1. Introduction
The use of insects has been of great interest lately due to their high nutritional value [
1,
2,
3,
4,
5,
6,
7] and relatively low rearing cost. European Union regulation 2017/893 allows the use of seven insect species for the production of aquafeed:
Hermetia illucens (L.),
Musca domestica L.,
Tenebrio molitor L.,
Alphitobius diaperinus Panzer,
Acheta domesticus (L.),
Gryllodes sigillatus (Walker), and
Gryllus assimilis (F.). Several studies have focused on the nutrition of various fish, such as tilapia [
8,
9,
10,
11,
12,
13], carp [
3], trout [
14,
15,
16], and sea bream [
17,
18], with feeds partially produced by various insect species. Replacement rates of 19.5–50% fishmeal with insect meal seem not to affect fish growth and basic characteristics [
15,
17,
19].
In aquaponics, the most important source of nutrients is the fish feed. Its protein composition, the amount, and the feeding frequency affect the digestibility of feed [
20] and therefore the rate of ammonia production and nitrate ions [
21]. Although fishmeal was, and in many cases remains, the primary protein source for the nutrition of farmed fish, the stagnant global availability of fishmeal necessitates the evaluation of alternative sources of protein. Vegetable proteins are associated with reduced taste [
22] and several anti-nutritional agents [
23,
24,
25,
26] that inhibit digestion of food by fish. Therefore, the use of insects as an alternative protein source in fish feed in an aquaponic system could be a sustainable and economical proposal; however, there is still inadequate information regarding this topic.
The superworm
Zophobas morio (F.) (Coleoptera: Tenebrionidae) is an insect species that is produced in large quantities in China and is used in the production of terrestrial animal and fish feeds [
27,
28,
29]. Its larvae have high nutritional value and are rich in protein (431.3–516.2 g/kg dry matter) and fatty acids (328.0–435.4 g/kg dry matter) [
30,
31,
32,
33]. Replacement of up to 50% of fishmeal with
Z. morio larvae resulted in satisfactory growth for several fish species [
11,
34,
35]. Moreover, replacement rates as high as 75% did not inhibit growth and showed a lower digestibility factor (ADC) for proteins and lipids than fishmeal [
11]. In addition, it was recently reported that the inclusion of
Z. morio in fish feeds may positively influence several innate immunity parameters [
36].
Sea bass,
Dicentrarchus labrax (L.), is an economically important fish in Europe, with especially high demand in the Mediterranean region [
37]. It is a euryhaline fish that is ideal for aquaponics, with several studies showing that it can grow in brackish water and freshwater [
38,
39,
40]. Despite the increased importance of this fish species, data that focus on the utilization of insects in sea bass nutrition are rather scarce, especially in the case of
Z. morio. Fish feed in aquaponics needs to fulfil the nutritional requirements of both fish and plants. For sustainability reasons, less dependence on fishmeal and fish oil ingredients must be achieved in fish feeds by substitution with novel high-energy and low-carbon footprint ingredients. Insects have high feed conversion efficiencies. The major environmental advantage of insect farming is that greenhouse gas emissions are lower because less land and water are required. This is the first study that evaluates the superworm
Z. morio as a fishmeal replacement in sea bass aquafeeds for aquaponics. Thus, the aim of this study was to investigate the effect of replacing fishmeal (10 and 20% fishmeal replacement) with
Z. morio insect meal on the growth performance, histological status, and water quality of sea bass reared in a freshwater aquaponic system. In addition, this study elucidates whether the effect of replacing fishmeal with insect meal has an impact on the growth performance of lettuce plants (
Lactuca sativa L.).
2. Materials and Methods
2.1. Aquaponic System and Experimental Set-Up
Three autonomous aquaponic systems were constructed. Each system consisted of three fish tanks, a hydroponic cultivation tank paved with clay pebble (8–16 mm) substrate, and a biological sump filter. The water volume of each of the fish tanks was 100 L, while the water volume of the hydroponic cultivation tank and the biological sump filter were 26 and 184 L, respectively. The total water volume of each aquaponic system was 500 L. More details about the dimensions and the function of the specific aquaponic systems were reported by Stathopoulou et al. [
40].
