The Use of Fish Scale Hormone Concentrations in the Assessment of Long-Term Stress and Associated Adverse Effects on Reproductive Endocrinology
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminary Hormone Assays
2.2. Treatment Groups
2.3. Serum Collection
2.4. Scale Collection
2.5. Scale Hormone Extraction and Quantitation
2.6. Serum Hormone Extraction and Quantitation
2.7. Assay Validation
2.8. Statistical Analyses
3. Results
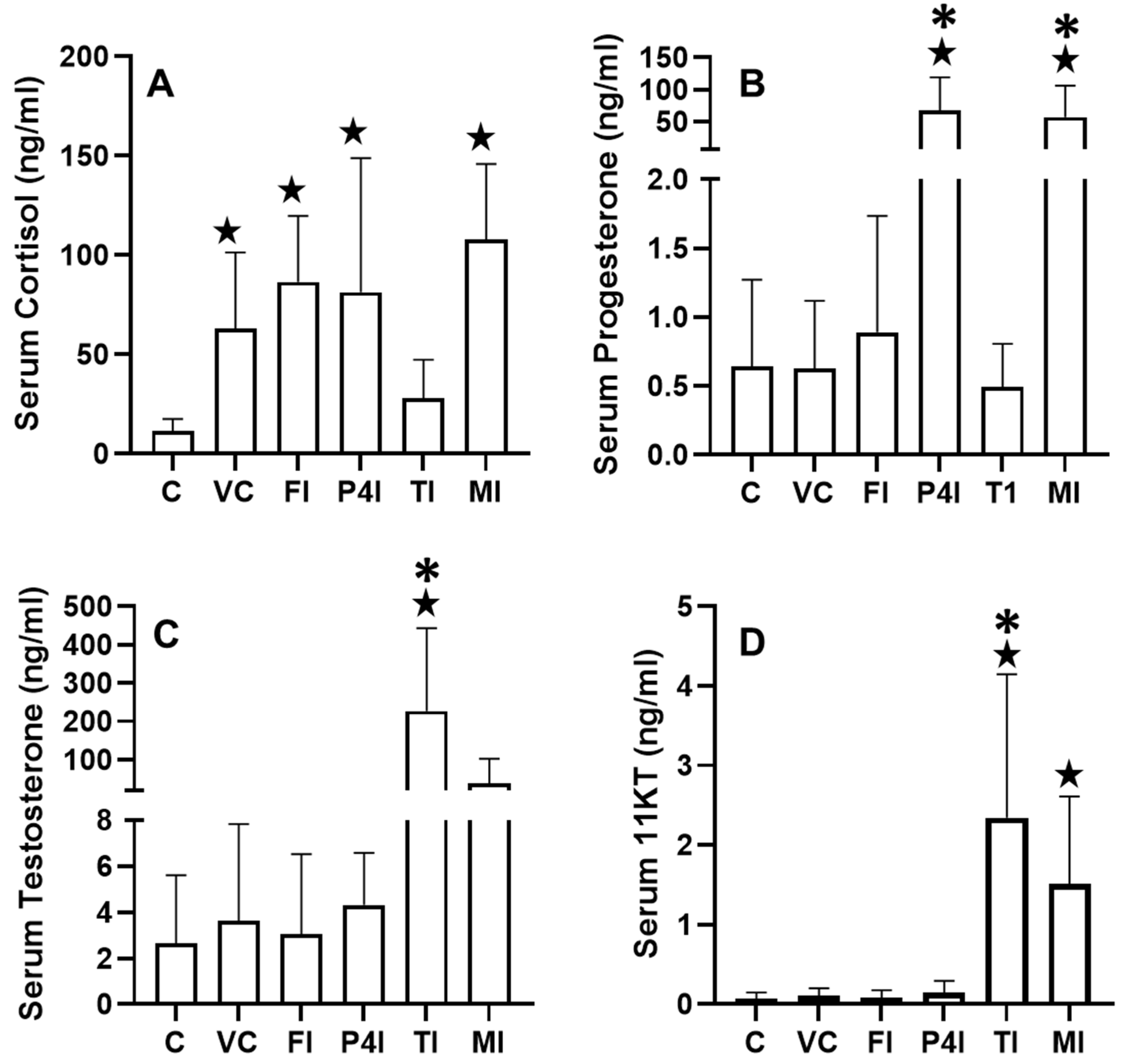
3.1. Serum Hormone Concentrations
3.1.1. Cortisol
3.1.2. Progesterone
3.1.3. Testosterone
3.1.4. 11KT
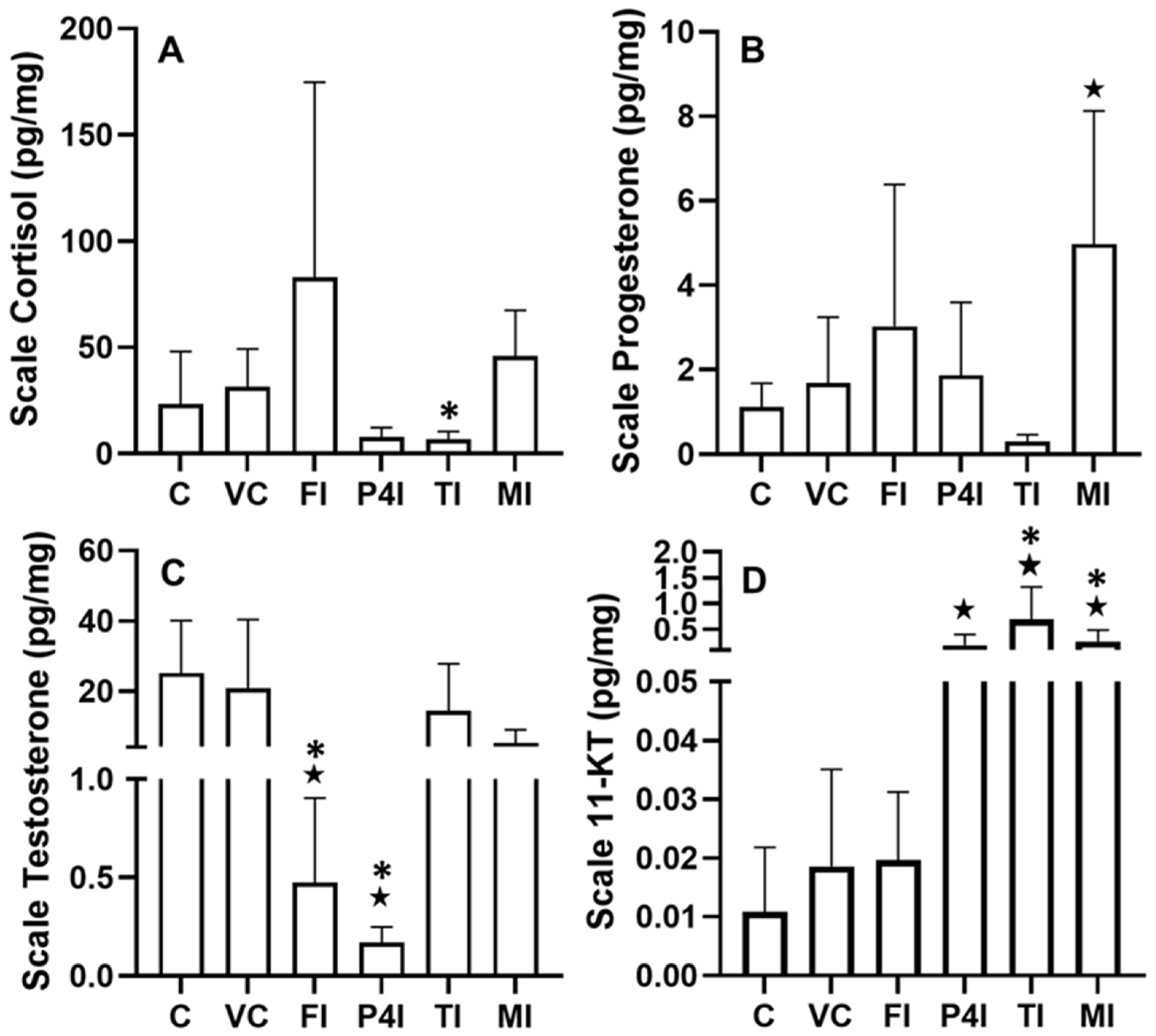
3.2. Scale Hormone Concentrations
3.2.1. Cortisol
3.2.2. Progesterone
3.2.3. Testosterone
3.2.4. 11KT
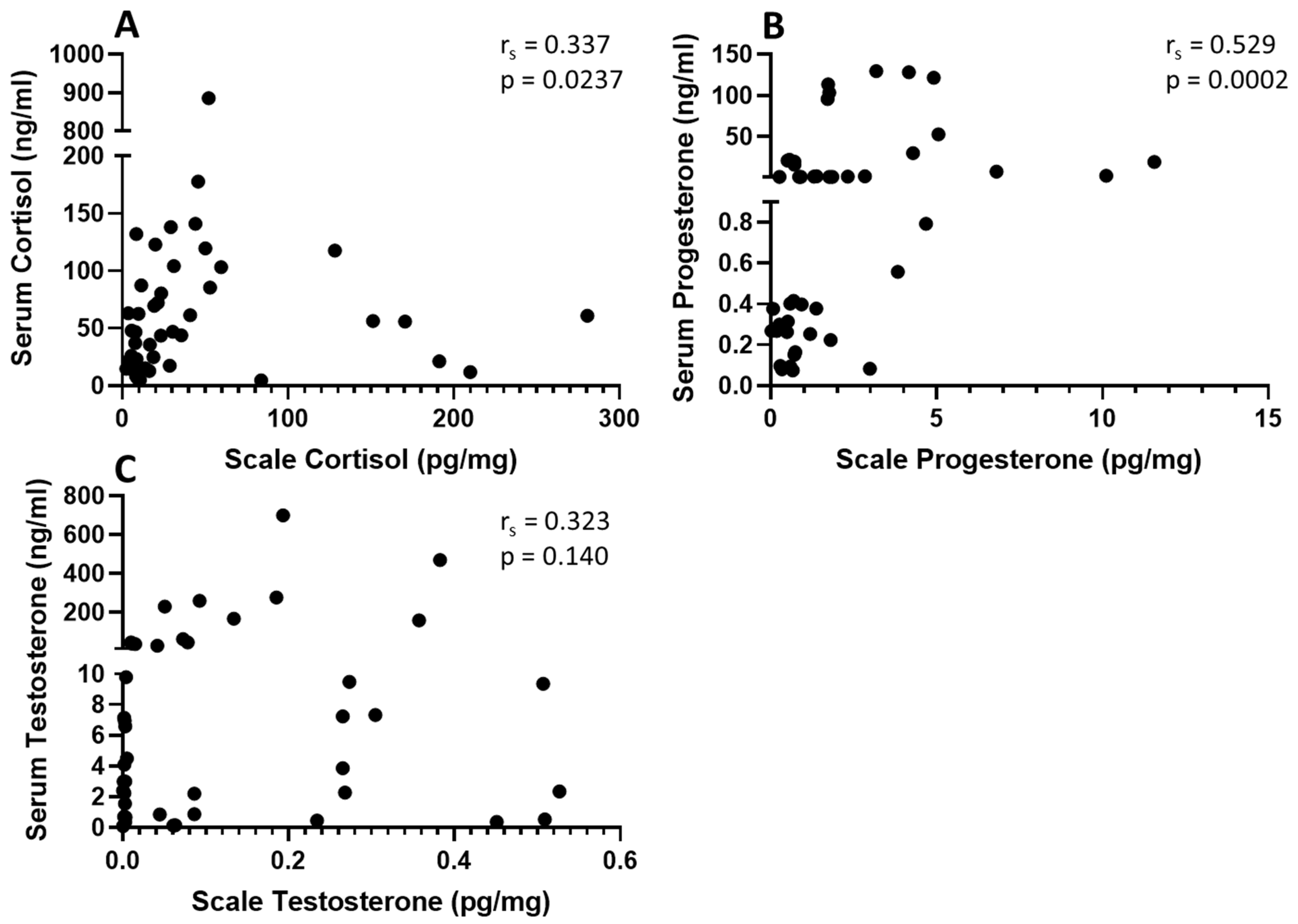
3.3. Serum-Scale Correlations
3.3.1. Cortisol
3.3.2. Progesterone
3.3.3. Testosterone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in Teleosts: Dynamics, Mechanisms of Action, and Metabolic Regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Rajakumar, A.; Senthilkumaran, B. Steroidogenesis and Its Regulation in Teleost-a Review. Fish Physiol. Biochem. 2020, 46, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar Bonga, S.E. The Stress Response in Fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Balasch, J.C.; Tort, L. Netting the Stress Responses in Fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish. In Fish Physiology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, pp. 1–34. ISBN 9780128027288. [Google Scholar]
- Laiz-Carrión, R.; Martín Del Río, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Influence of Cortisol on Osmoregulation and Energy Metabolism in Gilthead Seabream Sparus aurata. J. Exp. Zool. A Comp. Exp. Biol. 2003, 298, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.D. Growth and Stress in Fish Production. Aquaculture 1993, 111, 51–63. [Google Scholar] [CrossRef]
