Efficacy of Different Routes of Formalin-Killed Vaccine Administration on Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Challenged with Streptococcus agalactiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Preparation
2.2. Diet Preparation and Experimental Design
2.3. Experimental Procedure
2.4. Serum, Leukocytes, and Mucus Preparation
2.5. Immunological Assays
2.6. Nonspecific Immune-Related Gene Expression Analysis
2.7. Challenge Experiment
2.8. Statistical Analysis
3. Results
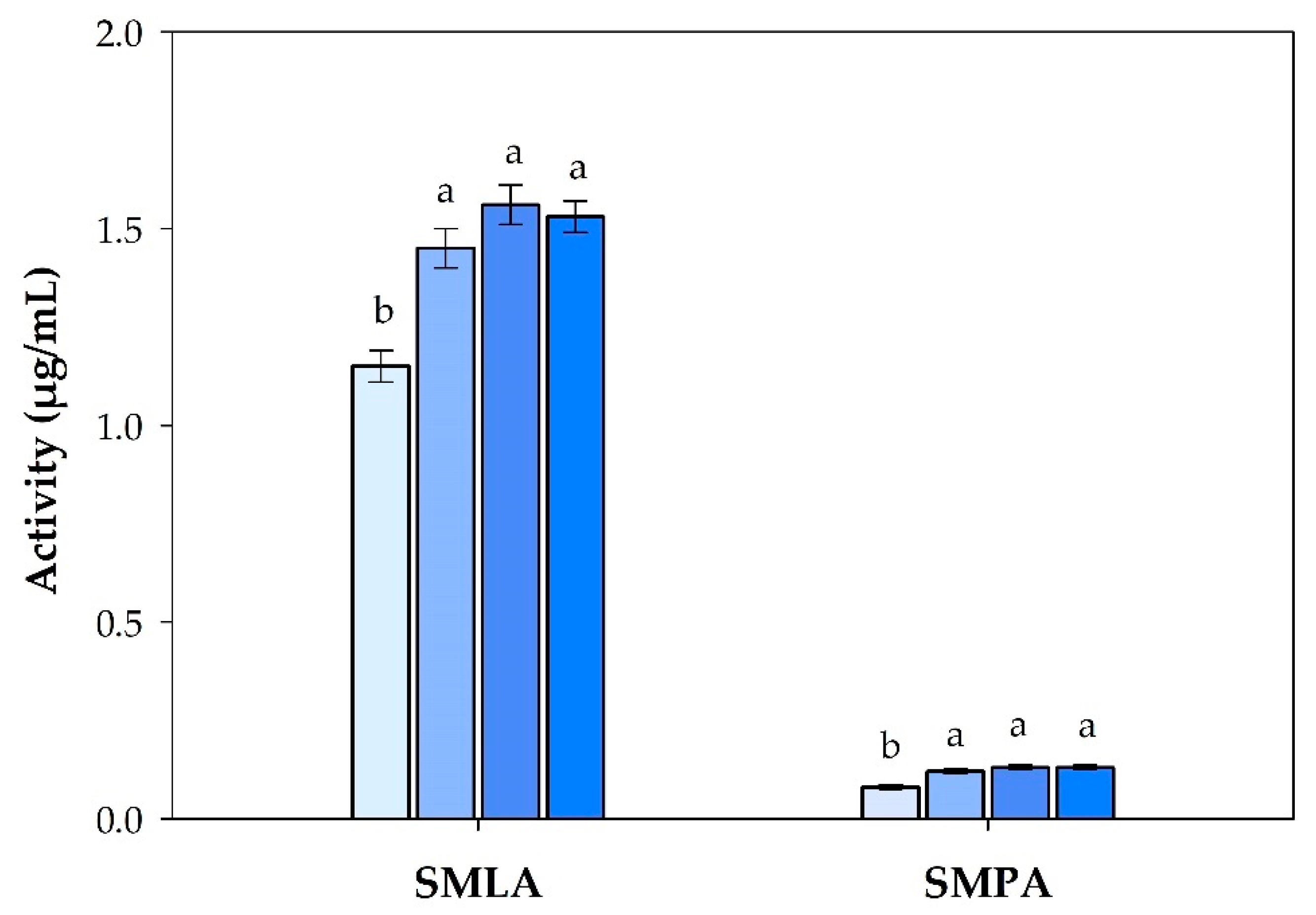
3.1. Mucosal Immune Response Analysis
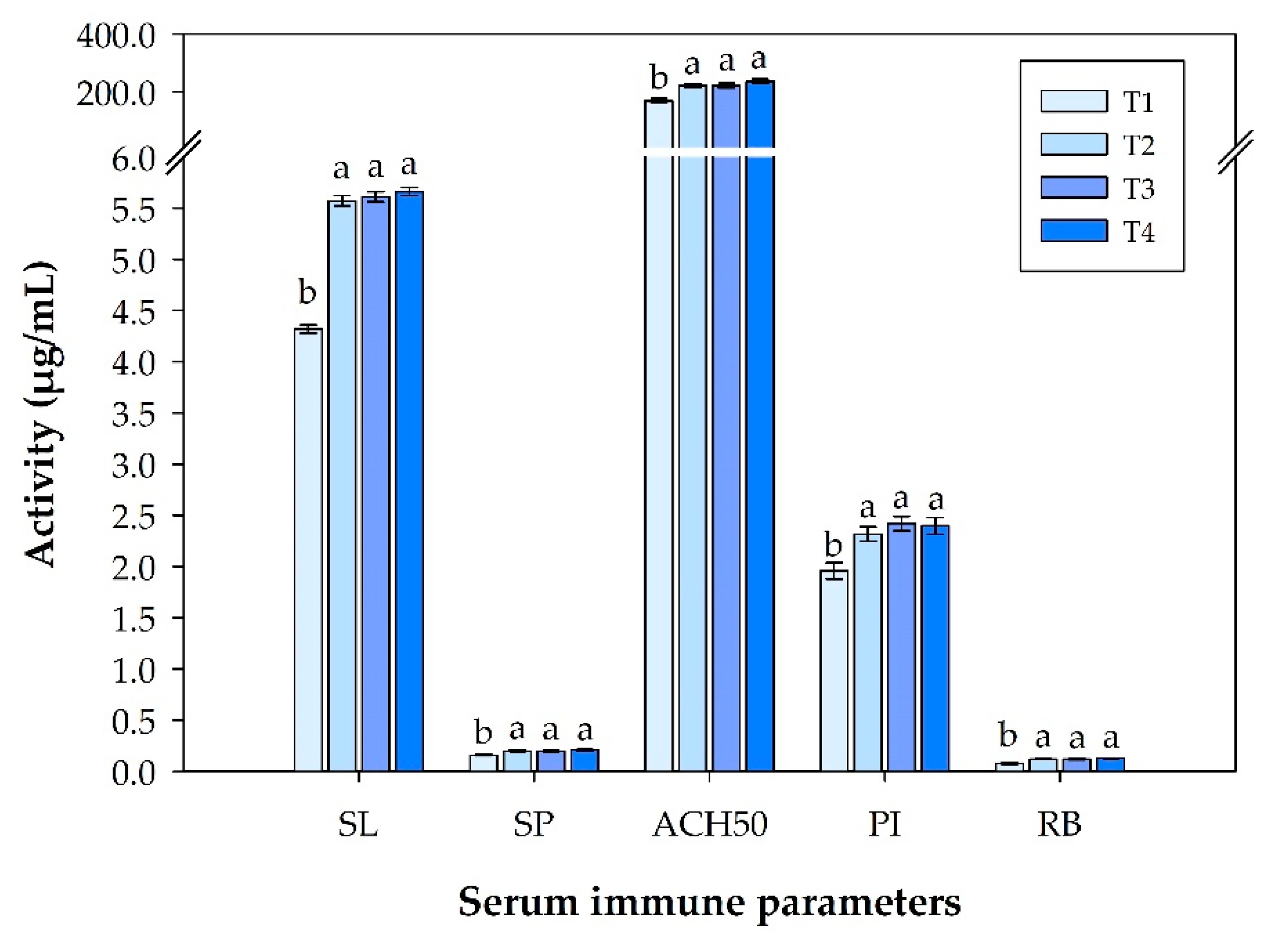
3.2. Immune Response Analysis in Serum
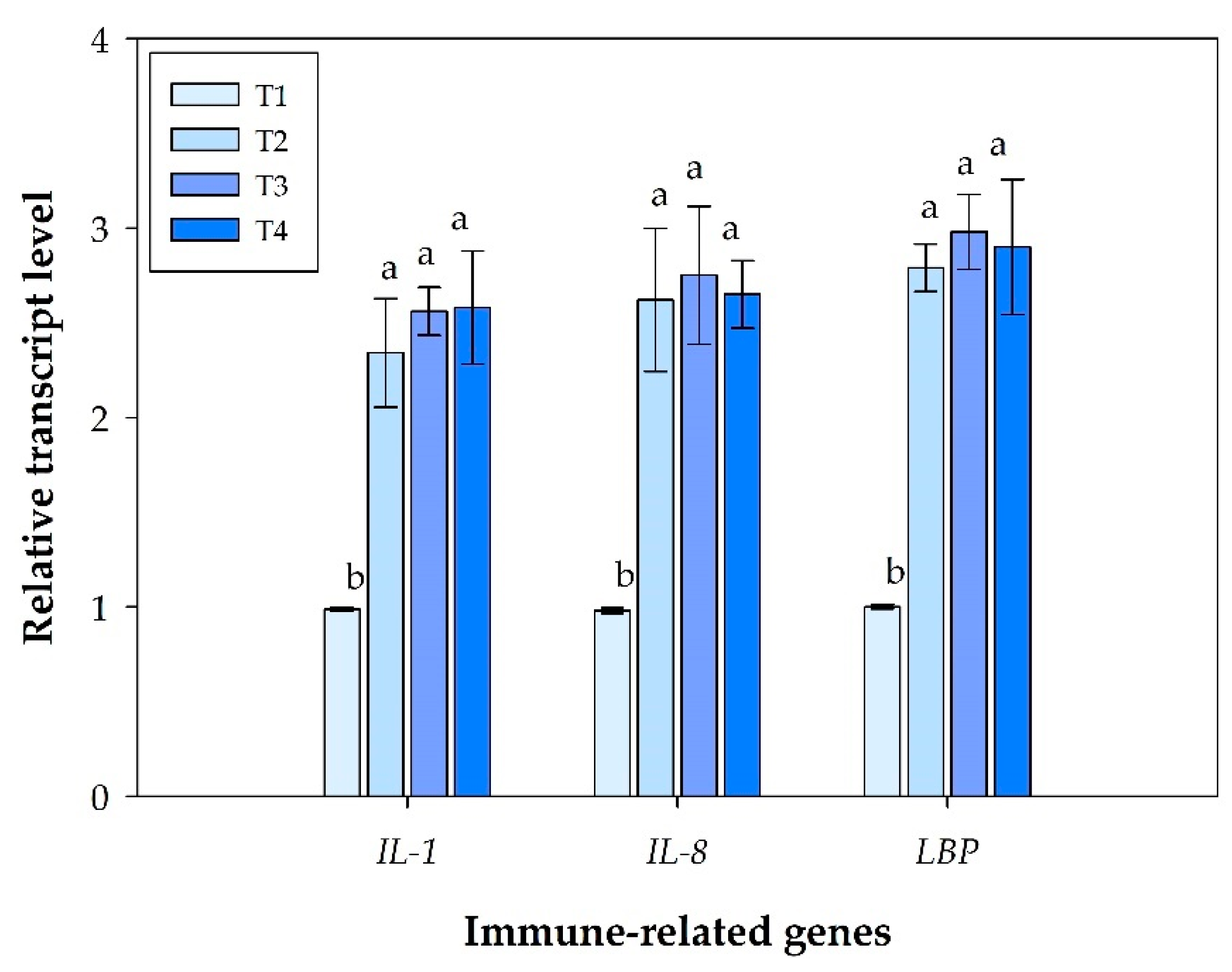
3.3. Immune Gene Expression Profiling
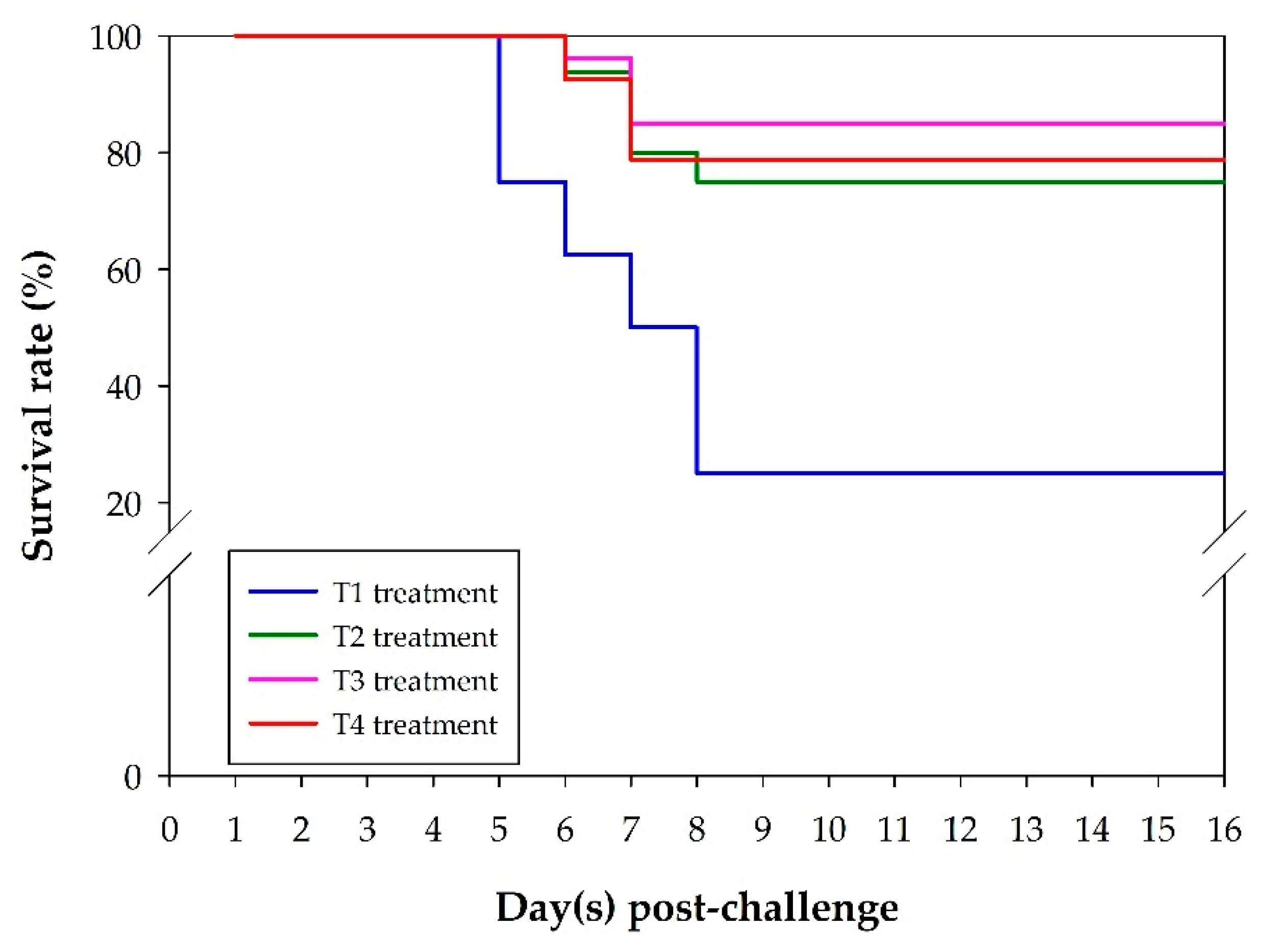
3.4. Fish Survival Rate after S. agalactiae Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shourbela, R.M.; Khatab, S.A.; Hassan, M.M.; Van Doan, H.; Dawood, M.A. The effect of stocking density and carbon sources on the oxidative status, and nonspecific immunity of Nile tilapia (Oreochromis niloticus) reared under biofloc conditions. Animals 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.-F.M. Tilapia Culture; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Subasinghe, R. World aquaculture 2015: A brief overview. In FAO Fisheries and Aquaculture Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Rico, A.; Oliveira, R.; McDonough, S.; Matser, A.; Khatikarn, J.; Satapornvanit, K.; Nogueira, A.J.; Soares, A.M.; Domingues, I.; Van den Brink, P.J. Use, fate and ecological risks of antibiotics applied in tilapia cage farming in Thailand. Environ. Pollut. 2014, 191, 8–16. [Google Scholar] [CrossRef]
- Sirimanapong, W.; Thompson, K.D.; Shinn, A.P.; Adams, A.; Withyachumnarnkul, B. Streptococcus agalactiae infection kills red tilapia with chronic Francisella noatunensis infection more rapidly than the fish without the infection. Fish Shellfish. Immunol. 2018, 81, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Wamala, S.P.; Mugimba, K.K.; Mutoloki, S.; Evensen, Ø.; Mdegela, R.; Byarugaba, D.K.; Sørum, H. Occurrence and antibiotic susceptibility of fish bacteria isolated from Oreochromis niloticus (Nile tilapia) and Clarias gariepinus (African catfish) in Uganda. Fish. Aquat. Sci. 2018, 21, 1–10. [Google Scholar] [CrossRef]
