Evaluation of the Effectiveness of Marking Juvenile Takifugu obscurus Otoliths with Strontium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Analysis
2.3. Statistical Analysis
3. Results
3.1. Survival and Growth in Different Strontium Concentrations
3.2. Quantitative Analysis of Otolith Marking
3.3. Changes in the Strontium Content of Fish
3.3.1. Changes in the Strontium Content in Muscle
3.3.2. Changes in the Strontium Content in Gill
3.3.3. Changes in the Strontium Content in Liver
4. Discussion
4.1. Survival and Growth in Different Strontium Concentrations
4.2. Quantitative Analysis of Otolith Marking
4.3. Changes in the Strontium Content of Fish
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoo, G.Y.; Park, I.S.; Lee, S. Effects of graded dietary lipid levels on growth performance, fatty acid profile, and hematological characteristics of hybrid pufferfish (Takifugu obscurus × T. rubripes) juveniles. Aquac. Rep. 2022, 24, 101120. [Google Scholar] [CrossRef]
- Ye, C.X.; Wu, Y.L.; Sun, Z.Z.; Wang, A.L. Dietary protein requirement of juvenile obscure puffer, Takifugu obscurus. Aquac. Res. 2016, 48, 2064–2073. [Google Scholar] [CrossRef]
- Qian, X.M.; Zhu, Y.X.; Sun, Z.L.; Xu, P.; Gu, R.B.; Xu, G.C. History and present situation of rare fishes in the Yangtze River and their exploitation and conservation strategies. China Fish. 2014, 24–27. (In Chinese) [Google Scholar] [CrossRef]
- Xu, D.P.; Jiang, S.L.; Zhao, C.S.; Fang, D.A.; Hu, H.Y. Comparative transcriptomics analysis of the river pufferfish (Takifugu obscurus) by tributyltin exposure: Clues for revealing its toxic injury mechanism. Fish Shellfish Immunol. 2018, 82, 536–543. [Google Scholar] [CrossRef]
- Shi, W.G. Technical Manual of Fishery Ecology and Proliferation and Release of Takifugu obscurus in the Lower Reaches of Yangtze River; Science Press: Beijing, China, 2014; pp. 156–157. (In Chinese) [Google Scholar]
