the head kidney of hybrid grouper (Epinephelus lanceolatus × Epinephelus fuscoguttatus
× Epinephelus fuscoguttatus ) by increasing antioxidant gene expression [62]. Consistent with these studies, our findings indicated that ELE supplementation in the diet could protect the hepatocytes and hemocytes from oxidative protein carbonation and lipid peroxidation by activating the antioxidant response in red claw crayfish [67].
) by increasing antioxidant gene expression [62]. Consistent with these studies, our findings indicated that ELE supplementation in the diet could protect the hepatocytes and hemocytes from oxidative protein carbonation and lipid peroxidation by activating the antioxidant response in red claw crayfish [67].
 × Epinephelus fuscoguttatus
× Epinephelus fuscoguttatus ) by increasing antioxidant gene expression [62]. Consistent with these studies, our findings indicated that ELE supplementation in the diet could protect the hepatocytes and hemocytes from oxidative protein carbonation and lipid peroxidation by activating the antioxidant response in red claw crayfish [67].
) by increasing antioxidant gene expression [62]. Consistent with these studies, our findings indicated that ELE supplementation in the diet could protect the hepatocytes and hemocytes from oxidative protein carbonation and lipid peroxidation by activating the antioxidant response in red claw crayfish [67].Phosphatases are crucial enzymes in the biological processes of aquatic animals and shape immune responses and reflect the immune system in aquatic animals [69,70]. ACP plays an essential role in substance metabolism. It is related to lysosomes and can be induced by exogenous substances [71,72]. AKP is the most important metabolic enzyme and plays a crucial role in the absorption and utilization of nutrients in aquatic animals, enhancing immunity of aquatic animals [73,74]. PO is an important enzyme in the prophenoloxidase (proPO) system, a key systemic enzyme for recognizing non-self substances in invertebrates. It is closely related to the immunity of crustaceans [75]. In this study, dietary ELE increased the activities of ACP, AKP, and PO of crayfish. A previous study observed that the administration of Mojave yucca (Yucca schidigera) extract enhanced ACP, AKP, and PO activities in whiteleg shrimp (Litopenaeus vannamei) [76]. Many plant extracts can improve the non-specific immunity of aquatic animals [54,68,77]. Yang et al. reported that dietary ELE significantly improved the muscle antioxidant capacity and flesh quality of grass carp [66]. No studies about the effects of dietary ELE on the non-specific immunity in crayfish have been reported. Further studies are needed to determine if the mechanisms underlying ELE-enhanced non-specific immunity are comparable with those found in other terrestrial animals.
Antimicrobial peptides (AMPs), such as HEM, lysozymes (LZM), and ALF, play important roles in the innate immunity of aquatic animals [78,79]. The regulation of immune genes is closely related to the regulation of immunity in aquatic animals [62]. Previous studies reported that E. ulmoides could promote immunity by regulating the secretion of proinflammatory cytokines [32,42]. Similarly, the high expression of AMPs implies an increase in disease resistance in aquatic animals [80,81,82]. In the present study, the results suggested that diet supplement ELE significantly up-regulated the mRNA levels of C-LZM and ALF in the hepatopancreas of crayfish compared with the control group. However, the regulation of AMP gene expression by dietary ELE at the level of mRNA translation was not reported previously in aquatic animals. We demonstrated that ELE induced the expression of the AMP gene. Thus, more research needs to be done to clarify the molecular mechanism.
