Effects of Different Oxytocin and Temperature on Reproductive Activity in Nile tilapia (Oreochromis niloticus): Based on Sex Steroid Hormone and GtHR Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments and Sampling
2.2. Total RNA Extraction and First-Strand cDNA Synthesis
2.3. Determination of GtHR Genes Expression
2.4. Determination of Estradiol (E2) and Testosterone (T) Content
3. Results
3.1. Template Preparation for Quantitative RT-PCR
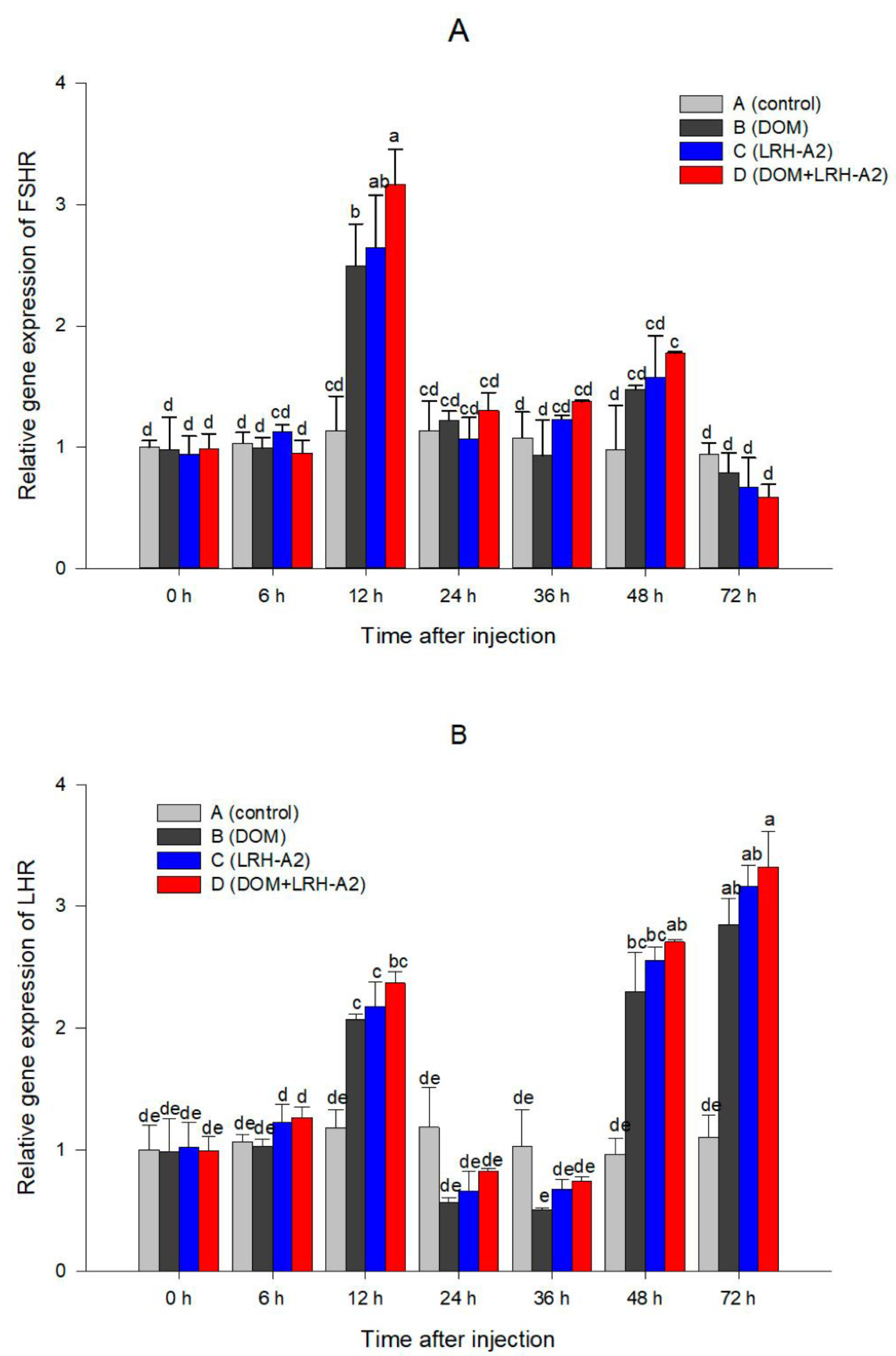
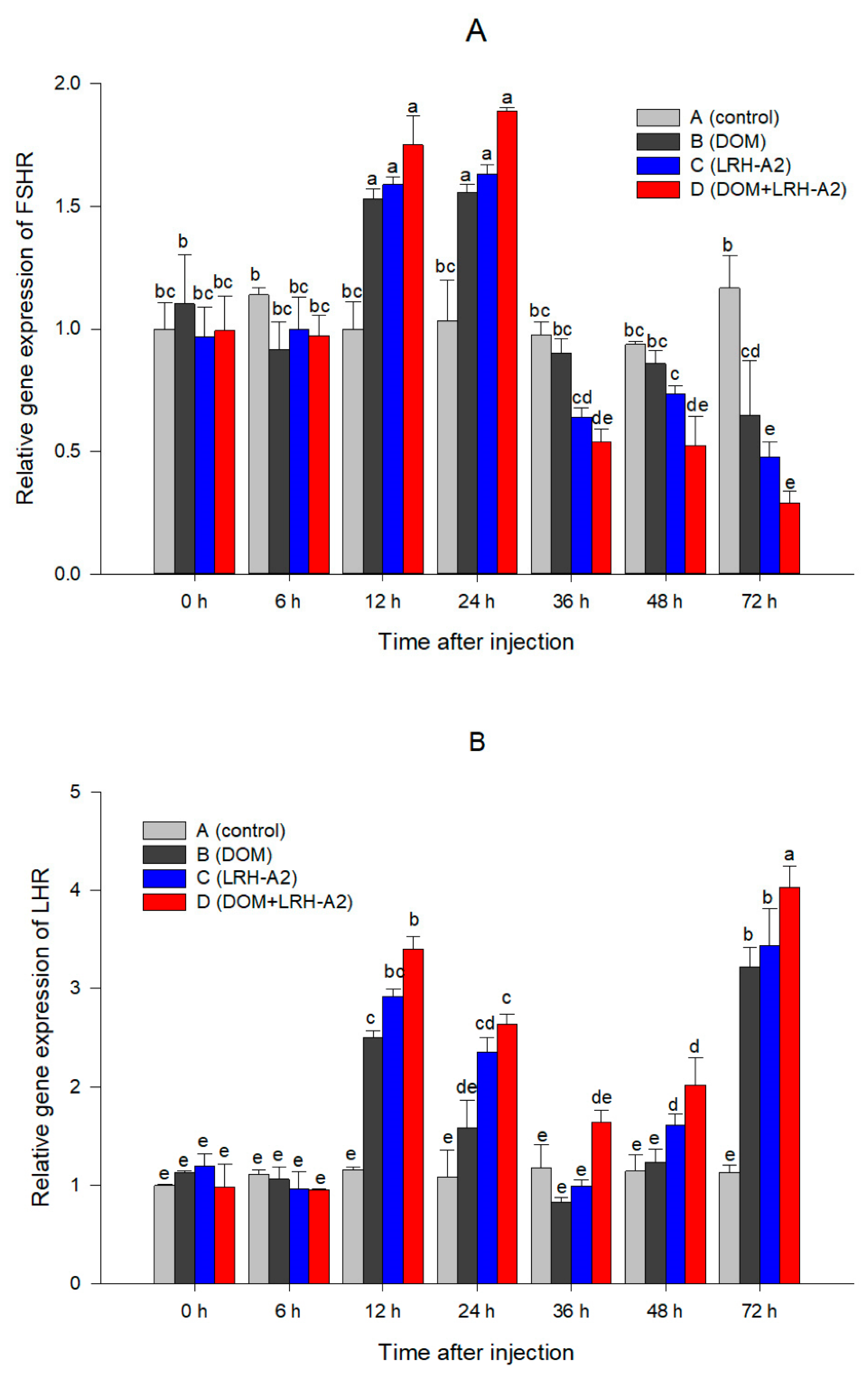
3.2. Oxytocin Injection and Variation of Serum Steroid Hormone Level and GtHR Genes
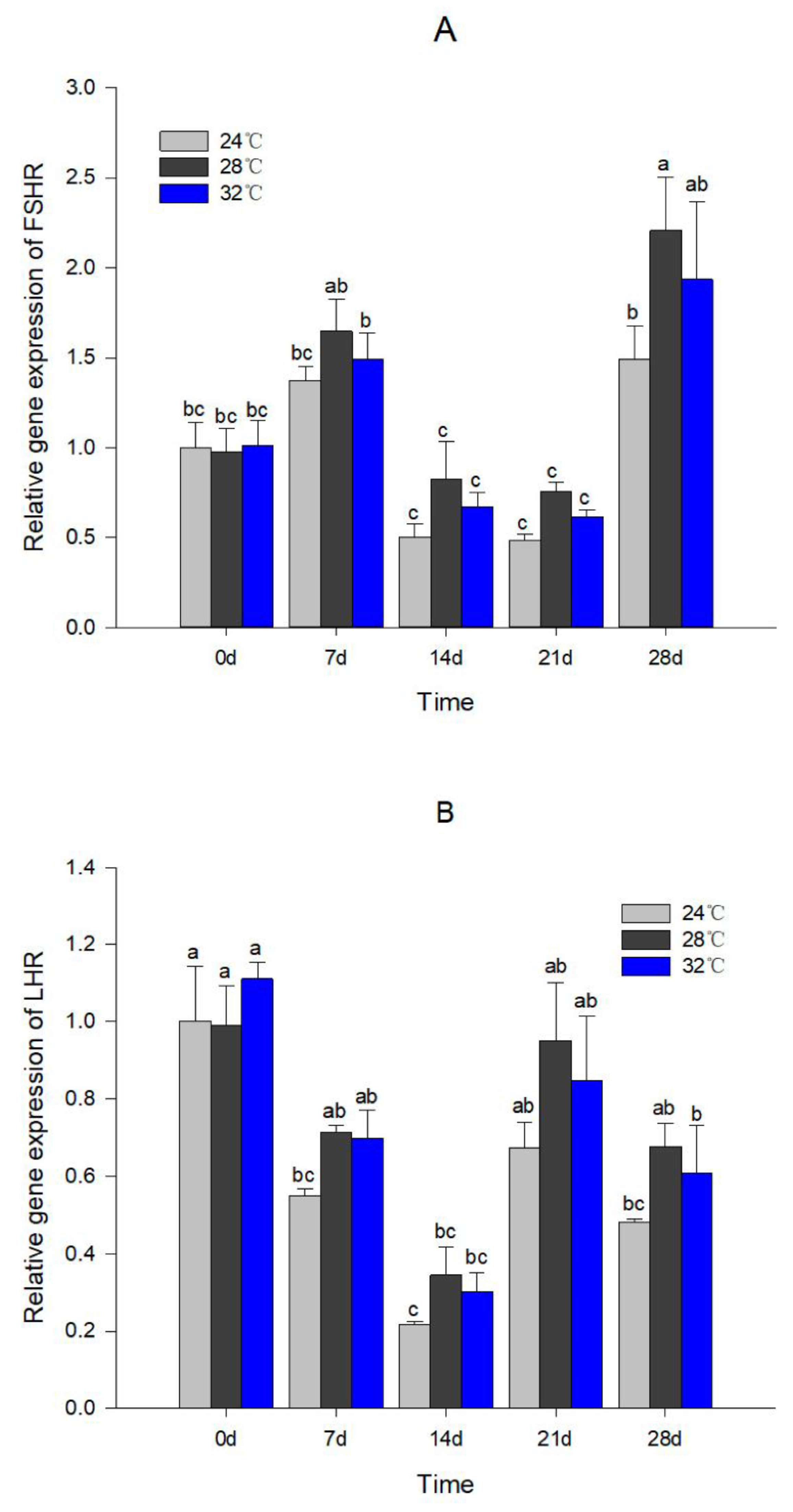
3.3. Different Temperature Treatments and Variations of Serum Steroid Hormone Level and GtHR Genes
4. Discussion
4.1. Variations of E2 and T Levels in Nile tilapia after Oxytocin Injection and Treatment at Different Temperatures
4.2. Effects of Oxytocin Injection and Different Temperature Treatments on the Expression of GtHR Genes in Nile tilapia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharib, S.D.; Wierman, M.E.; Shupnik, M.A.; Chin, W.W. Molecular Biology of the Pituitary Gonadotropins. Endocr. Rev. 1990, 11, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, B.; Ge, W. Genetic Analysis of Zebrafish Gonadotropin (FSH and LH) Functions by TALEN-Mediated Gene Disruption. Mol. Endocrinol. 2015, 29, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Kwok, H.-F.; So, W.-K.; Wang, Y.; Ge, W. Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone receptors—Evidence for their distinct functions in follicle development. Biol. Reprod. 2005, 72, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.D.; Lei, J.L. Molecular function of gonadotrophins and their receptors in the ovarian development of turbot (Scophthalmus maximus). Gen. Comp. Endocrinol. 2019, 277, 17–19. [Google Scholar] [CrossRef]