- LÜ, H.J.; Zhang, X.M.; Fu, M.; Xi, D.; Gao, T.X. Use of tetracycline hydrochloride and alizarin complexone for immersion marking black rockfish Sebastes schlegelii. Chin. J. Oceanol. Limnol. 2014, 32, 810–820. [Google Scholar] [CrossRef]
- Thomassen, S.; Pedersen, M.I.; Holdensgaard, G. Tagging the European eel Anguilla anguilla (L.) with coded wire tags. Aquaculture 2000, 185, 57–61. [Google Scholar] [CrossRef]
- Beentjes, M.P.; Jellyman, D.J. Enhanced growth of longfin eels, Anguilla dieffenbachia, transplanted into Lake Hawea, a high country lake in South Island, New Zealand. N. Z. J. Mar. Freshw. Res. 2010, 37, 1–11. [Google Scholar] [CrossRef]
- Daniel, C.; James, D.B. A terramycin 200 for fish (44.09% oxytetracycline dihydrate) treatment regimen proposed for the fluorescent marking of rainbow trout vertebrae. N. Am. J. Aquac. 2013, 75, 34–38. [Google Scholar]
- Stefanie, H.; Kate, M.Q.; Monica, M.; Magnus, A.; Annelie, H.; Jakob, O.H.; Anders, S.; Michele, C.; Karin, H.; Krzysztof, R.; et al. Short-term tagging mortality of Baltic cod (Gadus morhua). Fish. Res. 2021, 234, 7. [Google Scholar]
- Ruber, R.B.; Sergio, S.T.; Danay, M.R. A study of two tagging methods in the Caribbean sea cucumber Holothuria mexicana. Mar. Biodivers. Rec. 2014, 7, 118. [Google Scholar]
- Kurta, K.M.; Malysheva, O.O.; Spyrydonov, V.G. Comparative analysis of paddlefish (Polyodon spathula) populations’ genetic structure with regard to microsatellite DNA markers. Cytol. Genet. 2020, 54, 31–37. [Google Scholar] [CrossRef]
- Yang, J.X.; Pan, X.F.; Chen, X.Y.; Wang, X.A.; Zhao, Y.P.; Li, J.Y.; Li, Z.Y. Overview of the artificial enhancement and release of endemic freshwater fish in China. Zool. Res. 2013, 34, 267–280. [Google Scholar]
- Skalski, J.R.; Buchanan, R.A.; Griswold, J. Review of marking methods and release-recapture designs for estimating the survival of very small fish: Examples from the assessment of salmonid fry survival. Rev. Fish. Sci. 2009, 17, 391–401. [Google Scholar] [CrossRef]