Plants or plant extracts can improve the resistance of aquatic animals to environmental stress, such as low temperature, toxins, and heavy metals [73,83,84]. In the present study, supplementation of ELE to diets significantly improved the resistance of red claw crayfish against MC-LR stress. The increased SR of crayfish to environmental stress, as described for other aquaculture species, can result in increased health status and positive immune response of crayfish [85]. However, few studies have reported the effects of plant extracts on immune-related genes in crayfish under MC-LR stress. Therefore, we analyzed the immune status of crayfish in each feed group under MC-LR stress. In the current study, supplementation of ELE to diets significantly up-regulated the mRNA levels of SOD, GPx, Se-GPx, GST1, ALF, and HEM in the hepatopancreas of crayfish under MC-LR stress compared with Diet 1. The antioxidant system of crayfish neutralizes the damaging effects of ROS, and the antioxidant system with GSH plays a vital role in MC-LR depuration in crayfish [18]. In MC-LR stress experiments, the expression levels of SOD, CYP450, GPx, Se-GPx, and GST1 in crayfish were significantly increased in Diet 3 and Diet 4 groups. After a challenge with MC-LR, crayfish survival was significantly higher in Diet 3 and Diet 4 groups than in the Diet 1, which may be due to the increased effects of ELE on the immune system of crayfish [86]. These results are similar to those reported previously [87,88]. Plant extracts can improve the survival of aquatic animals in stressful environments by enhancing immunity [54,67,85,89]. Xie et al. reported that Tian-Dong-Tang-Gan powder could improve the resistance of whiteleg shrimp by inducing the expression levels of GPx, Mn-SOD, and ACP in the hepatopancreas of nitrite exposure [88]. Changes in SOD, GPx, GST, ALF, HEM, and LZM gene expression levels suggested that MC-LR induced ROS production and crayfish made the corresponding reaction by regulating these immune-related factors in vivo [18,87]. These results indicated that ELE could increase or maintain the mRNA expression levels of SOD, GPx, Se-GPx, GST1, ALF, HEM, and C-LZM in the hepatopancreas of juvenile red claw crayfish to regulate the ability of crayfish and resist MC-LR stress, reducing the damage of hepatopancreas caused by MC-LR in juvenile red claw crayfish.
5. Conclusions
This study aimed to assess the effect of dietary ELE on growth performance, muscle composition, hepatopancreas histology, immune responses, gene expression, and MC-LR resistance of red claw crayfish. This study showed that red claw crayfish fed a diet supplemented with 1–2 g dw ELE kg (dw)−1 diet for eight weeks promoted its growth performance and muscle crude protein, improved the T-AOC, SOD, GPx, and PO activities in the hepatopancreas and hemolymph, increased the mRNA expression levels of SOD, GPx, Se-GPx, ALF, and C-LZM in the hepatopancreas and improved the survival of red claw crayfish under MC-LR stress. ELE is considered an effective alternative to chemicals and antimicrobials for crayfish culture. Adding ELE at a level of 1–2 g dw ELE kg (dw)−1 diet over a feeding period is recommended for the achievement of rapid growth and resistance enhancement of juvenile red claw crayfish, matching market demands and farmer’s appreciation. Therefore, ELE can be used as a new green growth promotion agent and immunopotentiator for culturing crayfish.
Author Contributions
Y.-P.L.: Methodology, Writing—Original draft preparation, Data curation, Visualization, Investigation. P.-H.Z.: Data curation, Visualization, Investigation. J.-R.X.: Data curation, Software, Investigation. Y.-L.C.: Investigation, Validation. J.-T.L.: Investigation. C.-G.H.: Investigation. Z.-L.Z.: Investigation. J.-A.X.: Conceptualization, Methodology, Writing—Reviewing and Editing, Funding acquisition. X.-X.Z.: Conceptualization, Methodology, Writing—Reviewing and Editing. A.-L.W.: Conceptualization, Methodology, Writing—Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hainan Provincial Natural Science Foundation of China (No. 320RC710) and the Central Public-interest Scientific Institution Basal Research Fund for the Chinese Academy of Tropical Agricultural Sciences, China (No. 1630052019013).
