Polystyrene Microparticles and the Functional Traits of Invertebrates: A Case Study on Freshwater Shrimp Neocardina heteropoda
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design
2.3. Behavioural Assay
2.4. Metabolic Rates
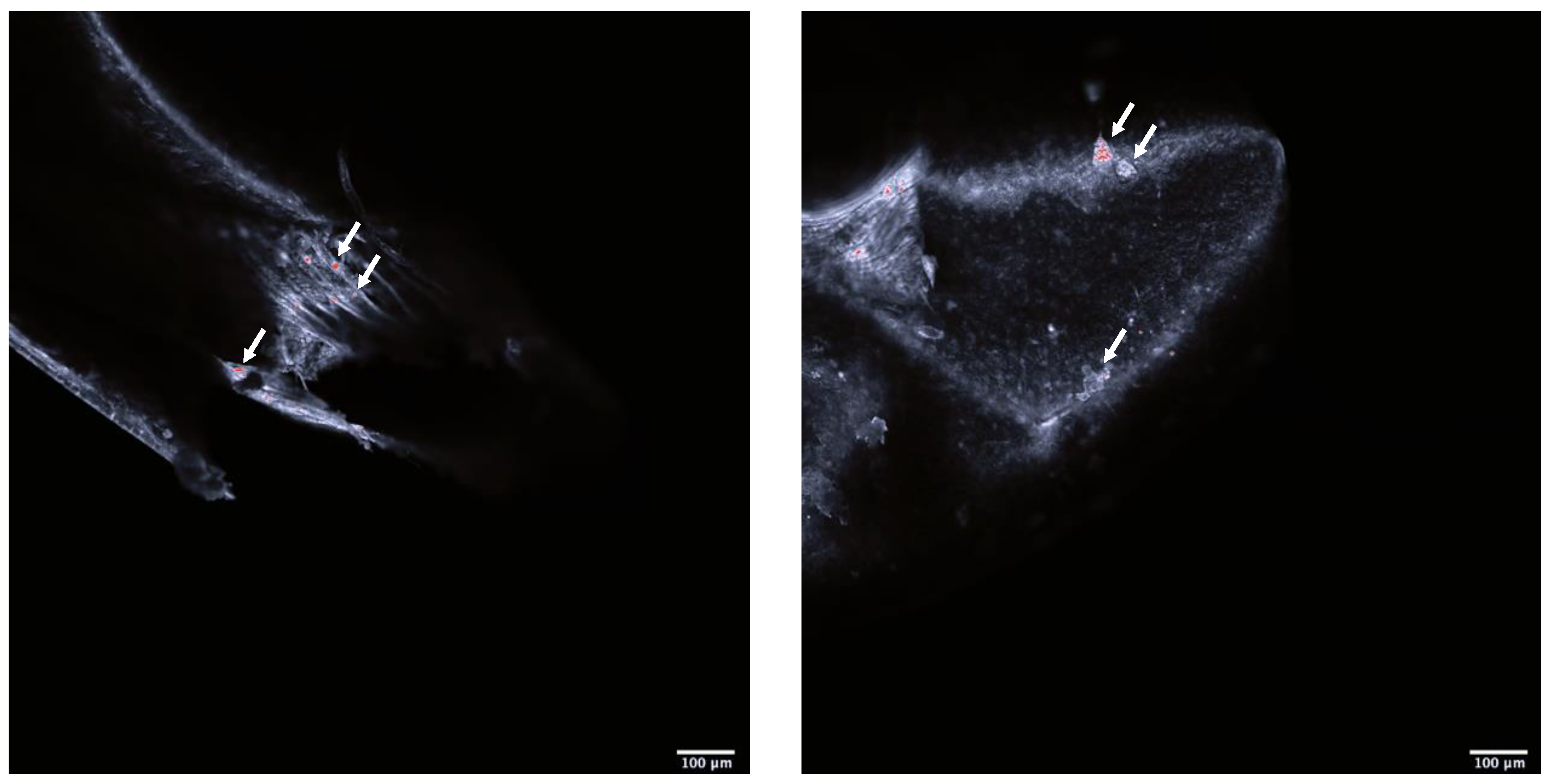
2.5. Presence of Polystyrene in Shrimps
2.6. Data Analysis
2.7. Statistical Analysis
3. Results
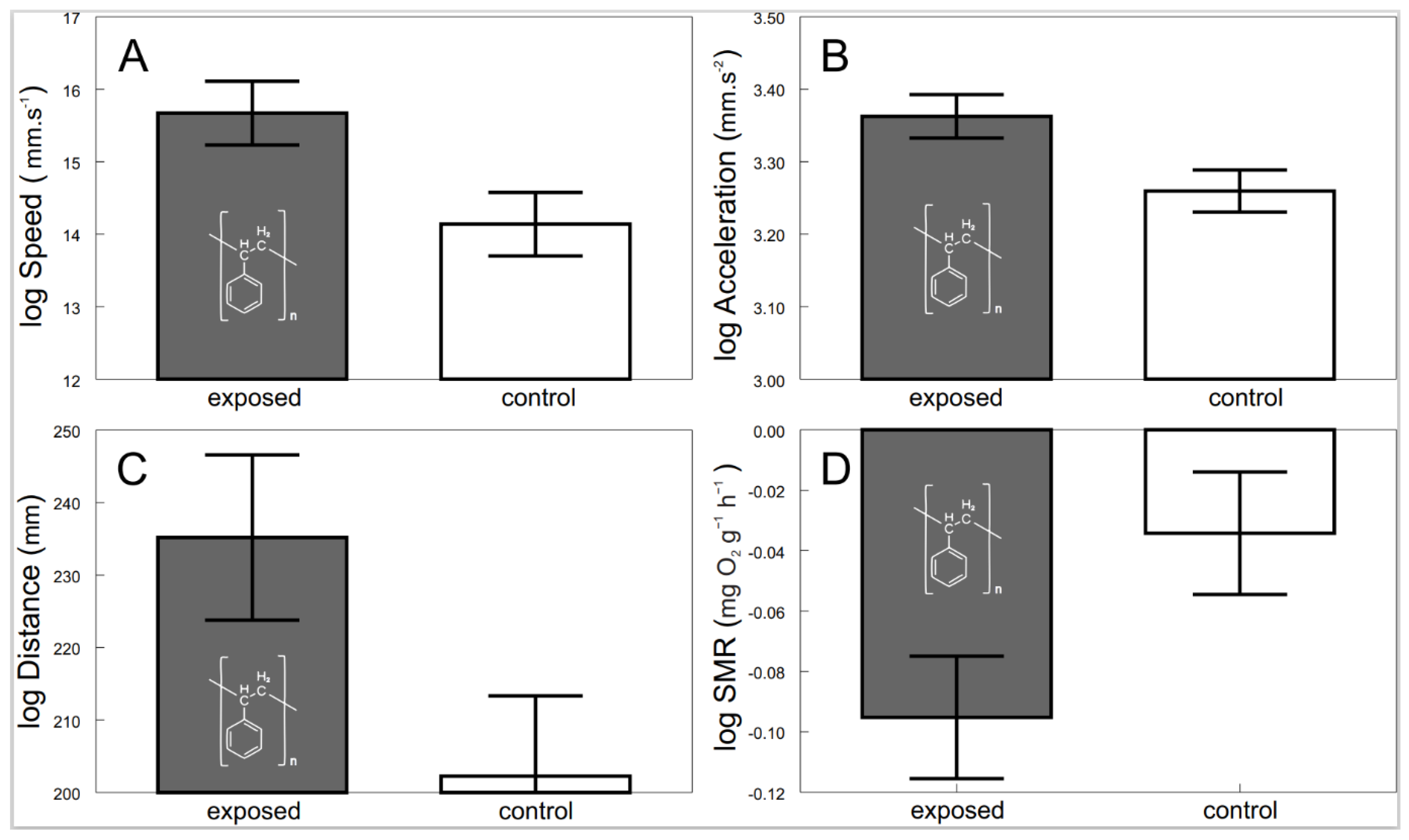
3.1. Overall Effects
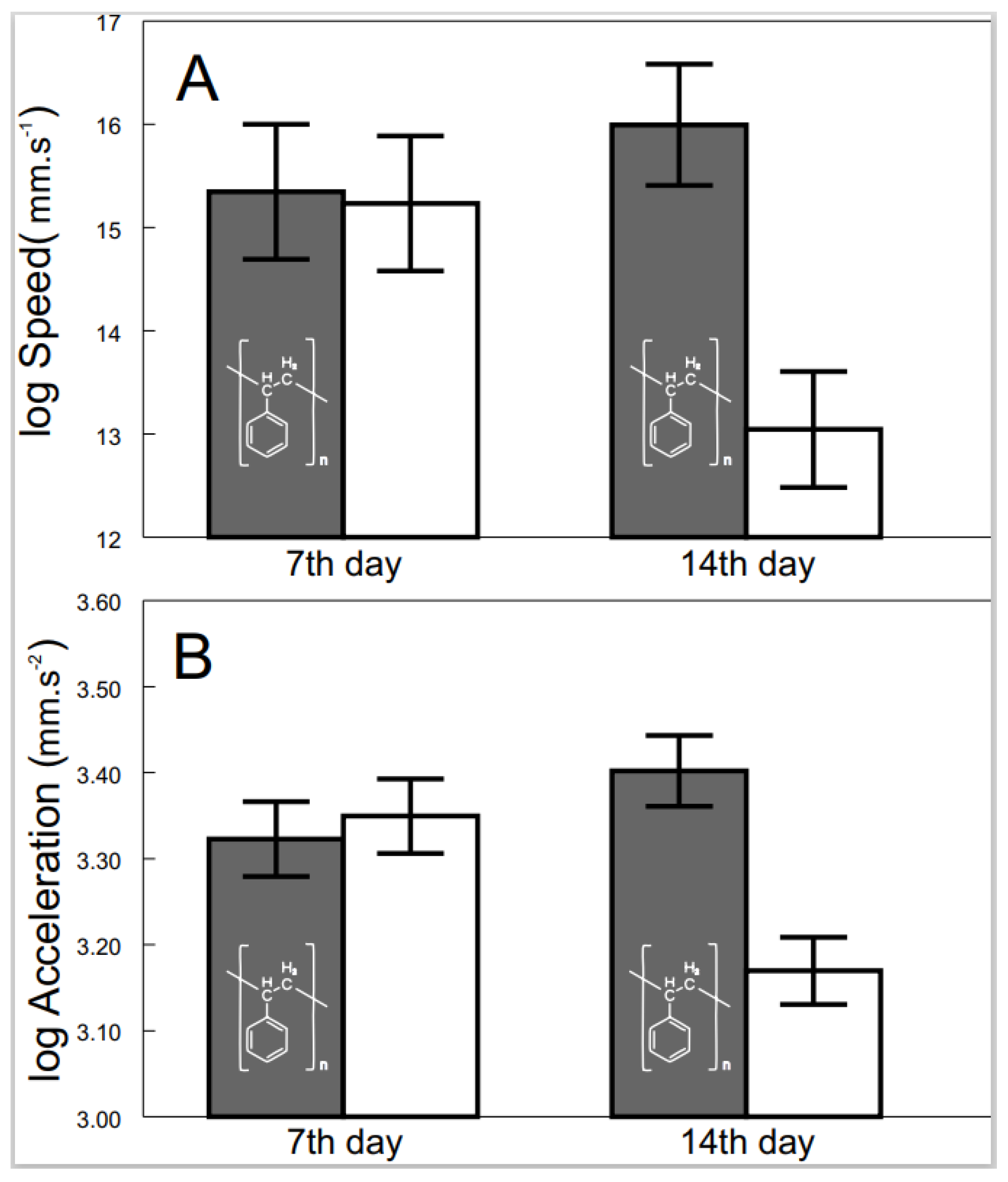
3.2. Progression of the Effects
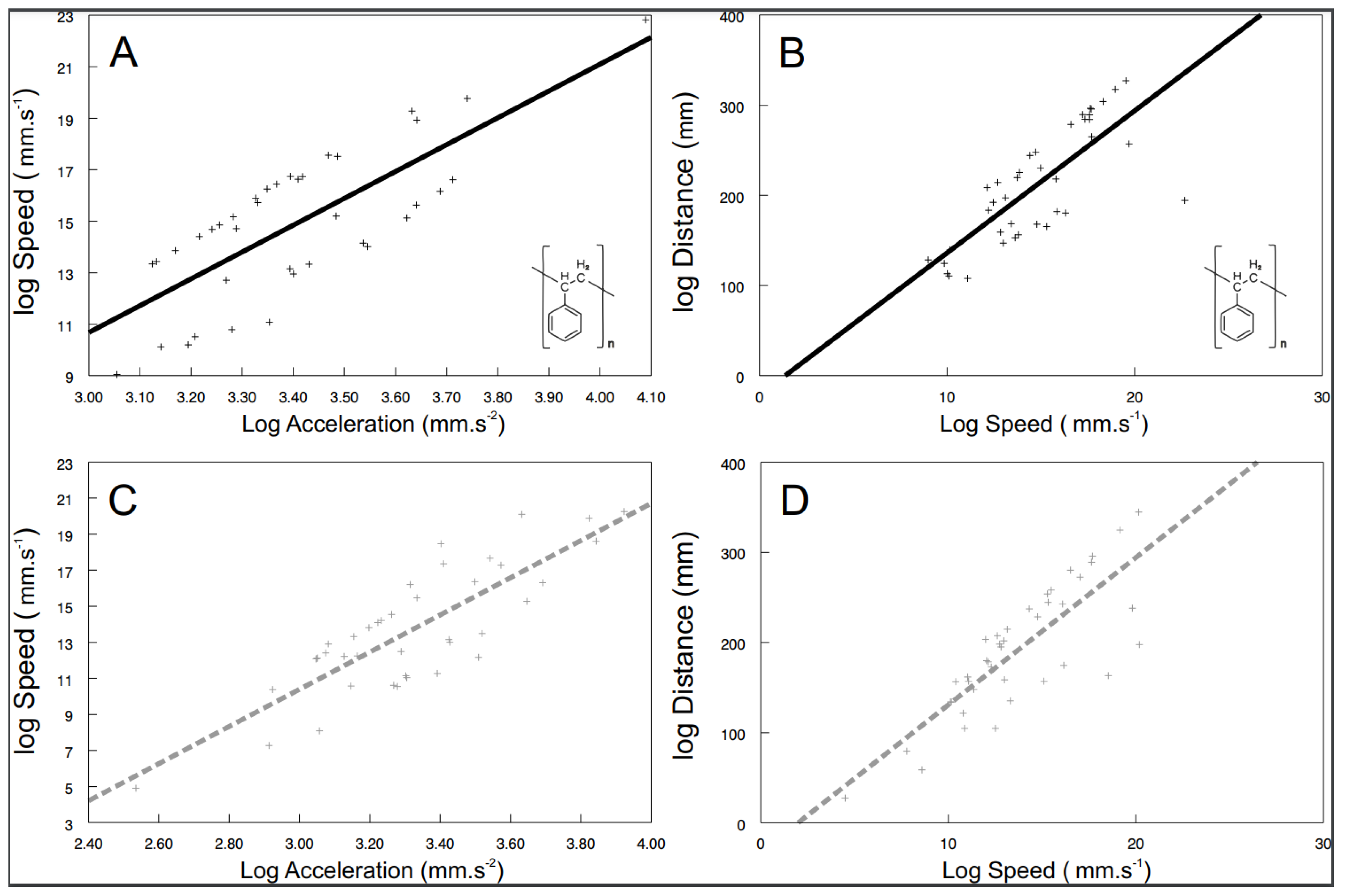
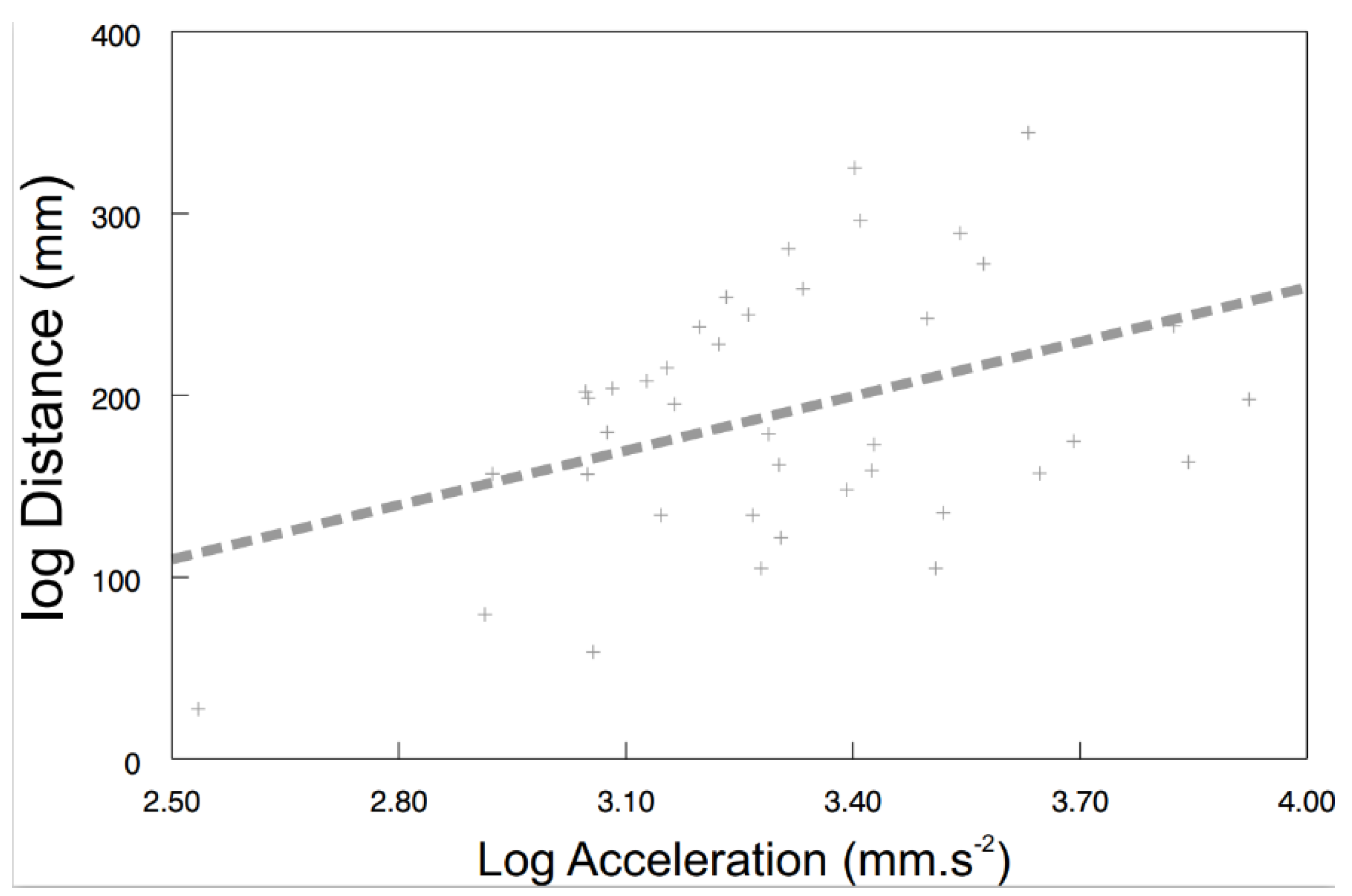
3.3. Behavioural Syndromes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Orb. Invisible-the Particles Inside Us; Tyree, C., Morrison, D., Eds.; 2017; Available online: https://orbmedia.org/the-invisibles (accessed on 17 July 2022).
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, W.; Chen, X.; He, Y.; Zhang, X.; Gong, H. A preliminary study of the association between colonization of microorganism on microplastics and intestinal microbiota in shrimp under natural conditions. J. Hazard Mater. 2021, 408, 124882. [Google Scholar] [CrossRef]
- Farrelly, T.; Shaw, I. Polystyrene as Hazardous Household Waste. In Household Hazardous Waste Management; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, occurrence, and degradation of microplastics in the aqueous environment. In Freshwater Microplastics; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef]
- Raza, A. Microplastics in Freshwater Systems: A Review on Its Accumulation and Effects on Fishes. Int. J. Res. Anal. Rev. -IJRAR 2018, 5. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and integrated strategies for the control and mitigation of plastic and microplastic pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef]
