Shark Provisioning Influences the Gut Microbiota of the Black-Tip Reef Shark in French Polynesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Shark and Gut-Content Sampling
2.2. Microbiota Metabarcoding
2.2.1. DNA Extraction and Controls
2.2.2. Library Construction and Sequencing
2.2.3. Filtering, Validation, and Taxonomic Assignment of 16S Sequences
2.3. Diversity of BRS Microbiota
2.4. Indicator Species Analysis
3. Results
3.1. Metabarcoding Data
3.2. Diversity of Gut Microbiota
3.2.1. Gut Microbiota Alpha Diversity
3.2.2. Gut Microbiota Beta Diversity
3.2.3. Indicator Genus and KO Functions
4. Discussion
4.1. Cetobacterium, Photobacterium, and Vibrio Dominated BRS Gut Microbiota
4.2. Influence of Seasons on Gut Microbiota
4.3. Influence of Shark Feeding on Gut Microbiota
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef]
- Roff, G.; Brown, C.J.; Priest, M.A.; Mumby, P.J. Decline of coastal apex shark populations over the past half century. Commun. Biol. 2018, 1, 223. [Google Scholar] [CrossRef] [PubMed]
- Hammerschlag, N.; Williams, L.; Fallows, M.; Fallows, C. Disappearance of white sharks leads to the novel emergence of an allopatric apex predator, the sevengill shark. Sci. Rep. 2019, 9, 1908. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Chapman, D.D.; Heupel, M.; Simpfendorfer, C.A.; Heithaus, M.; Meekan, M.; Harvey, E.; Goetze, J.; Kiszka, J.; Bond, M.E. Global status and conservation potential of reef sharks. Nature 2020, 583, 801–806. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Hammerschlag, N. Global Shark Currency: The Distribution, frequency, and economic value of shark ecotourism. Curr. Issues Tour. 2011, 14, 797–812. [Google Scholar] [CrossRef]
- Cisneros-Montemayor, A.M.; Barnes-Mauthe, M.; Al-Abdulrazzak, D.; Navarro-Holm, E.; Sumaila, U.R. Global economic value of shark ecotourism: Implications for conservation. Oryx 2013, 47, 381–388. [Google Scholar] [CrossRef]