Institutional Review Board Statement
The procedures and protocols for this study have been ethically reviewed and approved according to the guidelines of the relevant institutional committees and granted an Aquaculture Research Permit (ITBB20210401).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously and is not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
References
- Yuan, J.; Zheng, Y.; Gu, Z. Effects of cypermethrin on the hepatic transcriptome and proteome of the red claw crayfish Cherax quadricarinatus. Chemosphere 2020, 263, 128060. [Google Scholar] [CrossRef] [PubMed]
- Zuo, D.; Wu, D.-L.; Ma, C.-A.; Li, H.-X.; Huang, Y.-H.; Wang, D.-L.; Zhao, Y.-L. Effects of white spot syndrome virus infection and role of immune polysaccharides of juvenile Cherax quadricarinatus. Aquaculture 2015, 437, 235–242. [Google Scholar] [CrossRef]
- Jones, C.M. Production of juvenile redclaw crayfish, Cherax quadricarinatus (von Martens) (Decapoda, Parastacidae) III. Managed pond production trials. Aquaculture 1995, 138, 247–255. [Google Scholar] [CrossRef]
- Abdu, U.; Yehezkel, G.; Sagi, A. Oocyte development and polypeptide dynamics during ovarian maturation in the red-claw crayfish Cherax quadricarinatus. Invertebr. Reprod. Dev. 2000, 37, 75–83. [Google Scholar] [CrossRef]
- Ashour, M.; Alprol, A.; Heneash, A.; Saleh, H.; Abualnaja, K.; Alhashmialameer, D.; Mansour, A. Ammonia bioremediation from aquaculture wastewater effluents using Arthrospira platensis NIOF17/003: Impact of biodiesel residue and potential of ammonia-loaded biomass as rotifer feed. Materials 2021, 14, 5460. [Google Scholar] [CrossRef]
- Alprol, A.E.; Ashour, M.; Mansour, A.T.; Alzahrani, O.M.; Mahmoud, S.F.; Gharib, S.M. Assessment of Water Quality and Phytoplankton Structure of Eight Alexandria Beaches, Southeastern Mediterranean Sea, Egypt. J. Mar. Sci. Eng. 2021, 9, 1328. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Tong, T.; Tong, W.; Dong, L.; Xu, M.; Wang, Z. Dietary supplementation of Bacillus subtilis and fructooligosaccharide enhance the growth, non-specific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against Vibrio vulnificus. Fish Shellfish Immunol. 2014, 38, 7–14. [Google Scholar] [CrossRef]
- Mansour, A.T.; Ashour, M.; Alprol, A.E.; Alsaqufi, A.S. Aquatic Plants and Aquatic Animals in the Context of Sustainability: Cultivation Techniques, Integration, and Blue Revolution. Sustainability 2022, 14, 3257. [Google Scholar] [CrossRef]
- Ji, X.; Verspagen, J.M.H.; Van de Waal, D.B.; Rost, B.; Huisman, J. Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Sci. Adv. 2020, 6, eaax2926. [Google Scholar] [CrossRef]
- Gkelis, S.; Lanaras, T.; Sivonen, K. Cyanobacterial Toxic and Bioactive Peptides in Freshwater Bodies of Greece: Concentrations, Occurrence Patterns, and Implications for Human Health. Mar. Drugs 2015, 13, 6319–6335. [Google Scholar] [CrossRef]
- Wei, L.; Sun, B.; Chang, M.; Liu, Y.; Nie, P. Effects of cyanobacterial toxin microcystin-LR on the transcription levels of immune-related genes in grass carp Ctenopharyngodon idella. J. Appl. Phycol. 2009, 85, 231–238. [Google Scholar] [CrossRef]
- Abdel-Latif, H.; Khashaba, A. Subchronic toxicity of Nile tilapia with different exposure routes to Microcystis aeruginosa: Histopathology, liver functions, and oxidative stress biomarkers. Vet World 2017, 10, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, B.; Wu, H.; Nie, P. Effects of pure microcystin-LR on the transcription of immune related genes and heat shock proteins in larval stage of zebrafish (Danio rerio). Aquaculture 2009, 289, 154–160. [Google Scholar] [CrossRef]