At the beginning of the experiment, a 24 h period was used in order to allow the escape of any chlorine trace. The biological filter was set up as previously described by Stathopoulou et al. [
40]. Temperature, pH, and oxygen concentration were measured with multimeter sensors (HQ40d, Hach, Loveland, CO, USA), electrical conductivity was measured using a multimeter (CM35, Crison, Barcelona, Spain), and salinity was measured using an optical refractometer (ATC).
2.2. Experimental Diets
A defatted
Zophobas meal was used in the study.
Z. morio larvae were raised at the Laboratory of Entomology and Agricultural Zoology at the University of Thessaly, dried at 40 °C for 12 h, and milled to obtain a
Zophobas meal. As insect’s lipid quality is not suitable for fish nutrition [
2,
4], the
Zophobas meal was defatted using petroleum ether at a ratio of 5:1 (
v/
w) under stirring and heating at 40 °C for 2 h. The defatted
Zophobas meal contained 5.4% moisture, 69.4% crude protein, and 3.8% crude lipid. A fishmeal with 65.5% crude protein and 10.1% crude lipid content was used as the main protein source for the experimental diets.
Determination of the moisture of the ingredients and experimental feed content was performed through thermal drying to constant weight in an oven at 105 °C for 24 h. Kjeldahl analyses (N × 6.25; behr Labor-Technik, Düsseldorf, Germany) were used for determination of crude protein content, while crude fat was determined by exhaustive Soxhlet extraction with the use of petroleum ether (40–60 °C, BP) and a Soxtherm Multistat/SX PC (Sox-416 Macro, Gerhard, Germany). Ash content was determined through dry ashing in porcelain crucibles in a muffle furnace (Nabertherm L9/12/C6, Lilienthal, Germany) at 600 °C for 5 h. Finally, gross energy was determined adiabatically through the use of an IKA oxygen bomb calorimeter (C5000, IKA Werke, Staufen, Germany).
Three isonitrogenous (57% of dry matter, DM) and isoenergetic diets (22.3 MJ/Kg of DM) (
Table 1) were formulated wherein the fishmeal protein of the control diet (FM) was replaced by the defatted
Zophobas meal at 10% (ZM10) and 20% (ZM20), respectively [
41]. Lysine and methionine were added to ZM10 and ZM20 diets to counterbalance the lower levels of these amino acids compared to the control diet [
2,
42]. All diets were formulated to satisfy the known essential amino acid requirements of the species [
43], with these requirements based on the amino acid profiles of each feed ingredient according to those provided by the suppliers and those found in the feedipedia.org database. Corn gluten meal and sunflower meal were used as plant protein sources, while wheat meal was used as an energy source and filler ingredient for the protein replacements. Fish oil was used to satisfy the known n-3 essential fatty acid requirements of sea bass, while soybean oil was used as a supplementary lipid source. All diets had constant inclusion levels of vitamin and mineral premix, monocalcium phosphate, vitamin C, and vitamin E.
All dietary ingredients were ground in a grain feed mill (KoMo Fidibus, PGS, Leverkusen, Germany) and then mixed in a mixer (Bosch Maxxi MUM MUMXL20G, Bosch, Gerlingen, Germany) with the addition of fish oil and boiling water in order to produce a homogenous stiff dough. Feeds were pelletized by a pelleted machine (California Pellet Mill CL-2, IRMECO GmbH, Zaandam, The Netherlands) and 1.5 mm diameter pellets were produced. Pellets were dried at room temperature (20–22 °C) for 24 h with forced air under a fume hood and stored in air-sealed bags at 4 °C until used.
2.3. Experimental Design, Fish Rearing, and Plant Growth Conditions
Two hundred juvenile sea bass individuals weighing 1–2 g were transported to the Department of Ichthyology and Aquatic Environments (School of Agricultural Sciences, University of Thessaly) from a local commercial fish hatchery (SELONTA S.A., Peania, Greece). The fish were transported in special transport bags, which were oxygenated and filled with water of 25‰ salinity. Upon arrival at the laboratory, fish were placed for 3 h in fish tanks filled with water of the same salinity as the transport water. Thereafter, fish were allowed to gradually adapt to freshwater over a period of 2 months, as described previously by Stathopoulou et al. [
40].