- Sadoul, B.; Vijayan, M.M. Stress and Growth. In Fish Physiology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, pp. 167–205. ISBN 9780128027288. [Google Scholar]
- Small, B.C.; Bilodeau, A.L. Effects of Cortisol and Stress on Channel Catfish (Ictalurus punctatus) Pathogen Susceptibility and Lysozyme Activity Following Exposure to Edwardsiella ictaluri. Gen. Comp. Endocrinol. 2005, 142, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Contreras-Sanchez, W.; Fitzpatrick, M.S. Effects of Stress on Fish Reproduction, Gamete Quality, and Progeny. Aquaculture 2001, 197, 3–24. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and Fish Reproduction: The Roles of Allostasis and Hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Sollberger, S.; Ehlert, U. How to Use and Interpret Hormone Ratios. Psychoneuroendocrinology 2016, 63, 385–397. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Carrick, T.R.; Hughes, S.E.; Balm, P.H.M. Testosterone, 11-Ketotestosterone, and Estradiol-17β Modify Baseline and Stress-Induced Interrenal and Corticotropic Activity in Trout. Gen. Comp. Endocrinol. 1996, 104, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Thorarensen, H.; Davie, P.S. 11-Ketotestosterone Suppresses Interrenal Activity in Rainbow Trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 1996, 103, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, J.; Möller, G.; Hrabě De Angelis, M.; Adamski, J. Steroids in Teleost Fishes: A Functional Point of View. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in Teleosts: How Fish Eggs Are Formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Yamashita, M. Regulation of Oocyte Maturation in Fish. Dev. Growth Differ. 2008, 50, S195–S219. [Google Scholar] [CrossRef]
- Scott, A.P.; Sumpter, J.P.; Stacey, N. The Role of the Maturation-Inducing Steroid, 17,20β-Dihydroxypregn-4-En-3-One, in Male Fishes: A Review. J. Fish Biol. 2010, 76, 183–224. [Google Scholar] [CrossRef]