- Bøgwald, J.; Dalmo, R.A. Review on immersion vaccines for fish: An update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- Shahin, K.; Shinn, A.P.; Metselaar, M.; Ramirez-Paredes, J.G.; Monaghan, S.J.; Thompson, K.D.; Hoare, R.; Adams, A. Efficacy of an inactivated whole-cell injection vaccine for nile tilapia, Oreochromis niloticus (L.), against multiple isolates of Francisella noatunensis subsp. orientalis from diverse geographical regions. Fish Shellfish. Immunol. 2019, 89, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Suphoronski, S.; Chideroli, R.; Facimoto, C.; Mainardi, R.; Souza, F.; Lopera-Barrero, N.; Jesus, G.; Martins, M.; Di Santis, G.; De Oliveira, A. Effects of a phytogenic, alone and associated with potassium diformate, on tilapia growth, immunity, gut microbiome and resistance against francisellosis. Sci. Rep. 2019, 9, 6045. [Google Scholar] [CrossRef]
- Yun, S.; Giri, S.S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; Oh, W.T.; Chi, C. Enhanced bath immersion vaccination through microbubble treatment in the cyprinid loach. Fish Shellfish. Immunol. 2019, 91, 12–18. [Google Scholar] [CrossRef]
- Basri, L.; Nor, R.M.; Salleh, A.; Md. Yasin, I.S.; Saad, M.Z.; Abd. Rahaman, N.Y.; Barkham, T.; Amal, M.N.A. Co-infections of tilapia lake virus, Aeromonas hydrophila and Streptococcus agalactiae in farmed red hybrid tilapia. Animals 2020, 10, 2141. [Google Scholar] [CrossRef]
- Oh, W.T.; Jun, J.W.; Kim, H.J.; Giri, S.S.; Yun, S.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J. Characterization and pathological analysis of a virulent Edwardsiella anguillarum strain isolated from Nile tilapia (Oreochromis niloticus) in Korea. Front. Vet. Sci. 2020, 7, 14. [Google Scholar] [CrossRef]
- Rico, A.; Phu, T.M.; Satapornvanit, K.; Min, J.; Shahabuddin, A.; Henriksson, P.J.; Murray, F.J.; Little, D.C.; Dalsgaard, A.; Van den Brink, P.J. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 2013, 412, 231–243. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.R.; Ziouzenkova, O. Antibiotics in food chain: The consequences for antibiotic resistance. Antibiotics 2020, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108840. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef]
- Henriksson, P.J.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: A review from a systems perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current status of the use of antibiotics and the antimicrobial resistance in the Chilean salmon farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Qiang, J.; He, J.; Ma, X.Y.; Kpundeh, M.D.; Xu, P. Dietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia (GIFT). Fish Shellfish. Immunol. 2015, 44, 504–514. [Google Scholar] [CrossRef]