- Candelma, M.; Dalla Valle, L.; Colella, S.; Santojanni, A.; Carnevali, O. Cloning, characterization, and molecular expression of gonadotropin receptors in European hake (Merluccius merluccius), a multiple-spawning species. Fish Physiol. Biochem. 2018, 44, 895–910. [Google Scholar] [CrossRef]
- Levavi-Sivan, B.; Bogerd, J.; Mananos, E.L.; Gomez, A.; Lareyre, J.J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef]
- So, W.K.; Kwok, H.F.; Ge, W. Zebrafish gonadotropins and their receptors: II. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone subunits their spatial-temporal expression patterns and receptor specificity. Biol. Reprod. 2005, 72, 1382–1396. [Google Scholar] [CrossRef]
- Kobayashi, T.; Andersen, O. The gonadotropin receptors FSH-R and LH-R of Atlantic halibut (Hippoglossus hippoglossus), 1: Isolation of multiple transcripts encoding full-length and truncated variants of FSH-R. Gen. Comp. Endocrinol. 2008, 156, 584–594. [Google Scholar] [CrossRef]
- Sambroni, E.; Gac, F.L.; Breton, B.; Lareyre, J.J. Functional specificity of the rainbow trout (Oncorhynchus mykiss) gonadotropin receptors as assayed in a mammalian cell line. J. Endocrinol. 2007, 195, 213–228. [Google Scholar] [CrossRef][Green Version]
- Maugars, G.; Schmitz, M. Molecular cloning and characterization of FSH and LH receptors in Atlantic salmon (Salmo salar L.). Gen. Comp. Endocrinol. 2006, 149, 108–117. [Google Scholar] [CrossRef]
- Kusakabe, M.; Nakamura, I.; Evans, J.; Swanson, P.; Young, G. Changes in mRNAs encoding steroidogenic acute regulatory protein, steroidogenic enzymes and receptors for gonadotropins during spermatogenesis in rainbow trout testes. J. Endocrinol. 2006, 189, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Ijiri, S.; Trant, J.M. Molecular biology of channel catfish gonadotropin receptors: 1. Cloning of a functional luteinizing hormone receptor and preovulatory induction of gene expression. Biol. Reprod. 