- Orams, M.B. Feeding wildlife as a tourism attraction: A review of issues and impacts. Tour. Manag. 2002, 23, 281–293. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The Gut microbiota of marine fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Guivier, E.; Pech, N.; Chappaz, R.; Gilles, A. Microbiota associated with the skin, gills, and gut of the fish parachondrostoma toxostoma from the rhône basin. Freshw. Biol. 2020, 65, 446–459. [Google Scholar] [CrossRef]
- Romero, J.; Navarrete, P. 16S RDNA-based analysis of dominant bacterial populations associated with early life stages of coho salmon (Oncorhynchus kisutch). Microb. Ecol. 2006, 51, 422–430. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Uchii, K.; Matsui, K.; Yonekura, R.; Tani, K.; Kenzaka, T.; Nasu, M.; Kawabata, Z. Genetic and physiological characterization of the intestinal bacterial microbiota of bluegill (Lepomis macrochirus) with three different feeding habits. Microb. Ecol. 2006, 51, 277–284. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.O.B.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
- Ye, L.; Amberg, J.; Chapman, D.; Gaikowski, M.; Liu, W.-T. Fish gut microbiota analysis differentiates physiology and behavior of invasive Asian carp and indigenous American fish. ISME J. 2014, 8, 541–551. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Gooneratne, R.; Lai, R.; Zeng, C.; Zhan, F.; Wang, W. The Gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci. Rep. 2016, 6, 24340. [Google Scholar] [CrossRef]
- Jones, J.; DiBattista, J.D.; Stat, M.; Bunce, M.; Boyce, M.C.; Fairclough, D.V.; Travers, M.J.; Huggett, M.J. The Microbiome of the gastrointestinal tract of a range-shifting marine herbivorous fish. Front. Microbiol. 2018, 9, 2000. [Google Scholar] [CrossRef]
- Dehler, C.E.; Secombes, C.J.; Martin, S.A. Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L.). Aquaculture 2017, 467, 149–157. [Google Scholar] [CrossRef]