- Kwon, B.G.; Saido, K.; Koizumi, K.; Sato, H.; Ogawa, N.; Chung, S.Y.; Kusui, T.; Kodera, Y.; Kogure, K. Regional distribution of styrene analogues generated from polystyrene degradation along the coastlines of the North-East Pacific Ocean and Hawaii. Environ. Pollut. 2014, 188, 45–49. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, H.J.; Bertram, M.G.; Brodin, T.; Dang, Z.C.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The role of behavioral ecotoxicology in environmental protection. Environ. Sci. Technol. 2021, 55, 5620–5628. [Google Scholar] [CrossRef]
- Peterson, E.K.; Buchwalter, D.B.; Kerby, J.L.; LeFauve, M.K.; Varian-Ramos, C.W.; Swaddle, J.P. Integrative behavioral ecotoxicology: Bringing together fields to establish new insight to behavioural ecology, toxicology, and conservation. Curr. Zool. 2017, 63, 185–194. [Google Scholar] [CrossRef]
- Parrino, V.; Costa, G.; Giannetto, A.; De Marco, G.; Cammilleri, G.; Acar, Ü.; Piccione, G.; Fazio, F. Trace elements (Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in Mytilus galloprovincialis and Tapes decussatus from Faro and Ganzirri Lakes (Sicily, Italy): Flow cytometry applied for hemocytes analysis. J. Trace Elem. Med. Biol. 2021, 68, 126870. [Google Scholar] [CrossRef]
- Salerno, M.; Berlino, M.; Mangano, M.C.; Sara, G. Microplastics and the functional traits of fishes: A global meta-analysis. Glob. Change Biol. 2021, 27, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Andreou, D.; Green, I.D.; Britton, J.R. Microplastics in freshwater fishes: Occurrence, impacts and future perspectives. Fish Fish. 2021, 22, 467–488. [Google Scholar] [CrossRef]
- Saborowski, R.; Korez, Š.; Riesbeck, S.; Weidung, M.; Bickmeyer, U.; Gutow, L. Shrimp and microplastics: A case study with the Atlantic ditch shrimp Palaemon varians. Ecotoxicol. Environ. Saf. 2022, 234, 113394. [Google Scholar] [CrossRef]