- Dávila, M.S.; Latimer, M.F.; Dixon, B. Enhancing immune function and fish health in aquaculture. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 38, pp. 123–161. [Google Scholar]
- Paray, B.A.; El-Basuini, M.F.; Alagawany, M.; Albeshr, M.F.; Farah, M.A.; Dawood, M.A. Yucca schidigera usage for healthy aquatic animals: Potential roles for sustainability. Animals 2021, 11, 93. [Google Scholar] [CrossRef]
- Brandi, P.; Conejero, L.; Cueto, F.J.; Martínez-Cano, S.; Dunphy, G.; Gómez, M.J.; Relaño, C.; Saz-Leal, P.; Enamorado, M.; Quintas, A. Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections. Cell Rep. 2022, 38, 110184. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of immune responses to vaccination by the microbiota: Implications and potential mechanisms. Nat. Rev. Immunol. 2022, 22, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, A.; Netea, M.G. Trained immunity-related vaccines: Innate immune memory and heterologous protection against infections. Trends Mol. Med. 2022, 28, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Montuori, E.; de Pascale, D.; Lauritano, C. Recent Discoveries on Marine Organism Immunomodulatory Activities. Mar. Drugs 2022, 20, 422. [Google Scholar] [CrossRef]
- Mo, X.-B.; Wang, J.; Guo, S.; Li, A.-X. Potential of naturally attenuated Streptococcus agalactiae as a live vaccine in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 518, 734774. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Samir, A.; Wasfy, M.; Elsayed, M. Efficacy of injectable and immersion polyvalent vaccine against streptococcal infections in broodstock and offspring of Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2019, 88, 293–300. [Google Scholar] [CrossRef]
- Pumchan, A.; Krobthong, S.; Roytrakul, S.; Sawatdichaikul, O.; Kondo, H.; Hirono, I.; Areechon, N.; Unajak, S. Novel chimeric multiepitope vaccine for streptococcosis disease in Nile tilapia (Oreochromis niloticus Linn.). Sci. Rep. 2020, 10, 603. [Google Scholar] [CrossRef]
- Wang, Q.; Fu, T.; Li, X.; Luo, Q.; Huang, J.; Sun, Y.; Wang, X. Cross-immunity in Nile tilapia vaccinated with Streptococcus agalactiae and Streptococcus iniae vaccines. Fish Shellfish. Immunol. 2020, 97, 382–389. [Google Scholar] [CrossRef]