2001, 64, 1010–1018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitano, T.; Takenaka, T.; Takagi, H.; Yoshiura, Y.; Kazeto, Y.; Hirai, T.; Mukai, K.; Nozu, R. Roles of Gonadotropin Receptors in Sexual Development of Medaka. Cells 2022, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.B.; Liu, Q.W.; Zhou, Y.W.; Wang, C.Q.; Qin, H.; Zhao, C.; Liu, S.J. Differential expression of HPG-axis genes in autotetraploids derived from red crucian carp Carassius auratus red var., female x blunt snout bream Megalobrama amblycephala, male. J. Fish Biol. 2018, 93, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Fjelldal, P.G.; Hansen, T.; Huang, T.S. Continuous light and elevated temperature can trigger maturation both during and immediately after smoltification in male Atlantic salmon (Salmo salar). Aquaculture 2011, 321, 93–100. [Google Scholar] [CrossRef]
- Dadras, H.; Dzyuba, B.; Cosson, J.; Golpour, A.; Siddique, M.A.M.; Linhart, O. Effect of water temperature on the physiology of fish spermatozoon function: A brief review. Aquac. Res. 2017, 48, 729–740. [Google Scholar] [CrossRef]
- Mahanty, A.; Purohit, G.K.; Mohanty, S.; Mohanty, B.P. Heat stress-induced alterations in the expression of genes associated with gonadal integrity of the teleost Puntius sophore. Fish Physiol. Biochem. 2019, 45, 1409–1417. [Google Scholar] [CrossRef]
- Garcia-Lopez, A.; Couto, E.; Canario, A.V.M.; Sarasquete, C.; Martinez-Rodriguez, G. Ovarian development and plasma sex steroid levels in cultured female Senegalese sole Solea senegalensis. Comp. Biochem. Physiol. Part A-Mol. Integr. Physiol. 2007, 146, 342–354. [Google Scholar] [CrossRef]
- Li, G.L.; Liu, X.C.; Lin, H.R. Seasonal changes of serum sex steroids concentration and aromatase activity of gonad and brain in red-spotted grouper (Epinephelus akaara). Anim. Reprod. Sci. 2007, 99, 156–166. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. Aquaculture 2001, 197, 283–301. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H. Genetic basis of sex determination and application of sex control in fish: A review. J. Dalian Ocean. Univ. 2020, 35, 161–168. [Google Scholar] [CrossRef]