- Parrino, V.; Costa, G.; Cannavà, C.; Fazio, E.; Bonsignore, M.; Concetta, S.; Piccione, G.; Fazio, F. Flow cytometry and micro-Raman spectroscopy: Identification of hemocyte populations in the mussel Mytilus galloprovincialis (Bivalvia: Mytilidae) from Faro Lake and Tyrrhenian Sea (Sicily, Italy). Fish Shellfish. Immunol. 2019, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Cheng, J. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. [Google Scholar] [CrossRef]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Castañeda, G.; Ruiz-Fernández, A.C.; Frías-Espericueta, M.G.; Rivera-Hernández, J.R.; Green-Ruiz, C.R.; Páez-Osuna, F. Microplastics in the tissues of commercial semi-intensive shrimp pond-farmed Litopenaeus vannamei from the Gulf of California ecoregion. Chemosphere 2022, 297, 13419. [Google Scholar] [CrossRef]
- Biro, P.A.; Stamps, J.A. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 2008, 23, 361–368. [Google Scholar] [CrossRef]
- Herrera, M.; Castanheira, M.F.; Conceição, L.E.C.; Martins, C.I. Linking risk taking and the behavioral and metabolic responses to confinementstress in gilthead seabream Sparus aurata. Appl. Anim. Behav. Sci. 2014, 155, 101–108. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Y.; Liu, S.; Zhang, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: Are larger plastic particles more harmless? J. Hazard. Mater. 2020, 396, 122693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lv, W.; Jiao, Y.; Liu, Z.; Li, Y.; Cai, M.; Wu, D.; Zhou, W.; Zhao, Y. Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus. Aquat. Toxicol. 2020, 224, 105497. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.L.; Weinersmith, K.L.; Brodin, T.; Saltz, J.B.; Sih, A. Behavioural syndromes in fishes: A review with implications for ecology and fisheries management. J. Fish Biol. 2011, 78, 395–435. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.J.; Feeney, W.E.; Marshall, H.H.; Cowlishaw, G.; Heinsohn, R. Animal personality: What are behavioural ecologists measuring? Biol. Rev. 2013, 88, 465–475. [Google Scholar] [CrossRef]
- FAO. Current Bibliography for Aquatic Science and Fisheries; Taylor & Francis Ltd.: London, UK, 1961. [Google Scholar]
- Li, R.; Weng, J.; Ren, L.; Wang, X.; Meng, Q.; Wang, L.; Sun, J. A novel microRNA and its PFK target control growth length in the freshwater shrimp (Neocaridina heteropoda). J. Exp. Biol. 2020, 223, jeb.223529. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala, India. Sci. Total Environ. 2020, 737, 139839. [Google Scholar] [CrossRef]
- Eom, H.-J.; Nam, S.-E.; Rhee, J.-S. Polystyrene microplastics induce mortality through acute cell stress and inhibition of cholinergic activity in a brine shrimp. Mol. Cell. Toxicol. 2020, 16, 233–243. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.-A.; Linse, S.; Malmendal, A.; Cedervall, A.T. Altered Behavior, Physiology, and Metabolism in Fish Exposed to Polystyrene Nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef]
- Suman, T.Y.; Jia, P.-P.; Li, W.-G.; Junaid, M.; Xin, G.-Y.; Wang, Y.; Pei, D.-S. Acute and chronic effects of polystyrene microplastics on brine shrimp: First evidence highlighting the molecular mechanism through transcriptome analysis. J. Hazard. Mater. 2020, 400, 123220. [Google Scholar] [CrossRef]
- Han, C.-C.; Hsu, K.-C.; Fang, L.-S.; Cheng, I.-M.; Lin, H.-D. Geographical and temporal origins of Neocaridina species (Decapoda: Caridea: Atyidae) in Taiwan. BMC Genet 2019, 20, 86. [Google Scholar] [CrossRef]