- Dien, L.T.; Ngo, T.P.H.; Nguyen, T.V.; Kayansamruaj, P.; Salin, K.R.; Mohan, C.V.; Rodkhum, C.; Dong, H.T. Non-antibiotic approaches to combat motile Aeromonas infections in aquaculture: Current state of knowledge and future perspectives. Rev. Aquac. 2023, 15, 333–366. [Google Scholar] [CrossRef]
- Linh, N.V.; Dien, L.T.; Sangpo, P.; Senapin, S.; Thapinta, A.; Panphut, W.; St-Hilaire, S.; Rodkhum, C.; Dong, H.T. Pre-treatment of Nile tilapia (Oreochromis niloticus) with ozone nanobubbles improve efficacy of heat-killed Streptococcus agalactiae immersion vaccine. Fish Shellfish. Immunol. 2022, 123, 229–237. [Google Scholar] [CrossRef]
- Bedekar, M.K.; Kole, S. Fundamentals of fish vaccination. In Vaccine Design; Springer: New York, NY, USA, 2022; pp. 147–173. [Google Scholar]
- Dien, L.T.; Linh, N.V.; Mai, T.T.; Senapin, S.; St-Hilaire, S.; Rodkhum, C.; Dong, H.T. Impacts of oxygen and ozone nanobubbles on bacteriophage in aquaculture system. Aquaculture 2022, 551, 737894. [Google Scholar] [CrossRef]
- Novoa, B.; Pereiro, P. Editorial of Special Issue “The 2nd Edition: Vaccines for Aquaculture”. Vaccines 2022, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Vinay, T.; Jung, M.-H.; Patil, P.K.; Panigrahi, A.; Kallappa, G.S. Vaccines to Prevent Diseases in Aquaculture. Biotechnol. Adv. Aquac. Health Manag. 2022, 313, 313–323. [Google Scholar]
- Linh, N.V.; Panphut, W.; Thapinta, A.; Senapin, S.; St-Hilaire, S.; Rodkhum, C.; Dong, H.T. Ozone nanobubble modulates the innate defense system of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish. Immunol. 2021, 112, 64–73. [Google Scholar] [CrossRef]
- Thanh Dien, L.; Linh, N.V.; Sangpo, P.; Senapin, S.; St-Hilaire, S.; Rodkhum, C.; Dong, H.T. Ozone nanobubble treatments improve survivability of Nile tilapia (Oreochromis niloticus) challenged with a pathogenic multi-drug-resistant Aeromonas hydrophila. J. Fish Dis. 2021, 44, 1435–1447. [Google Scholar] [CrossRef]
- Thu Lan, N.G.; Salin, K.R.; Longyant, S.; Senapin, S.; Dong, H.T. Systemic and mucosal antibody response of freshwater cultured Asian seabass (Lates calcarifer) to monovalent and bivalent vaccines against Streptococcus agalactiae and Streptococcus iniae. Fish Shellfish. Immunol. 2021, 108, 7–13. [Google Scholar] [CrossRef]
