Identification and Characterization of microRNAs in the Gonads of Litopenaeus vannamei Using High-Throughput Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Shrimp and Sample Preparation
2.2. Small RNA cDNA Library Construction by High-Throughput Sequencing
2.3. Bioinformatics Analysis
2.4. Stem-Loop RT-qPCR
3. Results
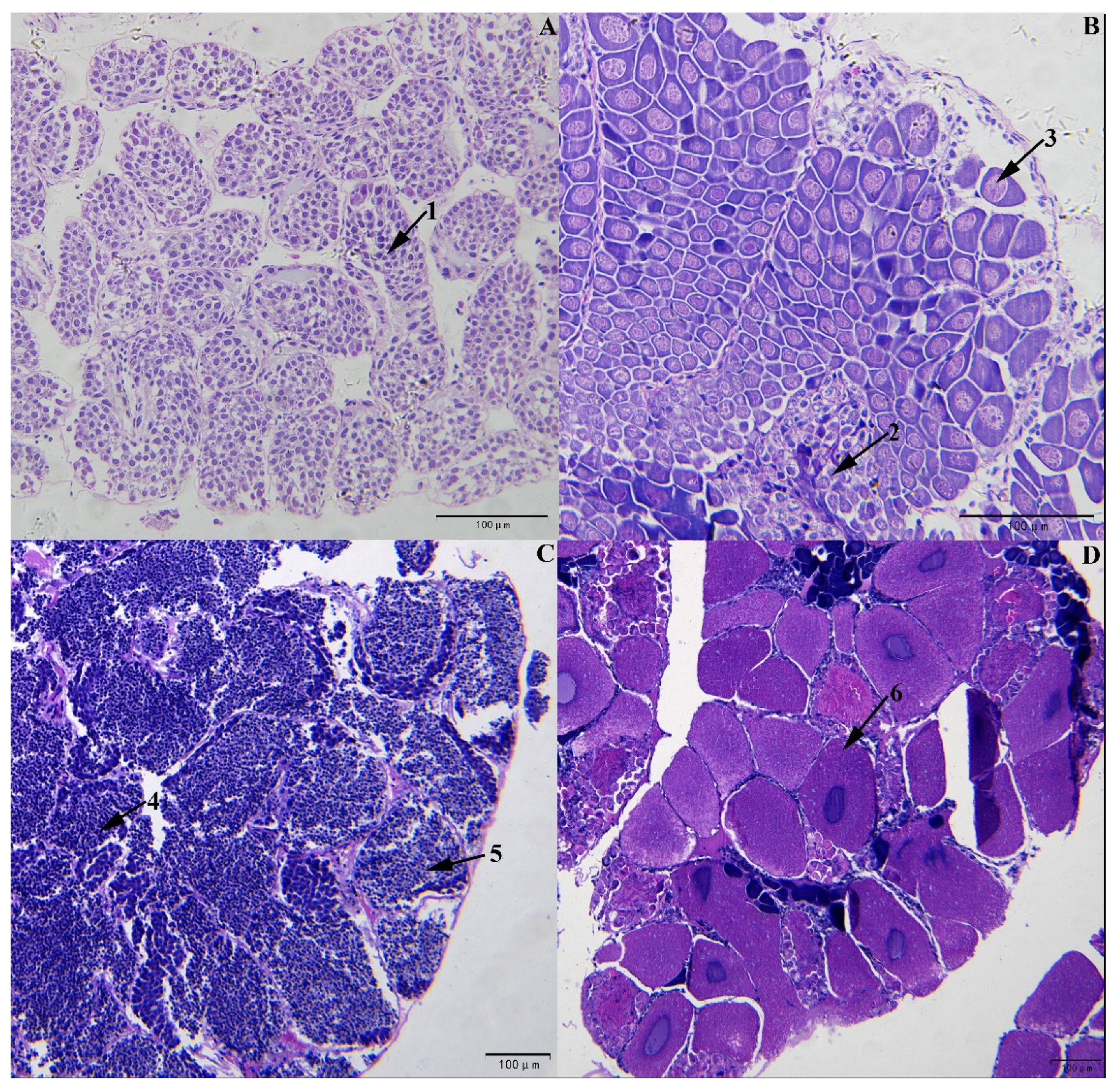
3.1. Determination of L. vannamei Sex and Development Stages Based on Gonad Sections
3.2. Summary of Sequencing Data in the Gonads of L. vannamei
3.3. Identification and Prediction of miRNAs in the L. vannamei Ovaries and Testes
3.4. Screening Differentially Expressed miRNAs in the Gonads of L. vannamei
3.5. The Differentially Expressed miRNAs Targets Prediction and Functional Annotation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, J.; Zhao, Y.; Gao, Y.; Xie, D.; Zhang, B.; Chen, X. Advances in breeding improved varieties of Litopenaeus vannamei. J. South. Agric. 2011, 42, 556–561. [Google Scholar]
- Guson, B.; Tanate, P.; Amornrat, P.; Warapond, W. Evaluation of the relationship between the 14-3-3ε protein and LvRab11 in the shrimp Litopenaeus vannamei during WSSV infection. Sci. Rep. 2021, 11, 19188. [Google Scholar]
- Cheng, A.; Shiu, Y.; Chiu, S.; Ballantyne, R.; Liu, C. Effects of chitin from Daphnia similis and its derivative, chitosan on the immune response and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish. Immunol. 2021, 119, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, C.; Wang, B.; Qin, H.; Zhang, S. Comparative transcriptome analysis of Litopenaeus vannamei reveals that triosephosphate isomerase-like genes play an important role during decapod iridescent virus 1 infection. Front. Immunol. 2020, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Liu, Q.H.; Wu, Y.; Huang, J. Litopenaeus vannamei clathrin coat AP17 involved in white spot syndrome virus infection. Fish Shellfish. Immunol. 2016, 52, 309–316. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Zhang, Q.; Luo, Z.; Li, F. The Polymorphism of LvMMD2 and Its Association with Growth Traits in Litopenaeus vannamei. Mar. Biotechnol. 2020, 22, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Luo, K.; Sheng, L.; Jie, K.; Chen, G. Evaluation of growth performance in Litopenaeus vannamei populations introduced from other. J. Fish. China 2013, 37, 34–42. [Google Scholar] [CrossRef]