- Richard, A.S.; Daniel, E.S.; Michael, J.P. Mark retention and fish survival associated with a low-cost marking technique for common carp Cyprinus carpio (Linnaeus, 1758). J. Appl. Ichthyol. 2020, 36, 693–698. [Google Scholar]
- Liu, Q.; Zhang, X.M.; Zhang, P.D.; Nwafili, S.A. The use of alizarin red S and alizarin complexone for immersion marking Japanese flounder Paralichthys olivaceus (T.). Fish. Res. 2009, 98, 67–74. [Google Scholar] [CrossRef]
- Meyer, S.; Sørensen, S.R.; Peck, M.A.; Støttrup, J.G. Sublethal effects of alizarin complexone marking on Baltic cod (Gadus morhua) eggs and larvae. Aquaculture 2012, 324, 158–164. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, R.K.; Gan, W.X.; Deng, L.J.; Song, Z.B. Otolith fluorescent and thermal marking of elongate loach (Leptobotia elongata) at early life stages. Environ. Biol. Fishes 2016, 99, 687–695. [Google Scholar] [CrossRef]
- Mirali, H.; Bani, A.; Fasihi, J. Application of enriched 137Ba tracer to mark juvenile Persian sturgeon (Acipenser persicus). Environ. Biol. Fishes 2017, 100, 163–169. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Fortuño, J.M.; Pérez-Mayol, S.; Grau, A. The use of energy dispersive X-ray spectroscopy to detect strontium marks in fish otoliths. Sci. Mar. 2011, 76, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Stötera, S.; Degen-Smyrek, A.K.; Krumme, U.; Stepputtis, D.; Bauer, R.; Limmer, B.; Hammer, C. Marking otoliths of Baltic cod (Gadus morhua Linnaeus, 1758) with tetracycline and strontium chloride. J. Appl. Ichthyol. 2019, 35, 427–435. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, W.; Li, P.L.; Tang, F.J.; Lu, W.Q.; Yang, J.; Jiang, T. Evidence of return of chum salmon released from Tangwang River by strontium marking method. Acta Oceanol. Sin. 2021, 40, 182–186. [Google Scholar] [CrossRef]
- Carriere, B.; Gillis, D.; Halden, N.; Anderson, G. Strontium metabolism in the juvenile Lake Sturgeon, Acipenser fulvescens (Rafinesque, 1817), and further evaluation of the isotope as a marking tool for stock discrimination. J. Appl. Ichthyol. 2016, 32, 258–266. [Google Scholar] [CrossRef]