- Rao, P.L.; Gupta, N.; Jayaraj, R.; Bhaskar, A.; Jatav, P. Age-dependent effects on biochemical variables and toxicity induced by cyclic peptide toxin microcystin-LR in mice. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 11–19. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Kaufononga, S.A.F.; Cary, S.C.; Hamilton, D.P. High Levels of Structural Diversity Observed in Microcystins from Microcystis CAWBG11 and Characterization of Six New Microcystin Congeners. Mar. Drugs 2014, 12, 5372–5395. [Google Scholar] [CrossRef]
- Fastner, J.; Codd, G.A.; Metcalf, J.S.; Woitke, P.; Wiedner, C.; Utkilen, H. An international intercomparison exercise for the determination of purified microcystin-LR and microcystins in cyanobacterial field material. Anal. Bioanal. Chem. 2002, 374, 437–444. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total. Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
- Yuan, J.; Gu, Z.; Zheng, Y.; Zhang, Y.; Gao, J.; Chen, S.; Wang, Z. Accumulation and detoxification dynamics of microcystin-LR and antioxidant responses in male red swamp crayfish Procambarus clarkii. Aquat. Toxicol. 2016, 177, 8–18. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, P.; Wang, W.; Li, D.; Li, L.; Tang, R.; Lei, H.; Shi, Z. Dose-dependent effects of extracted microcystins on embryonic development, larval growth and histopathological changes of southern catfish (Silurus meridionalis). Toxicon 2008, 51, 449–456. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, J.; Wu, J.; Han, X. The toxic effects of microcystin-LR on rat spermatogonia in vitro. Toxicol. Lett. 2012, 212, 48–56. [Google Scholar] [CrossRef]
- Wei, L.L.; Sun, B.J.; Nie, P. Ultrastructural alteration of lymphocytes in spleen and pronephros of grass carp (Ctenopharyngodon idella) experimentally exposed to microcystin-LR. Aquaculture 2008, 280, 270–275. [Google Scholar] [CrossRef]
- Hermansky, S.; Markin, R.; Fowler, E.; Stohs, S. Hepatic ultrastructual changes induced by the toxin microcystin-LR (MC-LR) in mice. J. Environ. Pathol. Toxicol. Oncol. 1993, 12, 101–106. [Google Scholar] [PubMed]
- Hooser, S.B.; Kuhlenschmidt, M.S.; Dahlem, A.M.; Beasley, V.R.; Carmichael, W.W.; Haschek, W.M. Uptake and subcellular localization of tritiated dihydro-microcystin-LR in rat liver. Toxicon 1991, 29, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Sun, J.; Lv, A.; Xian, J.-A.; Sung, Y.Y.; Sun, X.; Hu, X.; Xing, K. Transcriptome profiling of immune-responsive genes in the intestine of Cynoglossus semilaevis Günther challenged with Shewanella algae. Fish Shellfish Immunol. 2018, 80, 291–301. [Google Scholar] [CrossRef]
- Hou, J.; Li, L.; Xue, T.; Long, M.; Su, Y.; Wu, N. Hepatic positive and negative antioxidant responses in zebrafish after intraperitoneal administration of toxic microcystin-LR. Chemosphere 2015, 120, 729–736. [Google Scholar] [CrossRef]
- Wei, L.; Liu, Y.; Zhong, S.; Wu, H.; Ruan, J.; Liu, M.; Zhou, Q.; Zhong, Q. Transcriptome analysis of grass carp provides insights into the immune-related genes and pathways in response to MC-LR induction. Aquaculture 2018, 488, 207–216. [Google Scholar] [CrossRef]
- Chen, L.; Xie, P. Mechanisms of Microcystin-induced Cytotoxicity and Apoptosis. Mini-Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef]
- Sivasankar, P.; Anix Vivek Santhiya, A.; Kanaga, V. A review on plants and herbal extracts against viral diseases in aquaculture. J. Med. Plants Stud. 2015, 3, 75–79. [Google Scholar]