- Giatsis, C.; Sipkema, D.; Smidt, H.; Heilig, H.; Benvenuti, G.; Verreth, J.; Verdegem, M. The Impact of rearing environment on the development of gut microbiota in tilapia larvae. Sci. Rep. 2015, 5, 18206. [Google Scholar] [CrossRef]
- Givens, C.E.; Ransom, B.; Bano, N.; Hollibaugh, J.T. Comparison of the gut microbiomes of 12 bony fish and 3 shark species. Mar. Ecol. Prog. Ser. 2015, 518, 209–223. [Google Scholar] [CrossRef]
- Johny, T.K.; Saidumohamed, B.E.; Sasidharan, R.S.; Bhat, S.G. Metabarcoding data of bacterial diversity of the deep sea shark, Centroscyllium fabricii. Data Brief 2018, 21, 1029–1032. [Google Scholar] [CrossRef]
- Sherrill-Mix, S.; McCormick, K.; Lauder, A.; Bailey, A.; Zimmerman, L.; Li, Y.; Django, J.-B.N.; Bertolani, P.; Colin, C.; Hart, J.A. Allometry and ecology of the bilaterian gut microbiome. mBio 2018, 9, e00319-18. [Google Scholar] [CrossRef]
- Doane, M.P.; Morris, M.M.; Papudeshi, B.; Allen, L.; Pande, D.; Haggerty, J.M.; Johri, S.; Turnlund, A.C.; Peterson, M.; Kacev, D. The Skin microbiome of elasmobranchs follows phylosymbiosis, but in teleost fishes, the microbiomes converge. Microbiome 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Galeano, A.M.; Lozano, J.D.; Vives, M. Description of the microbiota in epidermal mucus and skin of sharks (Ginglymostoma cirratum and Negaprion brevirostris) and one stingray (Hypanus americanus). PeerJ 2020, 8, e10240. [Google Scholar] [CrossRef] [PubMed]
- Pratte, Z.A.; Perry, C.; Dove, A.D.; Hoopes, L.A.; Ritchie, K.B.; Hueter, R.E.; Fischer, C.; Newton, A.L.; Stewart, F.J. Microbiome structure in large pelagic sharks with distinct feeding ecologies. Anim. Microb. 2022, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.C.; Papastamatiou, Y.; German, D.P. The Nutritional physiology of sharks. Rev. Fish Biol. Fish. 2017, 27, 561–585. [Google Scholar] [CrossRef]
- Corse, E.; Meglécz, E.; Archambaud, G.; Ardisson, M.; Martin, J.-F.; Tougard, C.; Chappaz, R.; Dubut, V. A From-benchtop-to-desktop workflow for validating hts data and for taxonomic identification in diet metabarcoding studies. Mol. Ecol. Resour. 2017, 17, e146–e159. [Google Scholar] [CrossRef]