- Zhu, J.; Zou, Z.; Li, D.; Xiao, W.; Yu, J.; Chen, B.; Xue, L.; Yang, H. Transcriptome Profiling Revealed Basis for Growth Heterosis in Hybrid Tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Fishes 2022, 7, 43. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Sen, U.; Bhattacharyya, S.P.; Mukherjee, D. High performance liquid chromatographic separation of steroids from ovarian follicles of fresh water perch Anabas testudineus: Identification and characterization of the maturation-inducing hormone. J. Exp. Zool. 2002, 292, 565–572. [Google Scholar] [CrossRef]
- Comeau, L.A.; Campana, S.E.; Chouinard, G.A.; Hanson, J.M. Timing of Atlantic cod Gadus morhua seasonal migrations in relation to serum levels of gonadal and thyroidal hormones. Mar. Ecol. Prog. 2001, 221, 245–253. [Google Scholar] [CrossRef]
- Holland, M.C.; Hassin, S.; Zohar, Y. Gonadal development and plasma steroid levels during pubertal development in captive-reared striped bass, Morone saxatilis. J. Exp. Zool. 2000, 286, 49–63. [Google Scholar] [CrossRef]
- Kitano, H. Steroid hormones in fish reproduction. Nippon. Suisan Gakkaishi 2009, 75, 860–861. [Google Scholar] [CrossRef][Green Version]
- Tokarz, J.; Moller, G.; de Angelis, M.H.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef]
- Qin, Z.B.; Feng, J.; Sun, T.; He, Z.; Luo, B.; Pang, W. Change discioline of sex steroid hormoones in seum of loach (misgurnus anguillicaudayus) infected chorionic gonadotropin analogue (LHRHa). Acta Hydrobiol. Sin. 2009, 33, 1168–1174. [Google Scholar] [CrossRef]