- Mohammadi, G.; Rafiee, G.; El Basuini, M.F.; Van Doan, H.; Ahmed, H.A.; Dawood, M.A.; Abdel-Latif, H.M. Oregano (Origanum vulgare), St John’s-wort (Hypericum perforatum), and lemon balm (Melissa officinalis) extracts improved the growth rate, antioxidative, and immunological responses in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquac. Rep. 2020, 18, 100445. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Benefits of Dietary Polyphenols and Polyphenol-Rich Additives to Aquatic Animal Health: An Overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Abdel-Daim, M.M.; Shukry, M.; Nowosad, J.; Kucharczyk, D. Benefits and applications of Moringa oleifera as a plant protein source in Aquafeed: A review. Aquaculture 2021, 547, 737369. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, K.S.; Chang, I.-M. Elucidation of Anti-inflammatory Potencies of Eucommia ulmoides Bark and Plantago asiatica Seeds. J. Med. Food 2009, 12, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Ishimitsu, A.; Tojo, A.; Satonaka, H.; Ishimitsu, T. Eucommia ulmoides (Tochu) and its extract geniposidic acid reduced blood pressure and improved renal hemodynamics. Biomed. Pharmacother. 2021, 141, 111901. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.-H.; Ye, J.-Y.; Zhang, Y.-X.; Xu, P.; Xie, J. Effects of dietary reduced glutathione on growth performance, non-specific immunity, antioxidant capacity and expression levels of IGF-I and HSP70 mRNA of grass carp (Ctenopharyngodon idella). Aquaculture 2015, 438, 39–46. [Google Scholar] [CrossRef]
- Li, H.-D.; Tian, X.-L.; Dong, S.-L. Growth performance, non-specific immunity, intestinal histology and disease resistance of Litopenaeus vannamei fed on a diet supplemented with live cells of Clostridium butyricum. Aquaculture 2018, 498, 470–481. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Liu, G.; Oladele, O.A.; Rahu, N.; Tossou, M.C.; Yin, Y. Health-Promoting Properties of Eucommia ulmoides: A Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 5202908. [Google Scholar] [CrossRef]
- Xu, J.-K.; Li, M.-F.; Sun, R.-C. Identifying the impact of ultrasound-assisted extraction on polysaccharides and natural antioxidants from Eucommia ulmoides Oliver. Process. Biochem. 2015, 50, 473–481. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, W.; Liu, H. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019, 98, 3040–3049. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several Culinary and Medicinal Herbs Are Important Sources of Dietary Antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Kim, D.-S.; Kim, S.-J.; Park, J.; Kim, H.-L.; Kim, S.-Y.; Ahn, K.S.; Jang, H.-J.; Lee, S.-G.; Lee, K.-M.; et al. Eucommiae Cortex Inhibits TNF-α and IL-6 Through the Suppression of Caspase-1 in Lipopolysaccharide-Stimulated Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2012, 40, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Nakashima, H.; Itoh, Y. Anti-human immunodeficiency virus activity of oligosaccharides from rooibos tea (Aspalathus linearis) extracts in vitro. Leukemia 1997, 11, 128–130. [Google Scholar] [PubMed]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; de la Cruz, M.; Monteiro, M.C.; Melguizo, E.; et al. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.-G.; Tan, X.-P.; Cui, H.-B.; Qi, X.-Z.; Zhu, B.; Wang, G.-X. Antiviral activity of geniposidic acid against white spot syndrome virus replication in red swamp crayfish Procambarus clarkii. Aquaculture 2020, 528, 735533. [Google Scholar] [CrossRef]
- Lu, Y.-P.; Zheng, P.-H.; Zhang, X.-X.; Wang, L.; Li, J.-T.; Zhang, Z.-L.; Xu, J.-R.; Cao, Y.-L.; Xian, J.-A.; Wang, A.-L.; et al. Effects of dietary trehalose on growth, trehalose content, non-specific immunity, gene expression and desiccation resistance of juvenile red claw crayfish (Cherax quadricarinatus). Fish Shellfish Immunol. 2021, 119, 524–532. [Google Scholar] [CrossRef]