- Tropea, C.; Stumpf, L.; Greco, L.S.L. Effect of Temperature on Biochemical Composition, Growth and Reproduction of the Ornamental Red Cherry Shrimp Neocaridina heteropoda (Decapoda, Caridea). PLoS ONE. 2015, 10, e0119468. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Kooi, M.; Reisser, J.; Slat, B.; Ferrari, F.F.; Schmid, M.S.; Cunsolo, S.; Brambini, R.; Noble, K.; Sirks, L.-A.; Linders, T.E.W.; et al. The effect of particle properties on the depth profile of buoyant plastics in the ocean. Sci. Rep. 2016, 6, 33882. [Google Scholar] [CrossRef]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef]
- Tao, J.; Tamis, R.; Fink, K.; Williams, B.; Nelson-White, T.; Craig, R. The neglected morula/compact stage embryo transfer. Hum. Reprod. 2002, 17, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Kenward, M.G.; Roger, J.H. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Bondelind, M.; Sokolova, E.; Nguyen, A.; Karlsson, D.; Karlsson, A.; Björklund, K. Hydrodynamic modelling of traffic-related microplastics discharged with stormwater into the Göta River in Sweden. Environ. Sci. Pollut. Res. 2020, 27, 24218–24230. [Google Scholar] [CrossRef]
- Collard, F.; Gilbert, B.; Eppe, G.; Roos, L.; Compère, P.; Das, K.; Parmentier, E. Morphology of the filtration apparatus of three planktivorous fishes and relation with ingested anthropogenic particles. Mar. Pollut. Bull. 2017, 116, 182–191. [Google Scholar] [CrossRef]
- Jâms, I.B.; Windsor, F.M.; Poudevigne-Durance, T.; Ormerod, S.J.; Durance, I. Estimating the size distribution of plastics ingested by animals. Nat. Commun. 2020, 11, 1594. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garaventa, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef]
- Strungaru, S.A.; Jijie, R.; Nicoara, M.; Plavan, G.; Faggio, C. Micro-(nano) plastics in freshwater ecosystems: Abundance, toxicological impact and quantification methodology. TrAC–Trends Anal. Chem. 2019, 110, 116–128. [Google Scholar] [CrossRef]