- Sudo, R.; Tosaka, R.; Ijiri, S.; Adachi, S.; Suetake, H.; Suzuki, Y.; Horie, N.; Tanaka, S.; Aoyama, J.; Tsukamoto, K. Effect of temperature decrease on oocyte development, sex steroids, and gonadotropin beta-subunit mRNA expression levels in female Japanese eel Anguilla japonica. Fish. Sci. 2011, 77, 575–582. [Google Scholar] [CrossRef]
- Lin, H.R. Regulation mechanism of gonadotropin secretion in fish and a new efficient fish oxytocin. Life Sci. 1991, 003, 24–25. [Google Scholar]
- Qiang, L.; Lu, J.; Gao, Y.; Li, S.; Luo, J. The effect of temperature on gonad, embryonic development and survival rate of juvenile seahorses, Hippocampus kuda Bleeker. Aquaculture 2006, 254, 701–713. [Google Scholar]
- Rahman, M.A.; Ohta, K.; Yamaguchi, A.; Chuda, H.; Hirai, T.; Matsuyama, M. Gonadotropins, gonadotropin receptors and their expressions during sexual maturation in yellowtail, a carangid fish. Fish Physiol. Biochem. 2003, 28, 81–83. [Google Scholar] [CrossRef]
- Jia, Y.D.; Meng, Z.; Niu, H.X.; Hu, P.; Lei, J.L. Molecular cloning, characterization, and expression analysis of luteinizing hormone receptor gene in turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2014, 40, 1639–1650. [Google Scholar] [CrossRef]
- Chi, M.L.; Ni, M.; Li, J.F.; He, F.; Qian, K.; Zhang, P.; Chai, S.H.; Wen, H.S. Molecular cloning and characterization of gonadotropin subunits (GTHα, FSHβ and LHβ) and their regulation by hCG and GnRHa in Japanese sea bass (Lateolabrax japonicas) in vivo. Fish Physiol. Biochem. 2015, 41, 587–601. [Google Scholar] [CrossRef]
- Mu, W.J.; Wen, H.S.; He, F.; Li, J.F.; Liu, M.; Zhang, Y.Q.; Hu, J.; Qi, B.X. Cloning and expression analysis of follicle-stimulating hormone and luteinizing hormone receptor during the reproductive cycle in Korean rockfish (Sebastes schlegeli). Fish Physiol. Biochem. 2013, 39, 287–298. [Google Scholar] [CrossRef]
- Liu, M.; Wen, H.S.; He, F.; Li, J.F.; Ma, R.Q.; Hu, J.; Mu, W.J.; Zhang, Y.Q.; Qi, B.X. Cloning of LHR and expression analysis during the reproductive cycle in female yellow catfish (Pelteobagrus fulvidraco). J. Fish. China 2011, 35, 1770–1779. [Google Scholar]