- Fawzy, S.; Wang, W.; Wu, M.; Yi, G.; Huang, X. Effects of dietary different canthaxanthin levels on growth performance, antioxidant capacity, biochemical and immune-physiological parameters of white shrimp (Litopenaeus vannamei). Aquaculture 2022, 556, 738276. [Google Scholar] [CrossRef]
- Hirwitz, W.; Latimer, G. Official methods of analysis of AOAC International (16th edn). Trends Food Sci. Technol. 1995, 6, 382. [Google Scholar]
- Wu, D.; Huang, Y.; Chen, Q.; Jiang, Q.; Li, Y.; Zhao, Y. Effects and transcriptional responses in the hepatopancreas of red claw crayfish Cherax quadricarinatus under cold stress. J. Therm. Biol. 2019, 85, 102404. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Peng, M.; Wang, Z.; Peng, S.; Zhang, M.; Duan, Y.; Li, F.; Shi, S.; Yang, Q.; Zhang, C. Dietary supplementation with the extract from Eucommia ulmoides leaves changed epithelial restitution and gut microbial community and composition of weanling piglets. PLoS ONE 2019, 14, e0223002. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, P.; Caruso, D. Moving towards more sustainable aquaculture practices: A meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquacult. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Yousefi, M.; Zahedi, S.; Reverter, M.; Adineh, H.; Hoseini, S.M.; Van Doan, H.; El-Haroun, E.R.; Hoseinifar, S.H. Enhanced growth performance, oxidative capacity and immune responses of common carp, Cyprinus carpio fed with Artemisia absinthium extract-supplemented diet. Aquaculture 2021, 545, 737167. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Chen, S.; Chen, S.; Huang, Z.; Zhou, C.; Zou, C.; Liu, Q.; Ye, H.; Lin, H.; et al. Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2017, 66, 198–206. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Ye, H.; Zou, C.; Ye, C.; Wang, A. Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 73, 234–244. [Google Scholar]
- Ellis, R.; Parry, H.; Spicer, J.; Hutchinson, T.; Pipe, R.; Widdicombe, S. Immunological function in marine invertebrates: Responses to environmental perturbation. Fish Shellfish Immunol. 2011, 30, 1209–1222. [Google Scholar] [CrossRef]
- Rombout, J.H.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef]
- Najdegerami, E.H.; Bakhshi, F.; Lakani, F.B. Effects of biofloc on growth performance, digestive enzyme activities and liver histology of common carp (Cyprinus carpio L.) fingerlings in zero-water exchange system. Fish Physiol. Biochem. 2015, 42, 457–465. [Google Scholar] [CrossRef]
- Rašković, B.; Stanković, M.; Marković, Z.; Poleksić, V.D. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. 2011, 56, 87–100. [Google Scholar]
- Santoso, U.; Ohtani, S.; Tanaka, K. Tu-Chung Leaf Meal Supplementation Reduced an Increase in Lipid Accumulation of Chickens Stimulated by Dietary Cholesterol. Asian-Australas. J. Anim. Sci. 2000, 13, 1758–1763. [Google Scholar] [CrossRef]
- Jin, C.; Li, B.; Lin, S.; Yadav, R.; Kim, H.; Chae, H. Mechanism of the inhibitory effects of Eucommia ulmoides Oliv. Cortex Extracts (EUCE) in the CCl4 -induced acute liver lipid accumulation in rats. Int. J. Endocrinol. 2013, 2013, 751854. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Wang, A.; Lin, H. Effects of dietary Ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 72, 399–409. [Google Scholar] [PubMed]
- Xie, J.; Liu, B.; Zhou, Q.; Su, Y.; He, Y.; Pan, L.; Ge, X.; Xu, P. Effects of anthraquinone extract from rhubarb Rheum officinale Bail on the crowding stress response and growth of common carp Cyprinus carpio var. Jian. Aquaculture 2008, 281, 5–11. [Google Scholar] [CrossRef]