After successful adaptation to freshwater, 10 fish were placed in each fish tank of the aquaponics systems and left for 10 days to acclimatize. The number of fish was chosen according to the Hirayama equation [
44]. The equilibrium between water pollution caused by fish feeding and excretion and the purification of water was taken into account. At the end of the 10 day period, the weight and total length of each fish was measured. Thereafter, fish were placed in the fish tanks in such a way that there were no significant differences in initial weights and lengths among the aquaponic fish tanks.
At the end of the acclimation period, a total of 90 juvenile sea bass individuals with an average body weight of 21.55 ± 0.28 g and an average body length of 12.97 ± 0.06 cm, were placed in the aquaponic fish tanks (9 fish tanks in total, 10 individuals/tank). All experimental procedures were conducted according to the guidelines of EU Directive 2010/63/EU regarding the protection of animals used for scientific purposes and applied by FELASA accredited scientists (functions A–D). The experimental protocol was approved by the Ethics Committee of the Region of Thessaly, Veterinary Directorate, Department of Animal Protection-Medicines-Veterinary Applications (n. 18403/05-09-2019). The experiment was conducted at the registered experimental facility (EL-43BIO/exp-01) of the Laboratory of Aquaculture, Department of Ichthyology and Aquatic Environments, University of Thessaly.
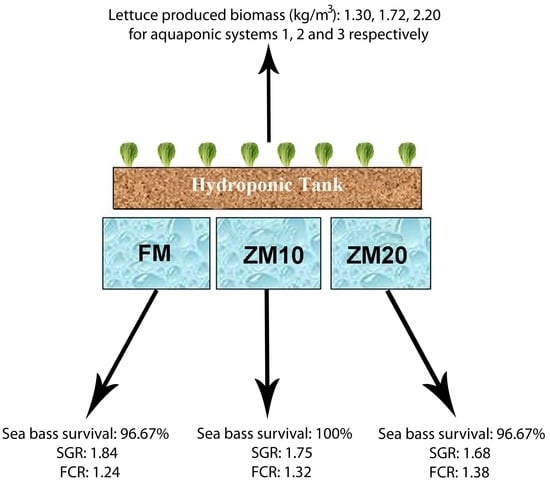
Fish were fed 5% of their body weight daily, and feeding rate was adjusted to fish weight every 15 days. Feed was distributed in four meals throughout the day (10:00 a.m.–16:00 p.m.–22:00 p.m.–04:00 a.m.) over 45 days. The time period of 45 days was chosen for the best growth of the lettuce plants [
45]. Each aquaponic system was represented by all three diets (
Figure 1). Feeding before 16:00 h was performed by hand, and the other meals were provided by automatic feeders. Fish tanks were cleaned through siphoning every morning (once per day) before the first feeding in order to remove uneaten food and feces. Daily feed consumption per fish tank was calculated by the difference between the provided feed and the amount of collected uneaten feed (corrected for leaching losses) [
46]. At the end of the experiment, the fish were anaesthetized with the use of Tricaine methanesulfonate (MS 222), and their final fish body weights and total lengths were measured.
Lettuce plants (var. Romana) were grown from seeds in a greenhouse until the 6 true-leaf stage. Five days before their transfer to the aquaponics systems, the plants were fertilized with 0.022 g Fe (Fe-DTPA), 0.12 mL Ca (foliar application), and 0.54 g K (KOH) [
40]. Twenty-four plants with similar height and numbers of leaves were chosen (average initial height of 8.90 ± 0.10 cm and an average number of leaves of 5.75 ± 0.12). In each of the three aquaponic systems, 8 lettuce plants were placed 20 cm apart [
40]. Plant positions were carefully chosen in order to ensure the homogeneity of the light environment, which reached 400–500 mmol m
–2 s
−1 of photosynthetically active radiation (PAR) [
40]. Since the aquaponics establishment was indoors, only artificial light was used, which was provided by 400 W HPS lamps (SYLVANIA, 230 V HID High Pressure Sodium) that were placed 65 cm above each hydroponic tank. A photoperiod of 10 h light and 14 h dark was constantly kept and controlled by a timer (Legrand, 3018 W44). No extra Ca, K, or Fe was added to the aquaponic systems. During the final harvest, the aerial tissues were cut and separated into leaves and stems. They were immediately weighed to measure fresh weight, and the number of leaves and stem height were then assessed. Separated aerial tissues and roots were stored at 70 °C until constant weight was reached and their dry biomass was then determined. Temperature was kept constant at 20 °C for each aquarium.