- Zhang, G.; Wang, W.; Duan, Z.Y.; Gui, L.; Su, M.L.; Mu, X.J.; Zhang, J.B. Gene cloning of gonadotropic hormone receptors and their mRNA expressions at different development stages of the gonad in Scatophagus argus. J. Fish. China 2017, 41, 993–1005. [Google Scholar]
- Gen, K.; Yamaguchi, S.; Okuzawa, K.; Kumakura, N.; Tanaka, H.; Kagawa, H. Physiological roles of FSH and LH in red seabream, Pagrus major. Fish Physiol. Biochem. 2003, 28, 77–80. [Google Scholar] [CrossRef]

| Primer Name | Prime Sequence | GeneBank No. |
|---|---|---|
| RT-FSHR-F | 5′-TGTGACAACCCCGAGGCTAAAAATA-3′ | MW353713 |
| RT-FSHR-R | 5′-GGCGAGGACAGAGATGATCCAGAT-3′ | |
| RT-LHR-F | 5′-CTCTGAGGTCTCTCCCCCCTAATG-3′ | MW353714 |
| RT-LHR-R | 5′-AGGCACTTTCCCTTTGTTTCCG-3′ | |
| β-actin-F | 5′-GTTGCCATCCAGGCTGTGCT-3′ | XM_003441527 |
| β-actin-R | 5′-TCTCGGCTGTGGTGGTGAAG-3′ |
| E2 Content (ng·mL−1) | Time after Injection (hour) | ||||||
|---|---|---|---|---|---|---|---|
| Group | 0 h | 6 h | 12 h | 24 h | 36 h | 48 h | 72 h |
| A | 6.4814 c | 6.2128 c | 6.5640 c | 6.5012 c | 6.4854 c | 6.5144 c | 6.4304 c |
| B | 6.3012 c | 6.2812 c | 6.1342 c | 7.6543 b | 6.9559 c | 6.4344 c | 6.2458 c |
| C | 6.3256 c | 6.3309 c | 6.2311 c | 8.7172 ab | 7.7369 b | 6.9167 c | 6.6169 c |
| D | 6.2927 c | 605665 c | 6.9203 bc | 9.4563 a | 8.6621 a | 7.5422 bc | 7.0136 c |
| T Content (nmol·mL−1) | Time after Injection (hour) | ||||||
|---|---|---|---|---|---|---|---|
| Group | 0 h | 6 h | 12 h | 24 h | 36 h | 48 h | 72 h |
| A | 15.8679 c | 16.3434 c | 16.8554 c | 16.5557 c | 16.1926 c | 15.8144 c | 17.3772 c |
| B | 16.2301 c | 16.5360 c | 17.2495 c | 22.1971 ab | 19.4445 bc | 18.7310 bc | 17.9607 c |
| C | 16.8832 c | 16.5583 c | 18.4922 c | 23.4629 a | 20.1876 bc | 19.1990 bc | 18.0240 c |
| D | 17.1265 c | 16.3253 c | 19.0393 c | 24.7363 a | 21.2823 b | 20.0361 b | 18.8451 c |