- Wickstrom, H.; Sjoberg, N.B. Traceability of stocked eels-the Swedish approach. Ecol. Freshw. Fish 2014, 23, 33–39. [Google Scholar] [CrossRef]
- Morris, J.A.; Rulifson, R.A.; Toburen, L.H. Life history strategies of striped bass, Morone saxatilis, populations inferred from otolith microchemistry. Fish. Res. 2003, 62, 53–63. [Google Scholar] [CrossRef]
- Limburg, K.E. Otolith strontium traces environmental history of subyearling American shad Alosa sapidissima. Mar. Ecol. Prog. Ser. 1995, 119, 25–35. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Hu, Y.H.; Chen, X.B.; Yang, J. Revealing population connectivity of the estuarine tapertail anchovy Coilia nasus in the Changjiang river estuary and its adjacent waters using otolith microchemistry. Fishes 2022, 7, 147. [Google Scholar] [CrossRef]
- Suyama, S.; Nakagami, M.; Naya, M.; Ueno, Y. Comparison of the growth of age-1 pacific saury Cololabis saira in the Western and the Central North Pacific. Fish. Sci. 2012, 78, 277–285. [Google Scholar] [CrossRef]
- Myers, F.W.; Dempster, T.; Fjelldal, P.G.; Hansen, T.; Jensen, A.J.; Swearer, S.E. Stable isotope marking of otoliths during vaccination: A novel method for mass-marking fish. Aquac. Environ. Interact. 2014, 5, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Getchell, R.G.; Bowser, P.R.; Cornwell, E.R.; Pavek, T.; Baneux, P.; Kirby, D.; Sams, K.L.; Marquis, H. Safety of strontium chloride as a skeletal marking agent for pacific salmon. J. Aquat. Anim. Health 2017, 29, 181–188. [Google Scholar] [CrossRef]
- Pyle, G.G.; Swanson, S.M.; Lehmkuhl, D.M. Toxicity of uranium mine receiving waters to early life stage fathead minnows (Pimephales promelas) in the laborator. Environ. Pollut. 2002, 116, 243–255. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Jiang, T.; Chen, X.B.; Liu, H.B.; Phelps, Q.; Yang, J. Inter-otolith differences in strontium markings: A case study on the juvenile crucian carp Carassius carassius. Fishes 2022, 7, 112. [Google Scholar] [CrossRef]