2.4. Water Quality Indicators
In fish tanks, the water temperature (°C) and pH were measured daily, while oxygen concentration (mg/L), electrical conductivity (mS/cm), and salinity (ppt) were measured every three days. Temperature, pH, and oxygen concentration were measured with multimeter sensors (HQ40d, Hach, Loveland, CO, USA), electrical conductivity was measured using a multimeter (CM35, Crison, Barcelona, Spain), and salinity was measured using an optical refractometer (ATC).
Nitrate (NO3−), phosphate (PO43−), un-ionized ammonia (NH3), and ammonium (NH4+) ions were monitored once a week (before the first daily fish feeding) by taking water samples at the water inlet point (GBin) and exit point (GBout) of the hydroponic tank. Measurements were performed using a Hach DR3900 model photometer with special pre-weighted reagents.
The filter’s functional characteristics were calculated by the following equations that Endut et al. [
47] and Huguenin and Colt [
48] previously described:
Hydraulic loading ratio, HLR (m/day) = flow rate (Q)/total surface area of the trough;
Hydraulic retention time, HRT (min) = (surface area water × depth × porosity of gravel trough/flow rate);
Specific surface area, SSA (m2/m3) = surface area of filter media/volume of the filter media;
Volume of filter media, Vmedia (m3) = surface area of the filter media/SSA;
Recycled ratio, r = volume of recycled water/volume of the system.
The production rate of ammonia nitrogen (PTAN) was calculated according to the below equation, which was previously described by Dediu et al. [
49].
where C
out and C
i represent the outlet and inlet ammonia concentration (mg/L), respectively, W represents mean fish body weight in the tank (g), and Q represents flow rate (L/h).
2.5. Fish and Plant Growth Performance Indicators
Fish growth performance was determined at the end of the 45-day period by the following equations:
Win and Wfin are the initial and final weight of the fish, respectively, and Δt is the duration of the experiment in days.
The growth performance of plants was assessed by the following measured or calculated parameters after the final harvest:
Number of leaves;
Leaf fresh weight (gr) = total fresh weight of leaves/number of leaves;
Stem height (cm);
Total fresh aerial biomass (gr) = total fresh weight of leaves + stem fresh weight;
Total dry aerial biomass (gr) = total dry weight of leaves + stem dry weight;
Root dry biomass (gr);
Total produced biomass (kg/m2) = total fresh weight of aerial part/cultivated area.
2.6. Fish Histology
Fish were euthanized according to EU Directive 2010/63/EU and FELASA guidelines. An overdose of Tricaine methanesulfonate (MS 222, 300+ mg/L) was used for this purpose. At the end of the 45-day period, 5 fish per tank were removed for histopathological examination of their liver and midgut, as previously described by Vlahos et al. [
46]. Tissue samples were fixed for 24 h in Davidson fixative. Dehydration in a graded series of ethanol, immersion in xylol, and embedding in paraffin followed. Sections of 4–7 μm in thickness were taken and stained with Hematoxylin–Eosin. Examination for histopathological lesions was performed under a microscope (Axiostar plus Carl Zeiss Light Microscopy, Carl Zeiss Ltd., Gottingen, Germany). A semi-quantitative grading system was used to quantify the histopathological lesions [
50]. Severity grading used the following system: Grade 0 (not remarkable), Grade 1 (minimal), Grade 2 (mild), Grade 3 (moderate), and Grade 4 (severe).
2.7. Statistical Analysis
Values are presented as means ± standard error of the mean (S.E.M.). Kolmogorov–Smirnov and Levene tests were used to check data for normality and homogeneity, respectively. For fish and plant growth performance, data were assessed by ANOVA, followed by Tukey’s post-hoc test. Independent t-tests for water quality at the inlet and outlet points of the grow beds of the three aquaponic systems were considered statistically significant at p < 0.05. The p value for all analyses was set at 0.05. Statistical analyses were carried out using the IBM SPSS Statistics V22 software package.