| E2 Content (pmol·mL−1) | Time Point (day) | ||||
|---|---|---|---|---|---|
| Group | 0 d | 7 d | 14 d | 21 d | 28 d |
| 24 °C | 18.4712 d | 26.0892 c | 28.7227 c | 42.2353 b | 53.7043 b |
| 28 °C | 18.7891 d | 31.4140 c | 34.2748 c | 50.5566 a | 56.1360 a |
| 32 °C | 18.4713 d | 29.2065 c | 31.3694 c | 48.8420 ab | 55.0063 a |
| T Content (pg·mL−1) | Time Point (day) | ||||
|---|---|---|---|---|---|
| Group | 0 d | 7 d | 14 d | 21 d | 28 d |
| 24 °C | 99.0988 d | 105.4493 d | 115.4272 cd | 132.1870 c | 169.0778 b |
| 28 °C | 99.3287 d | 118.3062 cd | 151.9149 b | 192.5650 a | 195.8710 a |
| 32 °C | 99.1389 d | 115.2353 cd | 130.5893 c | 166.1675 ab | 172.3760 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Li, D.; Zhu, J.; Zou, Z.; Xiao, W.; Chen, B.; Yang, H. Effects of Different Oxytocin and Temperature on Reproductive Activity in Nile tilapia (Oreochromis niloticus): Based on Sex Steroid Hormone and GtHR Gene Expression. Fishes 2022, 7, 316. https://doi.org/10.3390/fishes7060316
Yu J, Li D, Zhu J, Zou Z, Xiao W, Chen B, Yang H. Effects of Different Oxytocin and Temperature on Reproductive Activity in Nile tilapia (Oreochromis niloticus): Based on Sex Steroid Hormone and GtHR Gene Expression. Fishes. 2022; 7(6):316. https://doi.org/10.3390/fishes7060316
Chicago/Turabian StyleYu, Jie, Dayu Li, Jinglin Zhu, Zhiying Zou, Wei Xiao, Binglin Chen, and Hong Yang. 2022. "Effects of Different Oxytocin and Temperature on Reproductive Activity in Nile tilapia (Oreochromis niloticus): Based on Sex Steroid Hormone and GtHR Gene Expression" Fishes 7, no. 6: 316. https://doi.org/10.3390/fishes7060316
APA StyleYu, J., Li, D., Zhu, J., Zou, Z., Xiao, W., Chen, B., & Yang, H. (2022). Effects of Different Oxytocin and Temperature on Reproductive Activity in Nile tilapia (Oreochromis niloticus): Based on Sex Steroid Hormone and GtHR Gene Expression. Fishes, 7(6), 316. https://doi.org/10.3390/fishes7060316