- Monti, F.; Duriez, O.; Arnal, V.; Dominici, J.-M.; Sforzi, A.; Fusani, L.; Grémillet, D.; Montgelard, C. Being cosmopolitan: Evolutionary history and phylogeography of a specialized raptor, the osprey Pandion haliaetus. BMC Evolutionary Biology 2015, 15, 255. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Galan, M.; Pons, J.-B.; Tournayre, O.; Pierre, E.; Leuchtmann, M.; Pontier, D.; Charbonnel, N. Metabarcoding for the parallel identification of several hundred predators and their prey: Application to bat species diet analysis. Mol. Ecol. Resour. 2018, 18, 474–489. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU RRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef]
- González, A.; Dubut, V.; Corse, E.; Mekdad, R.; Dechatre, T.; Meglécz, E. VTAM: A Robust Pipeline for Validating Metabarcoding Data Using Internal Controls. Ecology 2020, preprint. [Google Scholar] [CrossRef]
- Robasky, K.; Lewis, N.E.; Church, G.M. The Role of replicates for error mitigation in next-generation sequencing. Nat. Rev. Genet. 2014, 15, 56–62. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: Prediction of habitat-specific functional profiles and functional redundancy based on 16S RRNA gene sequences. Environ. Microb. 2020, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.6.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The Vegan Package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H. LmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- MuMIn, B.K. Multi-Model Inference. R Package, Version 1.43.17; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Legendre, P.; Anderson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Roberts, D.W. Labdsv: Ordination and Multivariate Analysis for Ecology. R Package, Version 2.0-1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Maljković, A.; Côté, I.M. Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the caribbean reef shark. Biol. Conserv. 2011, 144, 859–865. [Google Scholar] [CrossRef]