4. Discussion
In the present study, an experimental aquaponic system for a Mediterranean fish species (sea bass) and a vegetable (lettuce) was studied for a duration of 45 days, with fishmeal replaced by
Z. morio meal. Although several studies have used sea bass in aquaponic systems [
40,
51,
52,
53], no tests have been performed with
Z. morio as an alternative fishmeal replacement. In a successful aquaponic system, less than 5% of the water is lost daily due to evaporation or daily functioning and has to be renewed [
54,
55]. Successful co-culture of fish and plants is related to the used diet and nutrient availability in the water.
The present study showed that sea bass can adapt very well to a freshwater aquaponics system given that no mortality was recorded throughout the two-month adaptation period. Similar results for comparable aquaponic systems were reported by Marino et al. [
56] and Stathopoulou et al. [
40], which are in agreement with the current results. Nevertheless, Dendrinos and Thorpe [
57] and Cataudella et al. [
58] reported high mortality levels when sea basses were transferred directly from seawater to freshwater, which could be mainly attributed to the absence of an actual adaptation period.
According to Lanari et al. [
59], seawater-cultured sea basses can achieve higher weight gain at temperatures between 22 and 28 °C, while in brackish water, maximum weight gain can be achieved at temperatures between 19 and 20 °C [
60]. In the present study, submersible aquarium heaters were used in order to keep water temperature constant at 20 °C, thus meeting the temperature requirements for both sea bass and lettuce.
Biswas et al. [
61] and Güroy et al. [
62] reported that dissolved oxygen (DO) levels of 7–8 mg/L can ensure adequate ventilation for sea bass respiration. In the present study, DO levels were kept between 7.27 and 7.57 mg/L to ensure fish respiration, plant root strengthening, the intake of nutrients, and the nitration process [
63,
64].
Management of pH is one of the most important parameters in an aquaponic system, as fish, bacteria, and plants require different pH values. Optimum nutrient uptake for plants is achieved in the 5.5 to 6.5 pH range, while fish require a pH of 6.5–8.5 and bacteria require a pH of 7.0–8.0 [
65]. In an aquaponic system, the optimal pH range is 6.5–7.0, as pH > 7.0 can reduce the solubility of phosphorus and micronutrients [
66]. In the present study, the mean pH values ranged between 7.14 and 7.19 for the three systems tested here, which is very close to the optimum pH range.