- Sedigh, E.; Heidari, B.; Roozati, A.; Valipour, A. The Effect of Different Intensities of Static Magnetic Field on Stress and Selected Reproductive Indices of the Zebrafish (Danio rerio) During Acute and Subacute Exposure. Bull. Environ. Contam. Toxicol. 2019, 102, 204–209. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Maternal Stress and Fish Reproduction: The Role of Cortisol Revisited. Fish Fish. 2018, 19, 1016–1030. [Google Scholar] [CrossRef]
- Campbell, P.M.; Pottinger, T.G.; Sumptef, J.P. Preliminary Evidence That Chronic Confinement Stress Reduces the Quality of Gametes Produced by Brown and Rainbow Trout. Aquaculture 1994, 120, 151–169. [Google Scholar] [CrossRef]
- Valdebenito, I.I.; Gallegos, P.C.; Effer, B.R. Gamete Quality in Fish: Evaluation Parameters and Determining Factors. Zygote 2013, 23, 177–197. [Google Scholar] [CrossRef]
- Eriksen, M.S.; Espmark, Å.; Braastad, B.O.; Salte, R.; Bakken, M. Long-Term Effects of Maternal Cortisol Exposure and Mild Hyperthermia during Embryogeny on Survival, Growth and Morphological Anomalies in Farmed Atlantic Salmon Salmo salar Offspring. J. Fish Biol. 2007, 70, 462–473. [Google Scholar] [CrossRef]
- Eriksen, M.S.; Bakken, M.; Espmark, Å.; Braastad, B.O.; Salte, R. Prespawning Stress in Farmed Atlantic Salmon Salmo salar: Maternal Cortisol Exposure and Hyperthermia during Embryonic Development Affect Offspring Survival, Growth and Incidence of Malformations. J. Fish Biol. 2006, 69, 114–129. [Google Scholar] [CrossRef]
- Mccormick, M.I. Behaviorally Induced Maternal Stress in a Fish Influences Progeny Quality by a Hormonal Mechanism. Ecology 1998, 79, 1873–1883. [Google Scholar] [CrossRef]
- Sloman, K.A. Exposure of Ova to Cortisol Pre-Fertilisation Affects Subsequent Behaviour and Physiology of Brown Trout. Horm. Behav. 2010, 58, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Stratholt, M.L.; Donaldson, E.M.; Liley, N.R. Stress Induced Elevation of Plasma Cortisol in Adult Female Coho Salmon (Oncorhynchus kisutch), Is Reflected in Egg Cortisol Content, but Does Not Appear to Affect Early Development. Aquaculture 1997, 158, 141–153. [Google Scholar] [CrossRef]