- Tan, X.; Lin, H.; Huang, Z.; Zhou, C.; Wang, A.; Qi, C.; Zhao, S. Effects of dietary leucine on growth performance, feed utilization, non-specific immune responses and gut morphology of juvenile golden pompano Trachinotus ovatus. Aquaculture 2016, 465, 100–107. [Google Scholar] [CrossRef]
- Muñoz, M.; Cedeño, R.; Rodrı, J.; Van der Knaap, W.; Mialhe, E.; Bachère, E. Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, Penaeus vannamei. Aquaculture 2000, 191, 89–107. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Li, X.-Q.; Tan, S.-M.; Cheng, Z.; Leng, X.-J. Influences of dietary Eucommia ulmoides extract on growth, flesh quality, antioxidant capacity and collagen-related genes expression in grass carp (Ctenopharyngodon idellus). Anim. Feed Sci. Technol. 2021, 277, 114965. [Google Scholar] [CrossRef]
- Liu, F.; Qu, Y.-K.; Geng, C.; Wang, A.-M.; Zhang, J.-H.; Chen, K.-J.; Liu, B.; Tian, H.-Y.; Yang, W.-P.; Yu, Y.-B. Effects of hesperidin on the growth performance, antioxidant capacity, immune responses and disease resistance of red swamp crayfish (Procambarus clarkii). Fish Shellfish. Immunol. 2020, 99, 154–166. [Google Scholar] [CrossRef]
- Cheng, Y. The growth performance and nonspecific immunity of red swamp crayfish Procambarus clarkia affected by dietary Rhodiola rosea polysaccharide. Fish Shellfish Immunol. 2019, 93, 796–800. [Google Scholar] [CrossRef]
- Murti, R.; Omkar; Shukla, G. Mercuric chloride intoxication in freshwater prawn: II. Effect on phosphatases activity. Ecotoxicol. Environ. Saf. 1984, 8, 581–586. [Google Scholar] [CrossRef]
- Sarlin, P.; Philip, R. Efficacy of marine yeasts and baker’s yeast as immunostimulants in Fenneropenaeus indicus: A comparative study. Aquaculture 2011, 321, 173–178. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Yang, H.-S.; Delaporte, M.; Zhao, S.-J.; Xing, K. Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. J. Exp. Mar. Biol. Ecol. 2007, 345, 52–60. [Google Scholar] [CrossRef]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Kim, S.W.; Yun, S.; Park, S.C. Effects of algal toxin okadaic acid on the non-specific immune and antioxidant response of bay scallop (Argopecten irradians). Fish Shellfish. Immunol. 2017, 65, 111–117. [Google Scholar] [CrossRef]
- Liu, B.; Ge, X.; Xie, J.; Xu, P.; He, Y.; Cui, Y.; Ming, J.; Zhou, Q.; Pan, L. Effects of anthraquinone extract from Rheum officinale Bail on the physiological responses and HSP70 gene expression of Megalobrama amblycephala under Aeromonas hydrophila infection. Fish Shellfish Immunol. 2012, 32, 1–7. [Google Scholar] [CrossRef]
- Pinoni, S.; Mañanes, A.L. Alkaline phosphatase activity sensitive to environmental salinity and dopamine in muscle of the euryhaline crab Cyrtograpsus angulatus. J. Exp. Mar. Biol. Ecol. 2004, 307, 35–46. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Söderhäll, K. The proPO and clotting system in crustaceans. Aquaculture 2000, 191, 53–69. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Tan, B.-P.; Dong, X.-H.; Chi, S.-Y.; Liu, H.-Y. Effects of different levels of Yucca schidigera extract on the growth and nonspecific immunity of Pacific white shrimp (Litopenaeus vannamei) and on culture water quality. Aquaculture 2015, 439, 39–44. [Google Scholar] [CrossRef]
- Wu, C.; Liu, C.; Chang, Y.; Hsieh, S. Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila in Oreochromis mosambicus. Fish Shellfish Immunol. 2010, 29, 258–263. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, Z.; Li, X.-C.; Du, J.; Hui, K.-M.; Zhang, C.-Y.; Wang, W. Three different anti-lipopolysaccharide factors identified from giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish. Immunol. 2012, 33, 766–774. [Google Scholar] [CrossRef]