- Rochman, C.M.; TKurobe IFlores, S.J. The Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Romano, N.; Ashikin, M.; Teh, J.C.; Syukri, F.; Karami, A. Effects of pristine polyvinyl chloride fragments on whole body histology and protease activity in silver barb Barbodes gonionotus fry. Environ. Pollut. 2018, 237, 1106–1111. [Google Scholar] [CrossRef]
- Athey, S.N.; Albotra, S.D.; Gordon, C.A.; Monteleone, B.; Seaton, P.; Andrady, A.L.; Taylor, A.R.; Brander, S.M. Trophic transfer of microplastics in an estuarine food chain and the effects of a sorbed legacy pollutant. Limnol. Oceanogr. Lett. 2020, 5, 154–162. [Google Scholar] [CrossRef]
- Roda, J.F.B.; Lauer, M.M.; Risso, W.E.; dos Reis, B.; Martinez, C. Microplastics and copper effects on the neotropical teleost Prochilodus lineatus: Is there any interaction? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110659. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, R.; Qu, H.; Zuo, Y.; Yu, Z.; Hu, G.; Li, Z.; Chen, H.; Lin, B.; Wang, B.; et al. Enhanced adsorption of tetrabromobisphenol a (TBBPA) on cosmetic-derived plastic microbeads and combined effects on zebrafish. Chemosphere 2020, 248, 126067. [Google Scholar] [CrossRef]
- Lay, S.L.; Simard, G.; Martinez, M.C.; Andriantsitohaina, R. Oxidative Stress and Metabolic Pathologies:From an Adipocentric Point of View. Oxidative Med. Cell. Longev. 2014, 2014, 908539. [Google Scholar]
- Betteridge, D.J. What Is Oxidative Stress? Metabolism 2000, 49 (Suppl. 1), 3–8. [Google Scholar] [CrossRef]
- Rossi, G.; Bamoud, J.; Monticelli, L. Polystyrene nanoparticles perturb lipid membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef]