- Caverzasio, J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008, 42, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Méo, S.C.; Leal, C.L.; Garcia, J.M. Activation and early parthenogenesis of bovine oocytes treated with ethanol and strontium. Anim. Reprod. Sci. 2004, 81, 35–46. [Google Scholar] [CrossRef]
- Camcı, H.; Doruk, C.; Ünver, S.S. Effect of strontium ranelate on condylar growth during mandibular advancement in rats. Turk. J. Orthod. 2020, 33, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.J.; Van Ginneken, L.; Blust, R. Kinetics of waterborne strontium uptake in the common carp, Cyprinus carpio, at different calcium levels. Environ. Toxicol. Chem. 2000, 19, 622–630. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Y.Z.; Yuan, X.W.; Zhang, Y.; Jiang, H.L.; Jiao, H.F.; Chen, J.H.; Li, S.F. Effects of strontium-enriched water on strontium content in the otolith and body of hatchery-reared Larimichthys crocea. Mar. Fish. 2019, 41, 338–345. (In Chinese) [Google Scholar]
- Elsdon, T.S.; Gillanders, B.M. Reconstructing migratory patterns of fish based on environmental influences on otolith chemistry. Rev. Fish Biol. Fish. 2003, 13, 217–235. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Gillanders, B.M. Strontium incorporation into calcified structures: Separating the effects of ambient water concentration and exposure time. Inter Res. Sci. Cent. 2005, 285, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Yokouchi, K.; Fukuda, N.; Shirai, K.; Aoyama, J.; Daverat, F.; Tsukamoto, K. Time lag of the response on the otolith strontium/calcium ratios of the Japanese eel, Anguilla japonica to changes in strontium/calcium ratios of ambient water. Environ. 2011, 92, 469–478. [Google Scholar] [CrossRef]