Electrical conductivity (EC) tolerance for hydroponically cultured lettuce ranges between 1.4 and 2.0 mS/cm [
67]. In aquaponics, an EC range of 0.3–1.1 mS/cm was previously recorded [
68,
69]. Our EC mean value was 1.59 mS/cm for all three systems. This value meets the requirements for hydroponic culture but is slightly higher than the values that Graber and Junge [
68] and Pantanella et al. [
69] suggest.
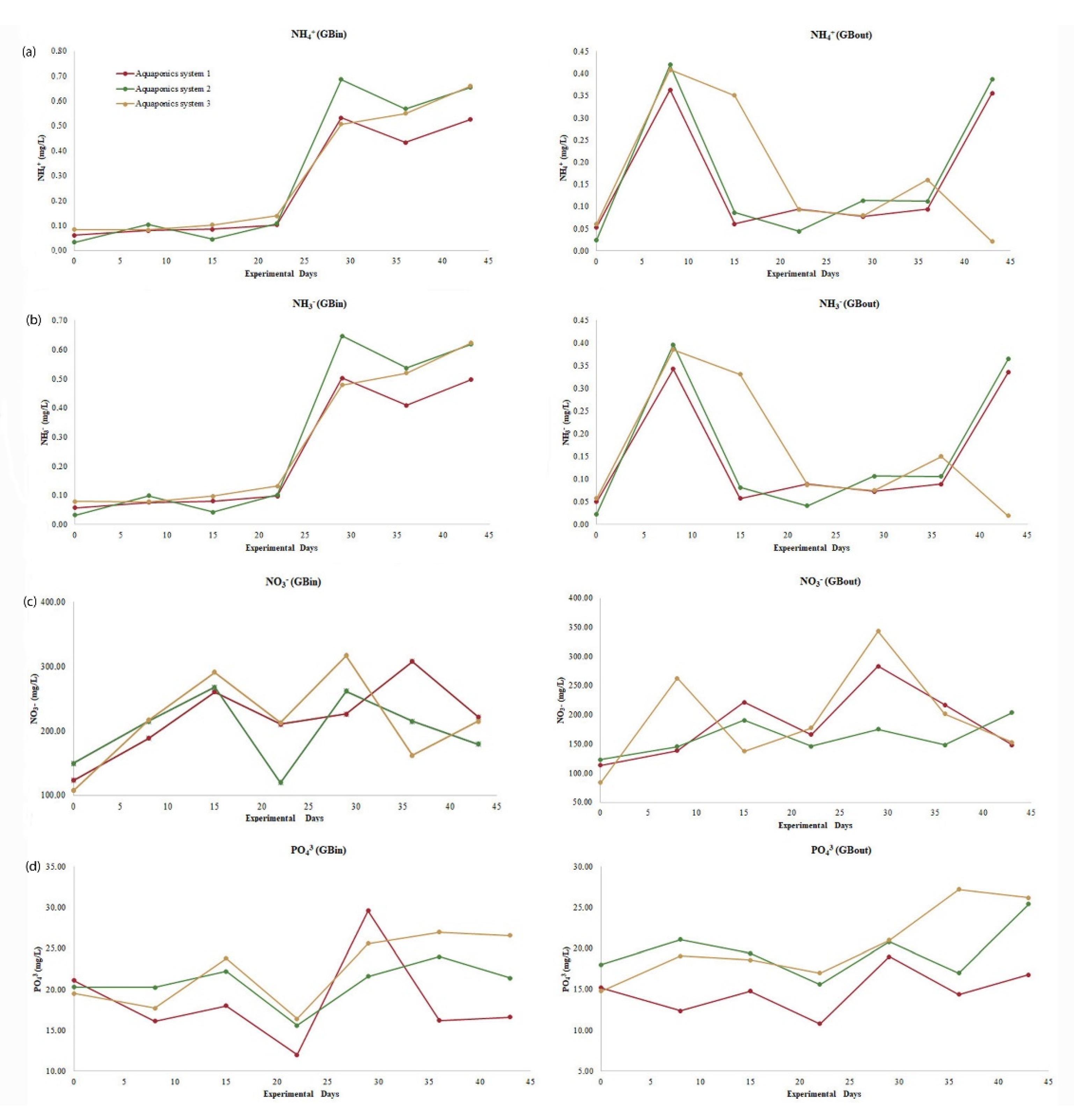
At the exit points (GB
out) of the cultivation tanks, lower concentrations of NH
3, NH
4+, NO
3−, and PO
43− were recorded than at the inlet points (GB
in). That fact indicates that lettuce plants absorbed the required nutrients for their growth through the water. Low water temperatures increase the absorbance of ammonium from plants and reduce the absorbance of the other nitrogen forms [
70]. In aquaponics systems, lettuce plants can absorb higher amounts of NH
3 and NH
4+ than NO
3− [
71]. Generally, when nitrogen concentrations are low, the preferred absorbed nitrogen source is ammonium, while when nitrogen concentrations are high, the preferred absorbed nitrogen source is nitrate [
72]. Aquaponic systems show ΝH
4+ concentrations between 0.47 and 2.8 mg/L [
49,
73,
74,
75], while NO
3− concentrations range between 10 and 250 mg/L [
76,
77,
78,
79,
80,
81] and PO
43− concentrations range between 3 and 52 mg/L [
73,
75,
82,
83]. In recirculating aquaculture systems (RAS), ΝH
3− concentrations of 1.86–2.79 mg/L can be toxic for fish [
84,
85]. In the present study, all three systems did not show statistical differences for ΝH
4+, ΝH
3, and NO
3− concentrations at the inlet or exit of the cultivation tanks (
Table 3). Both ΝH
4+ and ΝH
3 concentrations increased until the 29th day of the experiment, as the increased weight of fish was accompanied by an increased amount of food. The gradual rise in nitrate levels proved the efficiency of the filter in terms of its ability to oxidize produced ammonia. During the experiment, water supply (Q) was adjusted to 6.27 L/min and filtering speed (V) to 1.79 cm/min, ensuring successful nitration and maximum filter efficiency [
86,
87]. Moreover, the ΝH
4+ and ΝH
3 concentrations that we recorded here are within safe limits, while NO
3− concentration was between 126.6 and 272.7 mg/L and thus ensured adequate plant feeding. PO
43− concentration at the exit point of the hydroponic cultivation tank in the first system was lower than the other two systems (
Table 3) but still within the accepted limits (3–52 mg/L) [
73,
75,
82,
83].