- Hurt, R.; O’Reilly, C.M.; Perry, W.L. Microplastic prevalence in two fish species in two U.S. reservoirs. Limnol. Oceanogr. Lett. 2020, 5, 147–153. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.; Leung, J.Y.S.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X.; et al. Accumulation of microplastics in typical commercial aquatic species: A case study at a productive aquaculture site in China. Sci. Total Environ. 2020, 708, 135432. [Google Scholar] [CrossRef] [PubMed]
- Ariyomo, T.O.; Watt, P.J. The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim. Behav. 2012, 83, 41–46. [Google Scholar] [CrossRef]
- Sih, A.; Chang, A.T.; Wey, T.W. Effects of behavioural type, social skill and the social environment on male mating success in water striders. Anim. Behav. 2014, 94, 9–17. [Google Scholar] [CrossRef]
- Roche, D.G.; Careau, V.; Binning, S.A. Demystifying animal ‘personality’(or not): Why individual variation matters to experimental biologists. J. Exp. Biol. 2016, 219, 3832–3843. [Google Scholar] [CrossRef]
- Moscicki, M.K.; Hurd, P.L. Sex, boldness and stress experience affect convict cichlid, Amatitlania nigrofasciata, open field behaviour. Anim. Behav. 2015, 107, 105–114. [Google Scholar] [CrossRef]
- Sancho-Santos, M.E.; Horký, P.; Grabicová, K.; Hubená, P.; Slavík, O.; Grabic, R.; Douda, K.; Randák, T. Traces of tramadol impact behaviour in a native european fish. Ecotoxicol. Environ. Saf. 2021, 212, 111999. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef]
- Wolf, M.; Weissing, F.J. Animal personalities: Consequences for ecology and evolution. Trends Ecol. Evol. 2012, 27, 452–461. [Google Scholar] [CrossRef]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems-impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastic the Facts 2015-an Analysis of European Plastics Production, Demand and Waste Data. 2015. Available online: http://www.plasticseurope.org/Document/plastics—the-facts-2015.aspx?FolID¼2 (accessed on 17 July 2022).