- Li, X.Q.; Cong, X.R.; Shi, J.H.; Gao, Y.F.; Jiang, T.; Yang, J. Feasibility analysis of releasing individuals of Aristichthys nobilis identification based on otolith Sr markers. J. Lake Sci. 2017, 29, 914–922. (In Chinese) [Google Scholar]
- Liu, M.Z.; Jiang, R.J.; Zhang, H.; Yang, F.; Li, X.F.; Feng, G.P.; Yin, R.; Chen, F. Otolith marking with strontium for stock assessment in Coilia nasus. Front. Mar. Sci. 2022, 9, 882. [Google Scholar] [CrossRef]
- Secor, D.H.; Arzapalob, A.H.; Piccoli, P.M. Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes? J. Exp. Mar. Biol. Ecol. 1995, 192, 15–33. [Google Scholar] [CrossRef]
- Panfili, J.; Darnaude, A.M.; Vigliola, L.; Jacquart, A.; Labonne, M.; Gilles, S. Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths. J. Exp. Mar. Biol. Ecol. 2015, 467, 65–70. [Google Scholar] [CrossRef]
- Tzeng, W.N. Temperature effects on the incorporation of strontium in otolith of Japanese eel Anguilla japonica. J. Fish. Biol. 1994, 45, 1055–1066. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Trueman, C.N.; Milton, J.A.; Waring, C.P.; Cooper, M.J.; Hunter, E. Physiological influences can outweigh environmental signals in otolith microchemistry research. Mar. Ecol. Prog. Ser. 2014, 500, 245–264. [Google Scholar] [CrossRef] [Green Version]
- Sturrock, A.M.; Hunter, E.; Milton, J.A.; Johnson, R.C.; Waring, C.P.; Trueman, C.N.; Leder, E. Quantifying physiological influences on otolith microchemistry. Methods Ecol. Evol. 2015, 7, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Company, R.; Felícia, H.; Serafim, A.; Almeida, A.J.; Biscoito, M.; Bebianno, M.J. Metal concentrations and metallothionein-like protein levels in deep-sea fishes captured near hydrothermal vents in the Mid-Atlantic Ridge off Azores. Deep Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 893–908. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Q.Y.; Pan, Q.W.; Feng, Z.Q.; Li, Z.H.; Zhao, C.T.; Ran, B. Study on cytotoxicity of high strontium mineral water. Guangdong Med. J. 2013, 34, 2125–2128. (In Chinese) [Google Scholar]