- Grimes, D.; Brayton, P.; Colwell, R.; Gruber, S. Vibrios as autochthonous flora of neritic sharks. Syst. Appl. Microbiol. 1985, 6, 221–226. [Google Scholar] [CrossRef]
- Juste-Poinapen, N.; Yang, L.; Ferreira, M.; Poinapen, J.; Rico, C. Community profiling of the intestinal microbial community of juvenile hammerhead sharks (Sphyrna lewini) from the Rewa Delta, Fiji. Sci. Rep. 2019, 9, 7182. [Google Scholar] [CrossRef]
- Hovda, M.B.; Fontanillas, R.; McGurk, C.; Obach, A.; Rosnes, J.T. Seasonal variations in the intestinal microbiota of farmed atlantic salmon (Salmo salar L.). Aquac. Res. 2012, 43, 154–159. [Google Scholar] [CrossRef]
- Tarnecki, A.M.; Burgos, F.A.; Ray, C.L.; Arias, C.R. Fish intestinal microbiome: Diversity and symbiosis unravelled by metagenomics. J. Appl. Microbiol. 2017, 123, 2–17. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut microbiota and energy homeostasis in fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Semeniuk, C.A.; Speers-Roesch, B.; Rothley, K.D. Using fatty-acid profile analysis as an ecologic indicator in the management of tourist impacts on marine wildlife: A case of stingray-feeding in the caribbean. Environ. Manag. 2007, 40, 665–677. [Google Scholar] [CrossRef]
- Smith, C.C.; Snowberg, L.K.; Gregory Caporaso, J.; Knight, R.; Bolnick, D.I. Dietary input of microbes and host genetic variation shape among-population differences in stickleback gut microbiota. ISME J. 2015, 9, 2515–2526. [Google Scholar] [CrossRef]
- Bailey, M.T.; Coe, C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999, 35, 146–155. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.-M.