- Morales-Caselles, C.; Viejo, J.; Martí, E.; González-Fernández, D.; Pragnell-Raasch, H.; González-Gordillo, J.I.; Montero, E.; Arroyo, G.M.; Hanke, G.; Salvo, V.S.; et al. An inshore–offshore sorting system revealed from global classification of ocean litter. Nat. Sustain. 2021, 4, 484–493. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kučera, J.; Púček Belišová, N.; Mackuľak, T.; Ryba, J.; Douda, K.; Bondarev, D.; Slavík, O.; Tamáš, M.; Escobar Calderon, J.F.; Horký, P. Polystyrene Microparticles and the Functional Traits of Invertebrates: A Case Study on Freshwater Shrimp Neocardina heteropoda. Fishes 2022, 7, 323. https://doi.org/10.3390/fishes7060323
Kučera J, Púček Belišová N, Mackuľak T, Ryba J, Douda K, Bondarev D, Slavík O, Tamáš M, Escobar Calderon JF, Horký P. Polystyrene Microparticles and the Functional Traits of Invertebrates: A Case Study on Freshwater Shrimp Neocardina heteropoda. Fishes. 2022; 7(6):323. https://doi.org/10.3390/fishes7060323
Chicago/Turabian StyleKučera, Jozef, Noemi Púček Belišová, Tomáš Mackuľak, Jozef Ryba, Karel Douda, Dmitrij Bondarev, Ondrej Slavík, Michal Tamáš, Juan Felipe Escobar Calderon, and Pavel Horký. 2022. "Polystyrene Microparticles and the Functional Traits of Invertebrates: A Case Study on Freshwater Shrimp Neocardina heteropoda" Fishes 7, no. 6: 323. https://doi.org/10.3390/fishes7060323
APA StyleKučera, J., Púček Belišová, N., Mackuľak, T., Ryba, J., Douda, K., Bondarev, D., Slavík, O., Tamáš, M., Escobar Calderon, J. F., & Horký, P. (2022). Polystyrene Microparticles and the Functional Traits of Invertebrates: A Case Study on Freshwater Shrimp Neocardina heteropoda. Fishes, 7(6), 323. https://doi.org/10.3390/fishes7060323