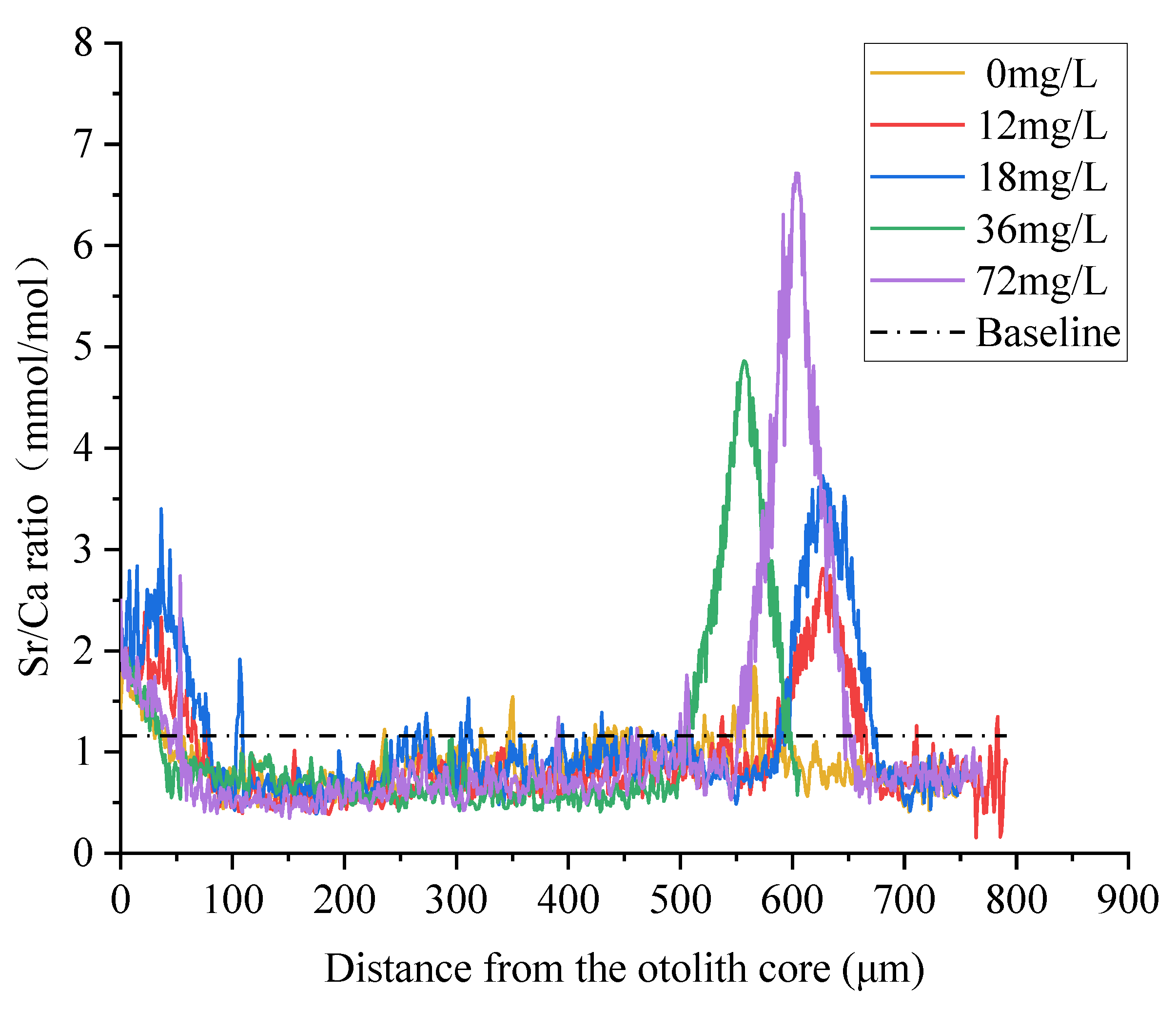

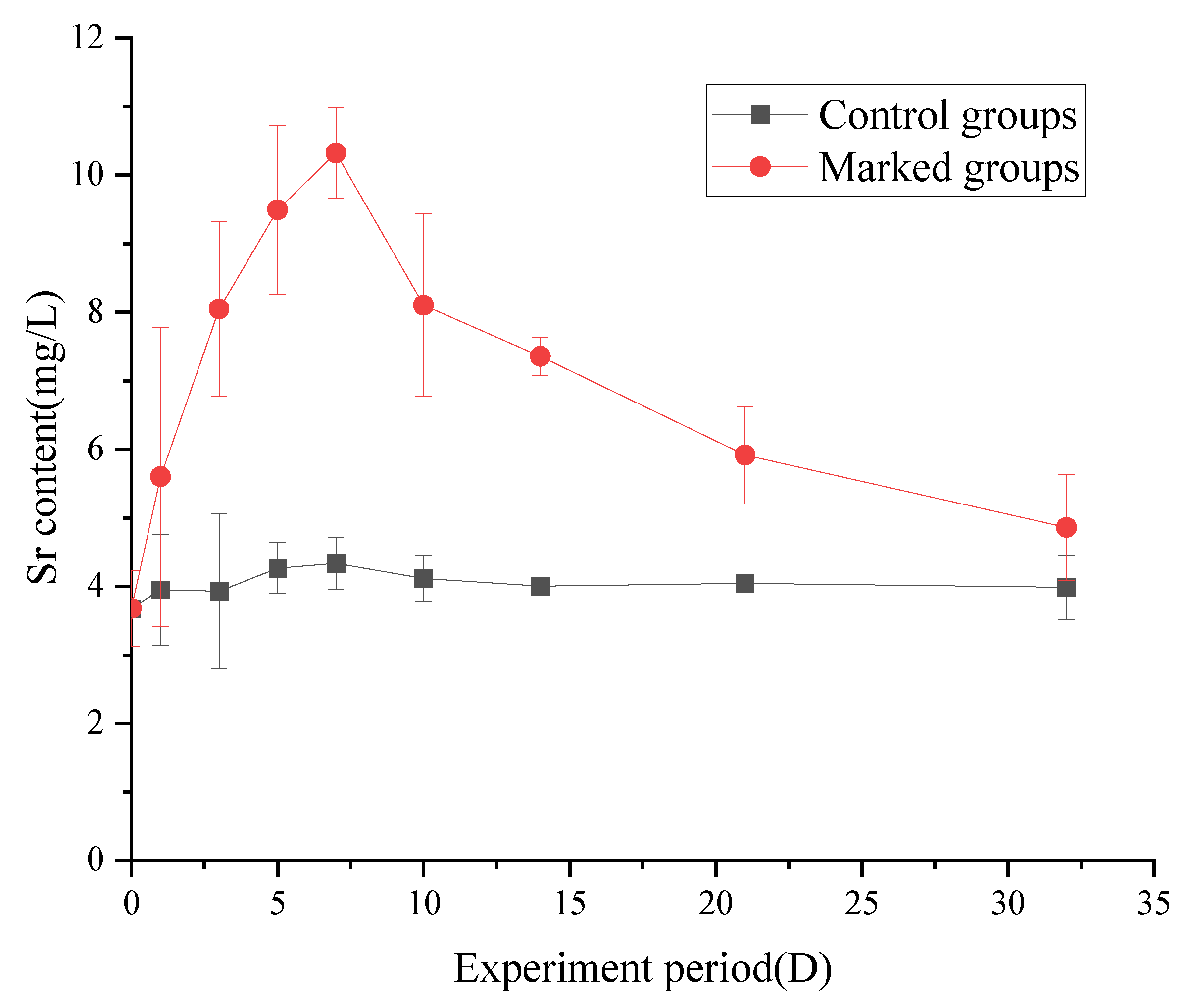
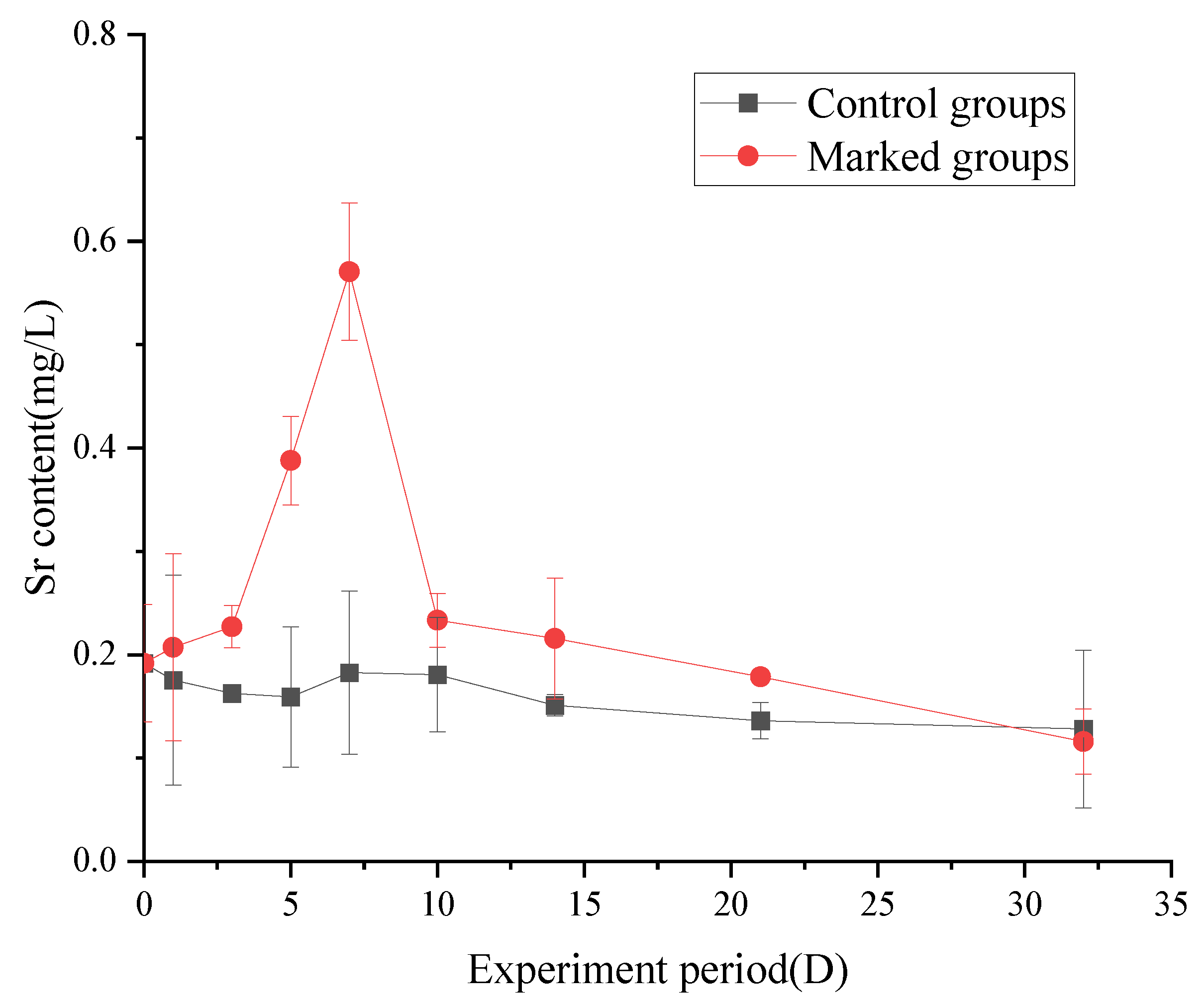
| Strontium Concentration (mg/L) | Survival Quantity | Survival Rate | Condition Factor | |
|---|---|---|---|---|
| 0 D | 7 D | |||
| 0 | 60 | 60 | 100 | 3.1405 ± 0.0839 a |
| 12 | 60 | 60 | 100 | 3.2892 ± 0.3201 ab |
| 18 | 60 | 60 | 100 | 3.5034 ± 0.4666 b |
| 36 | 60 | 60 | 100 | 3.2954 ± 0.2270 ab |
| 72 | 60 | 59 | 98.33 | 3.2543 ± 0.2517 ab |
| Strontium Concentration (mg/L) | Sr/Ca Ratios in Otoliths (mmol/mol) | |||
|---|---|---|---|---|
| 1 D | 3 D | 5 D | 7 D | |
| 0 | 0.81 ± 0.09 | 0.84 ± 0.13 | 0.82 ± 0.08 | 0.86 ± 0.09 |
| 12 | 0.87 ± 0.06 | 1.34 ± 0.17 | 1.76 ± 0.05 | 1.90 ± 0.09 |
| 18 | 1.12 ± 0.14 | 1.66 ± 0.07 | 1.99 ± 0.06 | 2.29 ± 0.02 |
| 36 | 1.66 ± 0.05 | 2.13 ± 0.17 | 2.49 ± 0.03 | 3.05 ± 0.22 |
| 72 | 2.00 ± 0.10 | 2.95 ± 0.13 | 3.03 ± 0.06 | 3.75 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Zhang, H.; Feng, G.; Liu, Y.; Han, Z.; Zhao, F.; Ye, Q.; Hu, W.; Song, C. Evaluation of the Effectiveness of Marking Juvenile Takifugu obscurus Otoliths with Strontium. Fishes 2022, 7, 371. https://doi.org/10.3390/fishes7060371
Gu L, Zhang H, Feng G, Liu Y, Han Z, Zhao F, Ye Q, Hu W, Song C. Evaluation of the Effectiveness of Marking Juvenile Takifugu obscurus Otoliths with Strontium. Fishes. 2022; 7(6):371. https://doi.org/10.3390/fishes7060371
Chicago/Turabian StyleGu, Lingling, Hui Zhang, Guangpeng Feng, Yong Liu, Zhiqiang Han, Feng Zhao, Qing Ye, Wangjiao Hu, and Chao Song. 2022. "Evaluation of the Effectiveness of Marking Juvenile Takifugu obscurus Otoliths with Strontium" Fishes 7, no. 6: 371. https://doi.org/10.3390/fishes7060371
APA StyleGu, L., Zhang, H., Feng, G., Liu, Y., Han, Z., Zhao, F., Ye, Q., Hu, W., & Song, C. (2022). Evaluation of the Effectiveness of Marking Juvenile Takifugu obscurus Otoliths with Strontium. Fishes, 7(6), 371. https://doi.org/10.3390/fishes7060371