- Hanke, I.; Ampe, B.; Kunzmann, A.; Gärdes, A.; Aerts, J. Thermal Stress Response of Juvenile Milkfish (Chanos chanos) Quantified by Ontogenetic and Regenerated Scale Cortisol. Aquaculture 2019, 500, 24–30. [Google Scholar] [CrossRef]
- Weirup, L.; Schulz, C.; Seibel, H.; Aerts, J. Scale Cortisol Is Positively Correlated to Fin Injuries in Rainbow Trout (Oncorhynchus mykiss) Reared in Commercial Flow through Systems. Aquaculture 2021, 543, 736924. [Google Scholar] [CrossRef]
- Hanke, I.; Hassenrück, C.; Ampe, B.; Kunzmann, A.; Gärdes, A.; Aerts, J. Chronic Stress under Commercial Aquaculture Conditions: Scale Cortisol to Identify and Quantify Potential Stressors in Milkfish (Chanos chanos) Mariculture. Aquaculture 2020, 526, 735352. [Google Scholar] [CrossRef]
- Aerts, J.; Metz, J.R.; Ampe, B.; Decostere, A.; Flik, G.; de Saeger, S. Scales Tell a Story on the Stress History of Fish. PLoS ONE 2015, 10, e0123411. [Google Scholar] [CrossRef]
- Goikoetxea, A.; Sadoul, B.; Blondeau-Bidet, E.; Aerts, J.; Blanc, M.O.; Parrinello, H.; Barrachina, C.; Pratlong, M.; Geffroy, B. Genetic Pathways Underpinning Hormonal Stress Responses in Fish Exposed to Short- and Long-Term Warm Ocean Temperatures. Ecol. Indic. 2021, 120, 106937. [Google Scholar] [CrossRef]
- Carbajal, A.; Reyes-López, F.E.; Tallo-Parra, O.; Lopez-Bejar, M.; Tort, L. Comparative Assessment of Cortisol in Plasma, Skin Mucus and Scales as a Measure of the Hypothalamic-Pituitary-Interrenal Axis Activity in Fish. Aquaculture 2019, 506, 410–416. [Google Scholar] [CrossRef]
- Carbajal, A.; Monclús, L.; Tallo-Parra, O.; Sabes-Alsina, M.; Vinyoles, D.; Lopez-Bejar, M. Cortisol Detection in Fish Scales by Enzyme Immunoassay: Biochemical and Methodological Validation. J. Appl. Ichthyol. 2018, 34, 967–970. [Google Scholar] [CrossRef]