- Attaya, A.; Veenstra, K.; Welsh, M.D.; Ahmed, M.; Torabi-Pour, N.; Saffie-Siebert, S.; Yoon, S.; Secombes, C.J. In vitro evaluation of novel (nanoparticle) oral delivery systems allow selection of gut immunomodulatory formulations. Fish Shellfish. Immunol. 2021, 113, 125–138. [Google Scholar] [CrossRef]
- Chung, S.; Secombes, C.J. Analysis of events occurring within teleost macrophages during the respiratory burst. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 89, 539–544. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; Ehab, E.-H.; Paolucci, M. Dietary inclusion of chestnut (Castanea sativa) polyphenols to Nile tilapia reared in biofloc technology: Impacts on growth, immunity, and disease resistance against Streptococcus agalactiae. Fish Shellfish. Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef]
- Parry Jr, R.M.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Cordero, H.; Cuesta, A.; Meseguer, J.; Esteban, M.Á. Changes in the levels of humoral immune activities after storage of gilthead seabream (Sparus aurata) skin mucus. Fish Shellfish. Immunol. 2016, 58, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kitao, T. The Opsonic Effect of Specific Immune Serum on the Phagocytic and Chemiluminescent Response in Rainbow Trout, Oncorhynchus mykiss Phagocytes. Fish Pathol. 1991, 26, 29–33. [Google Scholar] [CrossRef]
- Yano, T. Assays of hemolytic complement activity. Tech. Fish Immunol. 1992, 131–141. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Amend, D.F. Potency testing of fish vaccines. Fish Biol. Serodiagn. Vaccines 1981, 447–454. [Google Scholar]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Dhar, A.; Manna, S.; Thomas Allnutt, F. Viral vaccines for farmed finfish. Virus Dis. 2014, 25, 1e17. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Huang, P.; Weng, T.; Wen, Y.; Yang, H.; Liu, Y.; Xia, L. Induction of attenuated Nocardia seriolae and their use as live vaccine trials against fish nocardiosis. Fish Shellfish. Immunol. 2022, 131, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef] [PubMed]
- Hoare, R.; Leigh, W.; Limakom, T.; Wongwaradechkul, R.; Metselaar, M.; Shinn, A.P.; Ngo, T.P.H.; Thompson, K.D.; Adams, A. Oral vaccination of Nile tilapia (Oreochromis niloticus) against francisellosis elevates specific antibody titres in serum and mucus. Fish Shellfish. Immunol. 