Apparently, water quality is a critical parameter in aquaponic systems [
87], as only 30–40% of the total amount of nitrogen and phosphorus in fish feed is used by the fish for their growth, and the remaining 60–70% is excreted through the gills, faces, and urine [
88,
89,
90,
91]. In an aquaponic system, the hydraulic loading rate (HLR), the retention time of the water in the filter bed (HRT), the water flow rate (Q), and the water recirculation rate (r) influence ammonia removal, daily nutrient removal efficiency, and contact time between the nutrients and microbial communities in the plants [
47,
56]. In the present study, Q was set to 7.26–7.29 m
3/d, HLR ranged from 0.95 to 0.96 cm/d, and HRT ranged from 7.46 to 7.49 min. In aquaponics systems, HLR values of 1.28–2.56 m/d have been reported [
46,
47,
92]. According to Gichana et al. [
93], high HRT values may affect nutrient cycling in hydroponic tanks. HRT influences the filter’s carrying capacity and its ammonia removal efficiency [
46,
94,
95]. According to Timmons and Ebeling [
96], in large tanks (>1000 L), HRT tends to be around 45 min, while in small tanks, HRT tends to be lower than 10 min. Vlahos et al. [
46] reported an HRT of 9.7 min in a 135 L water volume. In the present study, with a 500 L total water volume, HRT values were in accordance with the values reported by Timmons and Ebeling [
96] and Vlahos et al. [
46].
In the present study, feeding was distributed across four meals throughout the day, as sea bass have very adjustable eating habits [
97,
98]. Sea bass prefer to be fed in the morning during spring and summer but prefer evening feeding in winter, as shown by a higher SGR and a lower FCR in contrast to morning feeding [
99,
100]. In aquaponics and RAS systems, increasing feeding frequency during the day promotes the most efficient utilization of nutrients in fish, increases SGR and WG, decreases FCR, and contributes to better water quality [
40,
61,
101,
102]. Fish were fed 5% of their body weight daily, as this rate met the nutritional requirements of both fish and plants in a recent study [
40]. Fishmeal substitution by
Z. morio larvae meal did not affect fish survival. This was in accordance with other studies using
H. illucens larvae (up to 50%) and
T. molitor larvae (up to 36%) as fishmeal substitutions for sea bass feeding [
19,
103,
104]. These results denote that dietary protein sources do not exert an effect on sea bass survival in aquaponic systems, and also suggest that
Z. morio meal is a suitable feedstuff for use in the aquaponic diets of sea bass.
As far as fish growth performance is concerned, the use of
Z. morio meal in diets did not affect feed acceptability; however, fish growth rates (WG and SGR) were significantly impaired and feed efficiency (FCR and FI) was also affected, though not significantly. These results indicate than even a 10% dietary fishmeal replacement with
Z. morio reduces fish growth performance. This in turn may imply that
Z. morio has lower digestibility than fishmeal for sea bass. Unfortunately, no other studies have been performed that use
Z. morio in the diets of sea bass, thus comparing results is challenging. However, the partial replacement of fishmeal protein by various insect species is generally possible without negative effects on fish growth and health [
19,
103,
104]. According to Magalhães et al. [
19] and Abdel-Tawwab et al. [
104], there were no significant differences in sea bass WG, feed utilization, SGR, or FCR following 6.5–50% substitution of fishmeal protein by
H. illucens larvae meal. In addition, when sea bass were fed diets with
T. molitor meal replacing up to 36% of fishmeal, fish weight was not affected and the hepato-somatic index value was improved [
103].