- Carbajal, A.; Tallo-Parra, O.; Monclús, L.; Vinyoles, D.; Solé, M.; Lacorte, S.; Lopez-Bejar, M. Variation in Scale Cortisol Concentrations of a Wild Freshwater Fish: Habitat Quality or Seasonal Influences? Gen. Comp. Endocrinol. 2019, 275, 44–50. [Google Scholar] [CrossRef]
- D’orbcastel, E.R.; Bettarel, Y.; Dellinger, M.; Sadoul, B.; Bouvier, T.; Amandé, J.M.; Dagorn, L.; Geffroy, B. Measuring Cortisol in Fish Scales to Study Stress in Wild Tropical Tuna. Environ. Biol. Fishes 2021, 104, 725–732. [Google Scholar] [CrossRef]
- Culbert, B.M.; Ligocki, I.Y.; Salena, M.G.; Wong, M.Y.L.; Hamilton, I.M.; Aubin-Horth, N.; Bernier, N.J.; Balshine, S. Rank- and Sex-Specific Differences in the Neuroendocrine Regulation of Glucocorticoids in a Wild Group-Living Fish. Horm. Behav. 2021, 136, 105079. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.K.C.; Janz, D.M. First Look into the Use of Fish Scales as a Medium for Multi-Hormone Stress Analyses. Fishes 2022, 7, 145. [Google Scholar] [CrossRef]
- Kennedy, E.K.C.; Janz, D.M. Chronic Stress Causes Cortisol, Cortisone and DHEA Elevations in Scales but Not Serum in Rainbow Trout. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 276, 111352. [Google Scholar] [CrossRef]
- Rasmussen, J.P.; Vo, N.T.; Sagasti, A. Fish Scales Dictate the Pattern of Adult Skin Innervation and Vascularization. Dev. Cell 2018, 46, 344–359.e4. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Vijayan, M.M.; Boutilier, R.G. Experimental Control of Stress Hormone Levels in Fishes: Techniques and Applications. Rev. Fish Biol. Fish. 1994, 4, 215–255. [Google Scholar] [CrossRef]
- Laberge, F.; Yin-Liao, I.; Bernier, N.J. Temporal Profiles of Cortisol Accumulation and Clearance Support Scale Cortisol Content as an Indicator of Chronic Stress in Fish. Conserv. Physiol. 2019, 7, coz052. [Google Scholar] [CrossRef]