2021, 113, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Thangsunan, P.; Kitiyodom, S.; Srisapoome, P.; Pirarat, N.; Yata, T.; Thangsunan, P.; Boonrungsiman, S.; Bunnoy, A.; Rodkhum, C. Novel development of cationic surfactant-based mucoadhesive nanovaccine for direct immersion vaccination against Francisella noatunensis subsp. orientalis in red tilapia (Oreochromis sp.). Fish Shellfish. Immunol. 2022, 127, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A.; Vaseeharan, B.; Ramasamy, P.; Jeyachandran, S. Oral vaccination for sustainable disease prevention in aquaculture—An encapsulation approach. Aquac. Int. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Embregts, C.W.E.; Forlenza, M. Oral vaccination of fish: Lessons from humans and veterinary species. Dev. Comp. Immunol. 2016, 64, 118–137. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; Cain, K.D. Prospects and challenges of developing and commercializing immersion vaccines for aquaculture. Int. Biol. Rev. 2017, 1, 1–20. [Google Scholar]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 2597. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Teiba, I.I.; Shahin, S.A.; Mourad, M.M.; Zaki, M.A.; Labib, E.M.; Azra, M.N.; Sewilam, H.; El-Dakroury, M.; Dawood, M.A. Dietary Guduchi (Tinospora cordifolia) enhanced the growth performance, antioxidative capacity, immune response and ameliorated stress-related markers induced by hypoxia stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2022, 120, 337–344. [Google Scholar] [CrossRef]
- Monir, M.S.; Yusoff, S.b.M.; Mohamad, A.; Ngoo, M.S.b.M.H.; Ina-Salwany, M.Y. Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus× O. niloticus). BMC Vet. Res. 2020, 16, 226. [Google Scholar] [CrossRef]
- Sirimanapong, W.; Thompson, K.D.; Kledmanee, K.; Thaijongrak, P.; Collet, B.; Ooi, E.L.; Adams, A. Optimisation and standardisation of functional immune assays for striped catfish (Pangasianodon hypophthalmus) to compare their immune response to live and heat killed Aeromonas hydrophila as models of infection and vaccination. Fish Shellfish. Immunol. 2014, 40, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, H.; Soliman, H.; Saleh, M.; El-Matbouli, M. CD4: A vital player in the teleost fish immune system. Vet. Res. 2019, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Martins, M.L.; Jatobá, A.; Buglione Neto, C.C.; Vieira, F.N.; Pereira, G.V.; Jerônimo, G.T.; Seiffert, W.Q.; Mouriño, J.L.P. Hematological and immunological responses of Nile tilapia after polyvalent vaccine administration by different routes. Pesqui. Veterinária Bras. 2009, 29, 874–880. [Google Scholar] [CrossRef]