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. Early life stress alters behavior, immunity, and microbiota in rats: Implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Graham, A.L.; Cooke, S.J. The Effects of noise disturbance from various recreational boating activities common to inland waters on the cardiac physiology of a freshwater fish, the largemouth bass (Micropterus salmoides). Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 1315–1324. [Google Scholar] [CrossRef]
- Lima, A.C.; Assis, J.; Sayanda, D.; Sabino, J.; Oliveira, R.F. Impact of ecotourism on the fish fauna of Bonito region (Mato Grosso Do Sul state, Brazil): Ecological, behavioural and physiological measures. Neotrop. Ichthyol. 2014, 12, 133–143. [Google Scholar] [CrossRef]
- Brooks, E.J.; Sloman, K.A.; Liss, S.; Hassan-Hassanein, L.; Danylchuk, A.J.; Cooke, S.J.; Mandelman, J.W.; Skomal, G.B.; Sims, D.W.; Suski, C.D. The Stress physiology of extended duration tonic immobility in the juvenile lemon shark, Negaprion brevirostris (Poey 1868). J. Exp. Mar. Biol. Ecol. 2011, 409, 351–360. [Google Scholar] [CrossRef]
- Goñi, N.; Arrizabalaga, H. Seasonal and interannual variability of fat content of juvenile albacore (Thunnus alalunga) and bluefin (Thunnus thynnus) tunas during their feeding migration to the Bay of Biscay. Prog. Oceanogr. 2010, 86, 115–123. [Google Scholar] [CrossRef]
- Navarro-Barrón, E.; Hernández, C.; Llera-Herrera, R.; García-Gasca, A.; Gómez-Gil, B. Overfeeding a high-fat diet promotes sex-specific alterations on the gut microbiota of the zebrafish (Danio rerio). Zebrafish 2019, 16, 268–279. [Google Scholar] [CrossRef]
- Soriano, E.L.; Ramírez, D.T.; Araujo, D.R.; Gómez-Gil, B.; Castro, L.I.; Sánchez, C.G. Effect of temperature and dietary lipid proportion on gut microbiota in yellowtail kingfish Seriola lalandi Juveniles. Aquaculture 2018, 497, 269–277. [Google Scholar] [CrossRef]