- Alderman, S.L.; Vijayan, M.M. 11β-Hydroxysteroid Dehydrogenase Type 2 in Zebrafish Brain: A Functional Role in Hypothalamus-Pituitary-Interrenal Axis Regulation. J. Endocrinol. 2012, 215, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Milla, S.; Wang, N.; Mandiki, S.N.M.; Kestemont, P. Corticosteroids: Friends or Foes of Teleost Fish Reproduction? Comp. Biochem. Physiol. 2009, 153, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Milla, S.; Jalabert, B.; Rime, H.; Prunet, P.; Bobe, J. Hydration of Rainbow Trout Oocyte during Meiotic Maturation and in Vitro Regulation by 17,20β-Dihydroxy-4-Pregnen-3-One and Cortisol. J. Exp. Biol. 2006, 209, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Z.; Levavi-Sivan, B. Hormonal Control of Reproduction and Growth|Endocrine Regulation of Fish Reproduction. In Encyclopedia of Fish Physiology; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 1, pp. 1500–1508. ISBN 9780080923239. [Google Scholar]
| Hormone | Dry Mass Required (mg) |
|---|---|
| Estradiol | 100+ |
| 17-α-OH progesterone | 100+ |
| Progesterone | 50–100 |
| Testosterone | 50–100 |
| 11-Ketotestosterone | 50–100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, E.K.C.; Janz, D.M. The Use of Fish Scale Hormone Concentrations in the Assessment of Long-Term Stress and Associated Adverse Effects on Reproductive Endocrinology. Fishes 2022, 7, 393. https://doi.org/10.3390/fishes7060393
Kennedy EKC, Janz DM. The Use of Fish Scale Hormone Concentrations in the Assessment of Long-Term Stress and Associated Adverse Effects on Reproductive Endocrinology. Fishes. 2022; 7(6):393. https://doi.org/10.3390/fishes7060393
Chicago/Turabian StyleKennedy, Emily K. C., and David M. Janz. 2022. "The Use of Fish Scale Hormone Concentrations in the Assessment of Long-Term Stress and Associated Adverse Effects on Reproductive Endocrinology" Fishes 7, no. 6: 393. https://doi.org/10.3390/fishes7060393
APA StyleKennedy, E. K. C., & Janz, D. M. (2022). The Use of Fish Scale Hormone Concentrations in the Assessment of Long-Term Stress and Associated Adverse Effects on Reproductive Endocrinology. Fishes, 7(6), 393. https://doi.org/10.3390/fishes7060393