- Engelsma, M.Y.; Huising, M.O.; Van Muiswinkel, W.B.; Flik, G.; Kwang, J.; Savelkoul, H.F.; Verburg-van Kemenade, B.L. Neuroendocrine–immune interactions in fish: A role for interleukin-1. Vet. Immunol. Immunopathol. 2002, 87, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Jun, J.W.; Kang, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Han, S.J.; Kwon, J.; Oh, W.T. Immunostimulation by starch hydrogel-based oral vaccine using formalin-killed cells against edwardsiellosis in Japanese eel, Anguilla japonica. Vaccine 2020, 38, 3847–3853. [Google Scholar] [CrossRef]
- Weiss, J. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): Structure, function and regulation in host defence against Gram-negative bacteria. Biochem. Soc. Trans. 2003, 31, 785–790. [Google Scholar] [CrossRef]
- Watson, J.; Riblet, R. Genetic Control of Responses to Bacterial Lipopolysaccharides in Mice: I. Evidence for a Single Gene that Influences Mitogenic and Immunogenic Respones to Lipopolysaccharides. J. Exp. Med. 1974, 140, 1147–1161. [Google Scholar] [CrossRef]
- Baba, T.; Imamura, J.; Izawa, K.; Ikeda, K. Immune protection in carp, Cyprinus carpio L., after immunization with Aeromonas hydrophila crude lipopolysaccharide. J. Fish Dis. 1988, 11, 237–244. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Y.; Li, Q.; Ke, X.; Liu, Z.; Lu, M.; Shi, C. An effective live attenuated vaccine against Streptococcus agalactiae infection in farmed Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2020, 98, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Beck, B.R.; Lee, S.M.; Jeon, J.; Lee, D.W.; Lee, J.I.; Song, S.K. Pellet feed adsorbed with the recombinant Lactococcus lactis BFE920 expressing SiMA antigen induced strong recall vaccine effects against Streptococcus iniae infection in olive flounder (Paralichthys olivaceus). Fish Shellfish. Immunol. 2016, 55, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Kole, S.; Qadiri, S.S.N.; Shin, S.-M.; Kim, W.-S.; Lee, J.; Jung, S.-J. Nanoencapsulation of inactivated-viral vaccine using chitosan nanoparticles: Evaluation of its protective efficacy and immune modulatory effects in olive flounder (Paralichthys olivaceus) against viral haemorrhagic septicaemia virus (VHSV) infection. Fish Shellfish. Immunol. 2019, 91, 136–147. [Google Scholar] [CrossRef] [PubMed]
| Constituents | Basal Diet |
|---|---|
| Soybean meal | 390 |
| Corn meal | 200 |
| Fish meal | 150 |