- Gan, H.M.; Hudson, A.O.; Rahman, A.Y.A.; Chan, K.G.; Savka, M.A. Comparative genomic analysis of six bacteria belonging to the genus Novosphingobium: Insights into marine adaptation, cell-cell signaling and bioremediation. BMC Genom. 2013, 14, 431. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Wang, J.; Linnenbrink, M.; Künzel, S.; Fernandes, R.; Nadeau, M.-J.; Rosenstiel, P.; Baines, J.F. Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc. Natl. Acad. Sci. USA 2014, 111, E2703–E2710. [Google Scholar] [CrossRef]
- Carmody, R.N.; Gerber, G.K.; Luevano, J.M., Jr.; Gatti, D.M.; Somes, L.; Svenson, K.L.; Turnbaugh, P.J. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015, 17, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.C.; Robinson, A.K.; Rodríguez-Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [Google Scholar] [CrossRef]
- Guerinot, M.L. Microbial iron transport. Annu. Rev. Microbiol. 1994, 48, 743–773. [Google Scholar] [CrossRef] [PubMed]
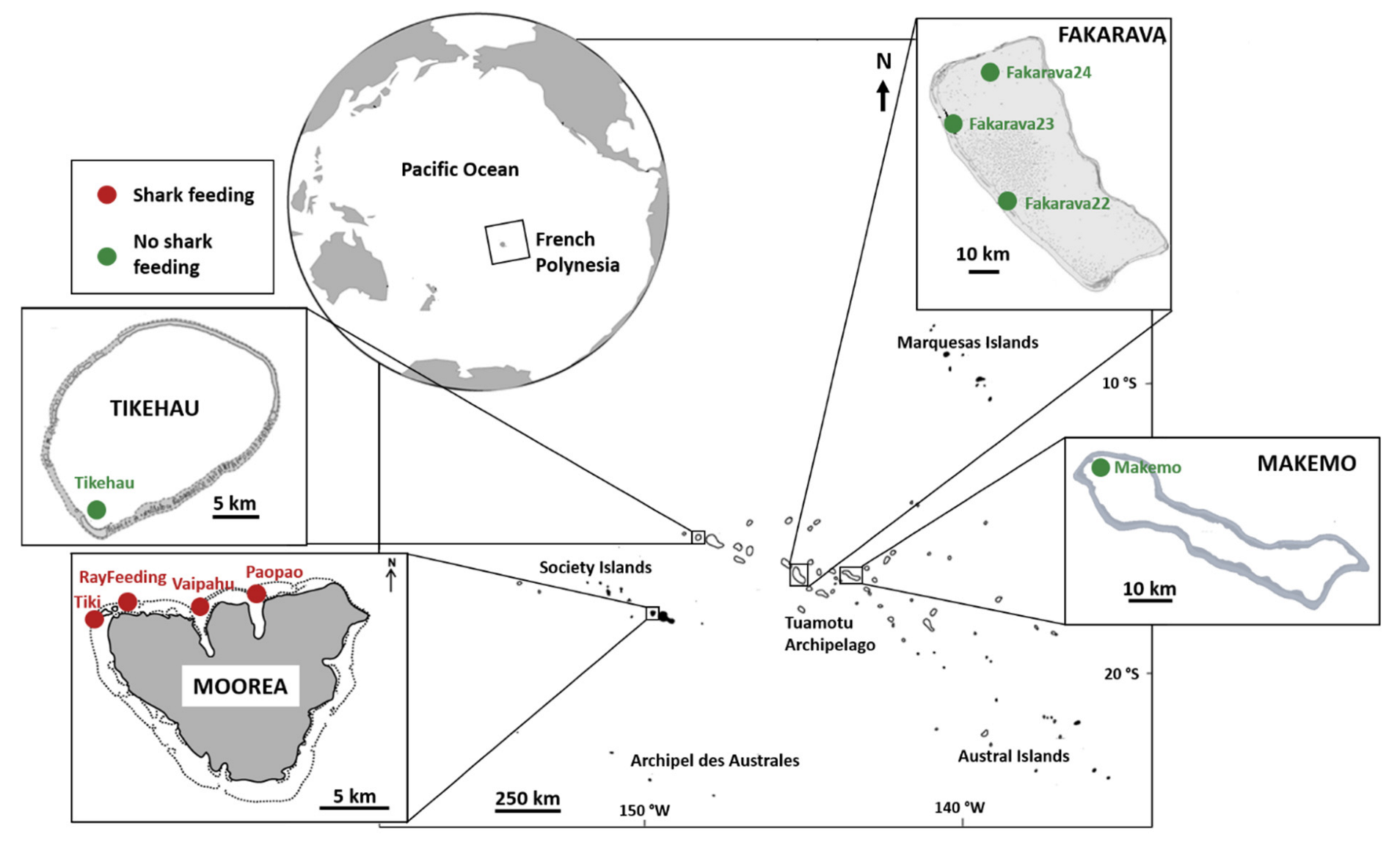
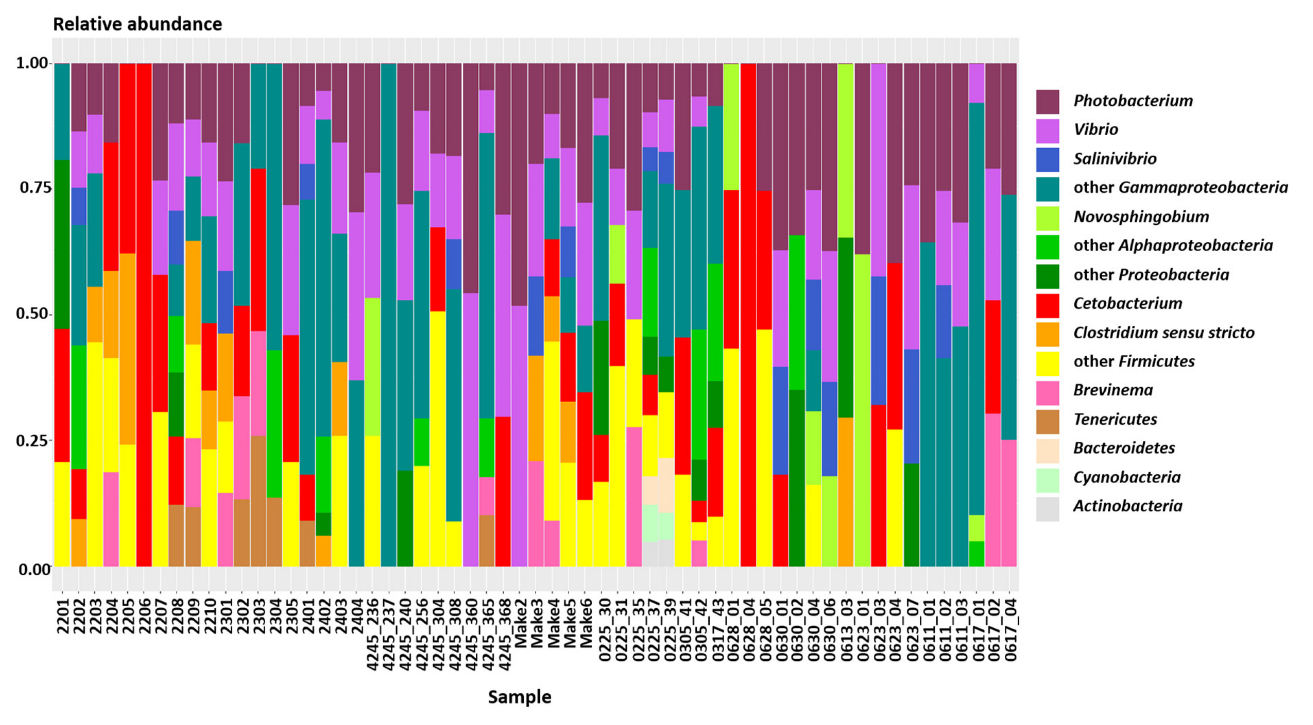
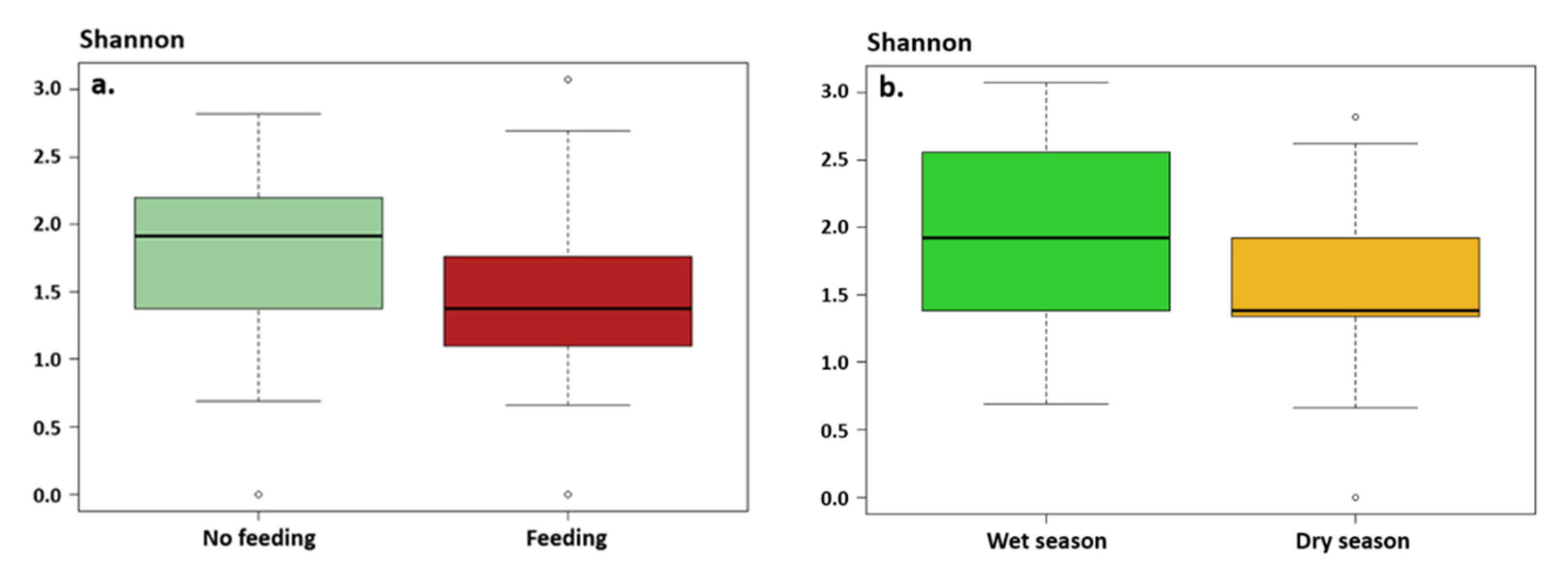
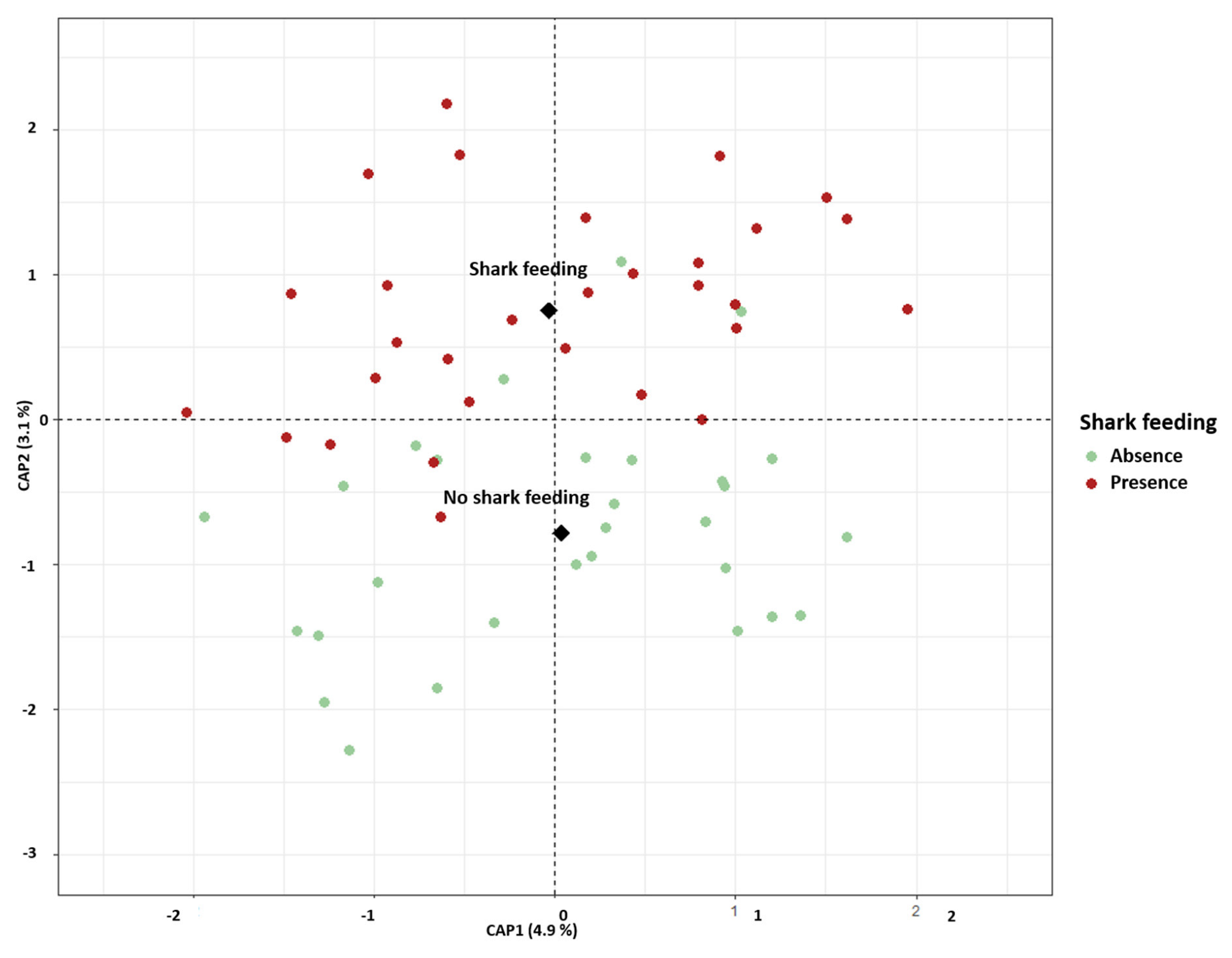
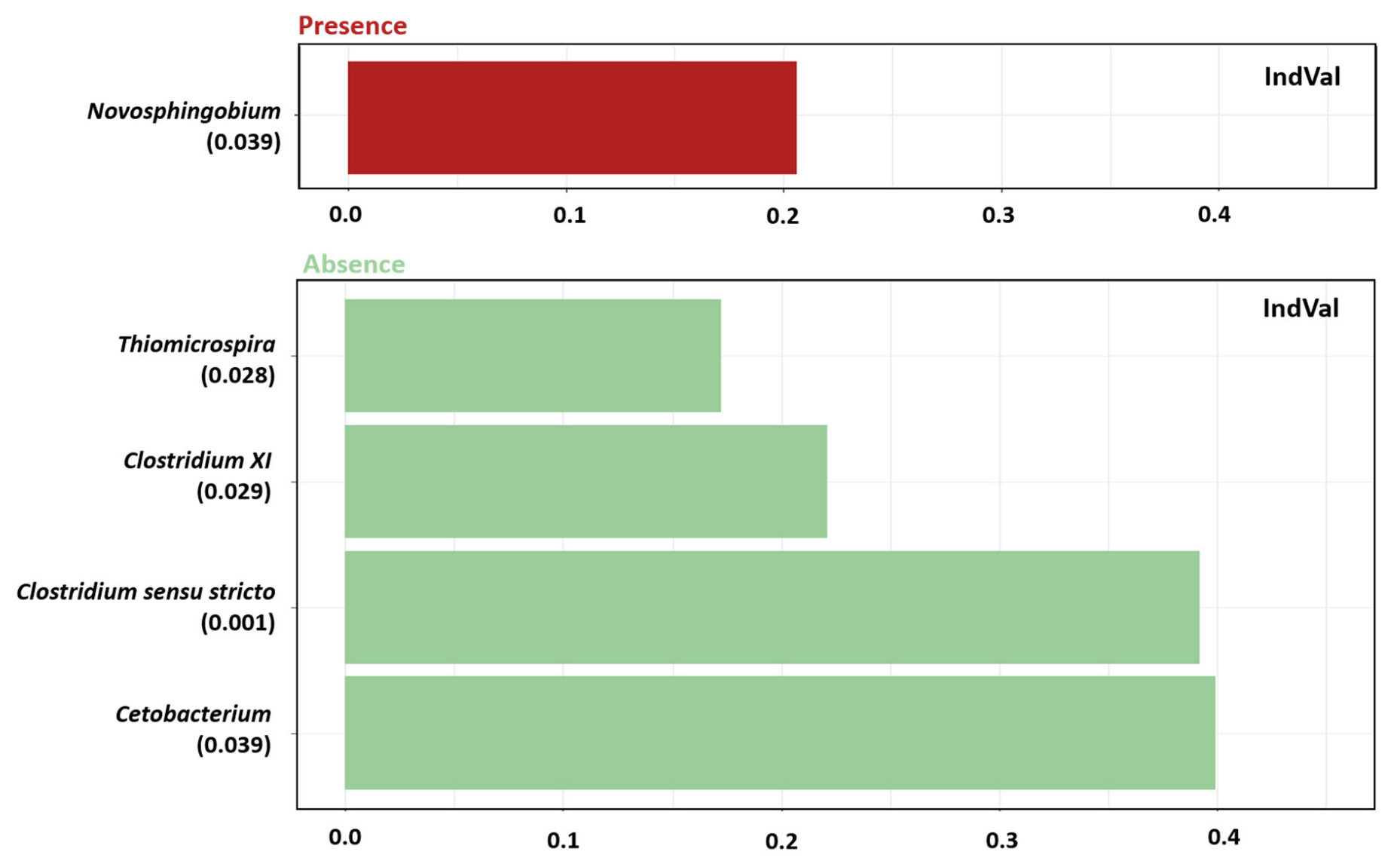
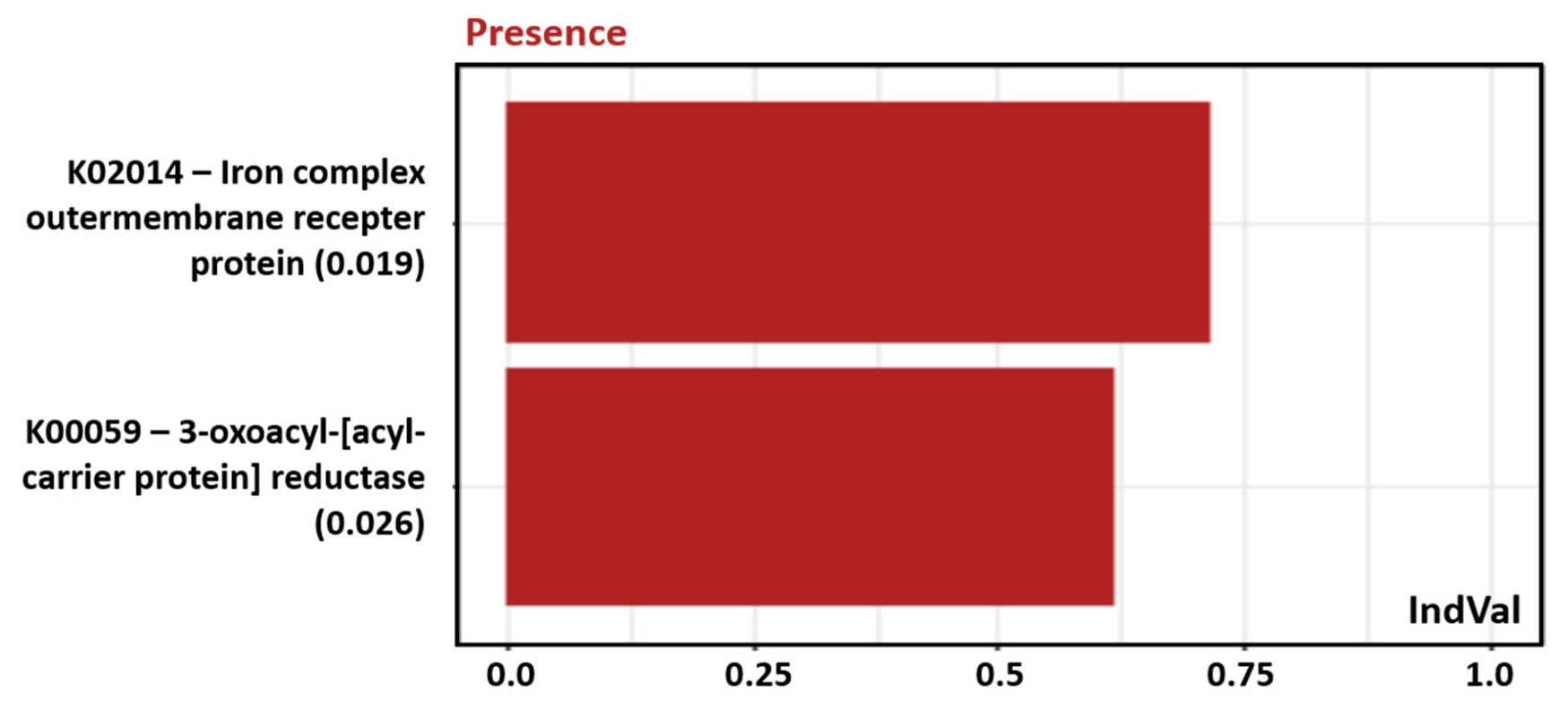
| Phylum | Class | Genus | Mean% ± sd |
|---|---|---|---|
| Proteobacteria | Gammaproteobacteria | Photobacterium | 17.6 ± 13.1 |
| Vibrio | 13.5 ± 13.4 | ||
| Salinivibrio | 3.4 ± 6.7 | ||
| Psychrobacter | 2.6 ± 6.1 | ||
| Acinetobacter | 2.5 ± 5.6 | ||
| Halomonas | 2.5 ± 5.1 | ||
| Shewanella | 2.2 ± 5.2 | ||
| Pseudoalteromonas | 1.8 ± 4.5 | ||
| Thiomicrospira | 1.1± 3.9 | ||
| Pseudomonas | 1.1 ± 3.6 | ||
| Stenotrophomonas | 1.1 ± 4.2 | ||
| Other Gammaproteobacteria (<1%) | 0.1 ± 0.1 | ||
| Alphaproteobacteria | Novosphingobium | 3.4 ± 10.6 | |
| Other Alphaproteobacteria (<1%) | 0.0 ± 0.1 | ||
| Betaproteobacteria | Burkholderia | 1.8 ± 7.1 | |
| Other Betaproteobacteria (<1%) | 0.0 ± 0.0 | ||
| Epsilonproteobacteria | Campylobacter | 1.3 ± 5.3 | |
| Firmicutes | Clostridia | Clostridium sensu stricto | 3.7 ± 8.1 |
| Cellulosilyticum | 2.9 ± 7.5 | ||
| Clostridium XI | 2.6 ± 6.5 | ||
| Clostridium XIVb | 1.4 ± 5.0 | ||
| Other Clostridia | 0.0 ± 0.0 | ||
| Bacilli | Bacillus | 1.3 ± 6.3 | |