Z. morio larval meal has been used for dietary fishmeal replacement in other fish species. Prachom et al. [
105] replaced 3–12% of fishmeal with
Z. morio larvae meal in a study on Asian sea bass (
Lales carcarifer) and reported no significant differences in WG, FCR, feed intake, or SGR. Nile tilapia fed with diets containing 25–50%
Z. morio larvae meal showed similar WG and SGR but a lower FCR than fish fed the control fishmeal-based diet. A 10% dietary inclusion of hydrolyzed
Z. morio meal ensured high survival rates and satisfactory growth performance and feed utilization parameters in sea trout (
Salmo trutta) [
35], while diets including up to 25% fishmeal replacement by
Z. morio had no adverse effects on rainbow trout (
Oncorhynchus mykiss) fingerlings [
28]. Asimaki et al. [
34], using a full-fat
Z. morio meal to replace up to 30% of dietary fishmeal, reported insignificant effects on feed utilization and growth performance in gilthead sea bream (
Sparus aurata), while similar results were obtained for the same species feeding on a defatted
Z. morio meal [
41]. In contrast, perch fed a diet of 25% FM with
Z. morio and
A. domesticus substitutions showed lower WG, a higher FCR, and lower SGR than perch fed a diet without FM substitution [
106].
Liver and intestine histological examination is important when evaluating the effectiveness of a new fish diet. Insect meals do not generally affect the intestine and gut morphology of fish. Doğankaya [
28] and Turek et al. [
107] did not detect any histopathological liver or intestine alterations in
O. mykiss fed with up to a 25% replacement of FM with
Z. morio meal. When mopane worm (
Imbrasia belina (Westwood)) was included as a protein source in the diet of Mozambique tilapia (
Oreochromis mossambicus (Peters)), liver and intestine histology revealed no histological alterations [
108]. Moreover, when Atlantic salmon,
Salmo salar L., were fed a diet including only insect meal, typical signs of enteritis were observed [
109]. Nevertheless, in the present study, no histological alterations were detected in the midgut of ZM10 and ZM20 groups, while more lipid droplets were detected in the liver of fish in the ZM20 group. Since the lipid level of the ZM20 diet was not higher than the rest of the diets, and since the feed intake of fish was similar among groups, the increased hepatic lipid droplets in ZM20 fish imply a lower lipid digestibility of
Z. morio meal when included at increased levels in the diet. This is interesting considering that the
Z. morio meal was a defatted meal with as low as 3.8% crude lipid. Certainly, future digestibility studies will enlighten such areas.
Many plant species, such as herbs and leafy vegetables, can be successfully cultivated in aquaponics. Lettuce is widely used in aquaponics as it is one of the most widely adopted vegetables worldwide, its harvest period is short, and it has medium nutritional requirements [
64]. Although there are many studies in the recent literature that assess lettuce growth performance in aquaponics systems, it is quite difficult to attempt comparisons due to the different experimental set-ups, system characteristics, and physicochemical parameters involved. However, it is noticeable that our results for total fresh biomass production (1.30–2.20 kg/m
2) are significantly higher than those of Castillo-Castellanos et al. [
110] (47.9 g/m
2) but lower than those reported by Lennard and Leonard [
82] (2006) and Stathopoulou et al. [
40] (average biomass 4.97 kg/m
2 and 3.63 kg/m
2, respectively), which could be attributed to experimental set-up variations among the different studies. This could be due to the different diets and amounts of feed given, which can differentiate metabolic products [
20,
91]. We observed a difference in the plant growth parameters measured for system C in terms of plant architecture and productivity compared to the two other systems. Lettuce plants in this system were shorter with fewer but heavier leaves, which resulted in higher productivity per surface unit. The parameters measured in the framework of the present study, either biophysical or concerning nutrient inputs, cannot explain this superior growth. Since caution was taken to ensure similar growth conditions in all three aquaponic systems, the above-mentioned differences may be ascribed to parameters of and/or substances in the recirculating water that were not determined in our experiment.
This study enhances scientific knowledge by providing evidence that insect meals can be used as an ingredient for fish species-specific aquadiets in aquaponics towards an ento-aquaponic approach. Since lettuce production parameters were within the ranges reported in the literature, the present study confirms the suitability of lettuce cultivation in aquaponics using insect Z. morio larvae meal. Thus, aquaponic cultivation could be proposed for commercial lettuce cultivation as it integrates aspects of the circular economy for fish and vegetable production. Additional experimental studies are needed to illustrate the parameters that determine the feasibility of such an integration.