- Destoumieux-Garzon, D.; Saulnier, D.; Garnier, J.; Jouffrey, C.; Bulet, P.; Bachere, E. Crustacean immunity. Antifungal peptides are generated from the C terminus of shrimp hemocyanin in response to microbial challenge. J. Biol. Chem. 2001, 276, 47070–47077. [Google Scholar]
- Shimizu, K.; Chen, W.; Ashique, A.M.; Moroi, R.; Li, Y.-P. Molecular cloning, developmental expression, promoter analysis and functional characterization of the mouse CNBP gene. Gene 2003, 307, 51–62. [Google Scholar] [CrossRef]
- Shi, X.-Z.; Zhao, X.-F.; Wang, J.-X. A new type antimicrobial peptide astacidin functions in antibacterial immune response in red swamp crayfish Procambarus clarkii. Dev. Comp. Immunol. 2014, 43, 121–128. [Google Scholar] [CrossRef]
- Sruthy, K.; Nair, A.; Puthumana, J.; Antony, S.P.; Singh, I.; Philip, R. Molecular cloning, recombinant expression and functional characterization of an antimicrobial peptide, Crustin from the Indian white shrimp, Fenneropenaeus indicus. Fish Shellfish Immunol. 2017, 71, 83–94. [Google Scholar] [CrossRef]
- Da Silva, A.; Brito, L.; Da Silva, D.; De Lima, P.; Da Silva Farias, R.; Gálvez, A.; Da Silva, S. Effect of Brachionus plicatilis and Navicula sp. on Pacific white shrimp growth performance, Vibrio, immunological responses and resistance to white spot virus (WSSV) in nursery biofloc system. Aquaculture 2021, 535, 736335. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; Khalil, S.R.; Moustafa, A.A.; Mahmoud, H.K.; Abdel-Latif, H.M. Astragalus membranaceus polysaccharides modulate growth, hemato-biochemical indices, hepatic antioxidants, and expression of HSP70 and apoptosis-related genes in Oreochromis niloticus exposed to sub-lethal thallium toxicity. Fish Shellfish Immunol. 2021, 118, 251–260. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Li, J.; Duan, Y.-F.; Niu, J.; Wang, J.; Huang, Z.; Lin, H.-Z. Effects of dietary chlorogenic acid on growth performance, antioxidant capacity of white shrimp Litopenaeus vannamei under normal condition and combined stress of low-salinity and nitrite. Fish Shellfish Immunol. 2015, 43, 337–345. [Google Scholar] [CrossRef]
- Raissy, M.; Ghafarifarsani, H.; Hoseinifar, S.H.; El-Haroun, E.R.; Naserabad, S.S.; Van Doan, H. The effect of dietary combined herbs extracts (oak acorn, coriander, and common mallow) on growth, digestive enzymes, antioxidant and immune response, and resistance against Aeromonas hydrophila infection in common carp, Cyprinus carpio. Aquaculture 2021, 546, 737287. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Gu, Z.; Zhang, Y.; Wang, Z. Activity and Transcriptional Responses of Hepatopancreatic Biotransformation and Antioxidant Enzymes in the Oriental River Prawn Macrobrachium nipponense Exposed to Microcystin-LR. Toxins 2015, 7, 4006–4022. [Google Scholar] [CrossRef]
- Xie, X.-D.; Cao, M.-X.; Chen, Q.; Yu, M.-L.; Liu, Q.-Y.; Zhao, Y.-Z.; Zhang, L.; Hu, T.-J. Effect of medical herbs in Tian-Dong-Tang-Gan powder on the oxidative stress induced by ammonia and nitrite in Litopenaeus vannamei. Aquaculture 2021, 548, 737584. [Google Scholar] [CrossRef]
- Wang, Q.-K.; Chen, C.-X.; Guo, Y.-J.; Zhao, H.-Y.; Sun, J.-F.; Ma, S.; Xing, K.-Z. Dietary polysaccharide from Angelica sinensis enhanced cellular defence responses and disease resistance of grouper Epinephelus malabaricus. Aquac. Int. 2011, 19, 945–956. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).