| Other bacilli | 0.0 ± 0.1 | ||
| Other Firmicutes (<1%) | 0.8 ± 2.9 | ||
| Fusobacteria | Fusobacteriia | Cetobacterium | 13.2 ± 20.3 |
| Spirochaetes | Spirochaetia | Brevinema | 3.6 ± 8.0 |
| Tenericutes | Mollicutes | Mycoplasma | 1.1 ± 4.3 |
| Other Mollicutes | 0.5 ± 2.3 | ||
| Other Bacteria (including Archaea) (<1%) | 0.0 ± 0.0 | ||
| Estimate | Sum of Squares | Degree of Freedom | F | p-Value | |
|---|---|---|---|---|---|
| Shannon | |||||
| Number of reads | 5.67 | 1 | 22.89 | 1.32 × 10−5 | |
| Season | 2.46 | 1 | 9.95 | 2.61 × 10−3 | |
| Provisioning | 5.67 | 1 | 5.31 | 0.03 | |
| Intercept | 1.48 | 55 | 1.02 × 10−8 | ||
| Number of reads | 6.3 × 10−3 | 55 | 1.33 × 10−5 | ||
| Season Dry | −0.47 | 55 | 2.61 × 10−3 | ||
| Provisioning presence | −0.31 | 55 | 0.03 | ||
| Sum of Squares | Degree of Freedom | F | p-Value | |
|---|---|---|---|---|
| Number of reads | 1.02 | 1 | 2.97 | 0.001 |
| Shark provisioning | 0.66 | 1 | 1.93 | 0.006 |
| Residual | 19.18 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, A.; Sasal, P.; Clua, É.; Meglécz, E.; Clerissi, C. Shark Provisioning Influences the Gut Microbiota of the Black-Tip Reef Shark in French Polynesia. Fishes 2022, 7, 312. https://doi.org/10.3390/fishes7060312
Esposito A, Sasal P, Clua É, Meglécz E, Clerissi C. Shark Provisioning Influences the Gut Microbiota of the Black-Tip Reef Shark in French Polynesia. Fishes. 2022; 7(6):312. https://doi.org/10.3390/fishes7060312
Chicago/Turabian StyleEsposito, Anaïs, Pierre Sasal, Éric Clua, Emese Meglécz, and Camille Clerissi. 2022. "Shark Provisioning Influences the Gut Microbiota of the Black-Tip Reef Shark in French Polynesia" Fishes 7, no. 6: 312. https://doi.org/10.3390/fishes7060312
APA StyleEsposito, A., Sasal, P., Clua, É., Meglécz, E., & Clerissi, C. (2022). Shark Provisioning Influences the Gut Microbiota of the Black-Tip Reef Shark in French Polynesia. Fishes, 7(6), 312. https://doi.org/10.3390/fishes7060312