| Rice bran | 150 |
| Wheat flour | 70 |
| Cellulose | 20 |
| Premix | 10 |
| Vitamin C 98% | 5 |
| Soybean oil | 5 |
| Approximate component of dietary treatment (g kg−1 dry matter basis) | |
| Gross energy (Cal/g) | 3892 |
| Dry matter | 991.83 |
| Crude protein | 322.28 |
| Ash | 84.90 |
| Fiber | 43.47 |
| Crude lipid | 38.56 |
| Primers | Oligo Sequence (5′-3′) | Genes | Tm (°C) | Size (bp) |
|---|---|---|---|---|
| 18S rRNA | F: GTGCATGGCCGTTCTTAGTT R: CTCAATCTCGTGTGGCTGAA | 18S RNA | 60 | 150 |
| IL-1 | F: GTCTGTCAAGGATAAGCGCTG R: ACTCTGGAGCTGGATGTTGA | IL-1 | 59 | 200 |
| IL-8 | F: CTGTGAAGGCATGGGTGTG R: GATCACTTTCTTCACCCAGGG | IL-8 | 59 | 196 |
| LBP | F: ACCAGAAACTGCGAGAAGGA R: GATTGGTGGTCGGAGGTTTG | LBP | 59 | 200 |
| Treatment Groups | Statistical Analysis | ||
|---|---|---|---|
| T1 | T2 | T3 | |
| T2 | 0.000 * | ||
| T3 | 0.000 * | 0.120 ns | |
| T4 | 0.000 * | 0.624 ns | 0.290 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linh, N.V.; Dien, L.T.; Dong, H.T.; Khongdee, N.; Hoseinifar, S.H.; Musthafa, M.S.; Dawood, M.A.O.; Van Doan, H. Efficacy of Different Routes of Formalin-Killed Vaccine Administration on Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Challenged with Streptococcus agalactiae. Fishes 2022, 7, 398. https://doi.org/10.3390/fishes7060398
Linh NV, Dien LT, Dong HT, Khongdee N, Hoseinifar SH, Musthafa MS, Dawood MAO, Van Doan H. Efficacy of Different Routes of Formalin-Killed Vaccine Administration on Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Challenged with Streptococcus agalactiae. Fishes. 2022; 7(6):398. https://doi.org/10.3390/fishes7060398
Chicago/Turabian StyleLinh, Nguyen Vu, Le Thanh Dien, Ha Thanh Dong, Nuttapon Khongdee, Seyed Hossein Hoseinifar, Mohamed Saiyad Musthafa, Mahmoud A. O. Dawood, and Hien Van Doan. 2022. "Efficacy of Different Routes of Formalin-Killed Vaccine Administration on Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Challenged with Streptococcus agalactiae" Fishes 7, no. 6: 398. https://doi.org/10.3390/fishes7060398
APA StyleLinh, N. V., Dien, L. T., Dong, H. T., Khongdee, N., Hoseinifar, S. H., Musthafa, M. S., Dawood, M. A. O., & Van Doan, H. (2022). Efficacy of Different Routes of Formalin-Killed Vaccine Administration on Immunity and Disease Resistance of Nile Tilapia (Oreochromis niloticus) Challenged with Streptococcus agalactiae. Fishes, 7(6), 398. https://doi.org/10.3390/fishes7060398