- Wang, Z. The healthy culture techniques of the first generation of SPF Litopenaeus vannamei in earth pond. J. Fish. Res. 2017, 39, 245. [Google Scholar]
- Valencia-Castañeda, G.; Millán-Almaraz, M.I.; Fierro-Sañudo, J.F.; Fregoso-López, M.G.; Páez-Osuna, F. Monitoring of inland waters for culturing shrimp Litopenaeus vannamei: Application of a method based on survival and chemical composition. Environ. Monit. Assess. 2017, 189, 395. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.A.; Schach, U.; Ordaz, A.; Steinfeld, J.S.; Draper, B.W.; Siegfried, K.R. Dmrt1 is necessary for male sexual development in zebrafish. Dev. Biol. 2017, 422, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Gui, J.F. Diverse and variable sex determination mechanisms in vertebrates. Sci. China Life Sci. 2018, 61, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Gui, J.F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Del, R.P.; Pavneesh, M. Does miRNA Expression in the Spent Media Change during Early Embryo Development. Front. Vet. Sci. 2021, 8, 658968. [Google Scholar]
- Yong, C.; Jing, Z.; Ying, L.; Hong, G.; Wei, L. Inhibitory effects of miRNA-200c on chemotherapy-resistance and cell proliferation of gastric cancer SGC7901/DDP cells. Chin. J. Cancer 2010, 29, 1006–1011. [Google Scholar]
- Zhang, J.; Luo, H.; Xiong, Z.; Wan, K.; He, H. High-throughput sequencing reveals biofluid exosomal miRNAs associated with immunity in pigs. Biosci. Biotechnol. Biochem. 2019, 84, 53–62. [Google Scholar] [CrossRef]
- Sun, X.; Xu, L.; Wang, Y.; Yu, R.; Zhu, X.; Luo, X.; Gong, Y.; Wang, R.; Limera, C.; Zhang, K.; et al. Identification of novel and salt-responsive miRNAs to explore miRNA-mediated regulatory network of salt stress response in radish (Raphanus sativus L.). BMC Genom. 2015, 16, 197. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Duan, S.H.; Wang, G.L.; Li, J.L. Integrated mRNA and miRNA expression profile analysis of female and male gonads in Hyriopsis cumingii. Sci. Rep. 2021, 11, 665. [Google Scholar] [CrossRef]
- Li, S.; Lin, G.; Fang, W.; Gao, D.; Huang, J.; Xie, J.; Lu, J. Identification and Comparison of microRNAs in the Gonad of the Yellowfin Seabream (Acanthopagrus latus). Int. J. Mol. Sci. 2020, 21, 5690. [Google Scholar] [CrossRef]
- Wei, P.Y.; He, P.P.; Zhang, X.Z.; Li, W.; Zhang, L.; Guan, J.L.; Chen, X.H.; Lin, Y.; Zhuo, X.F.; Li, Q.; et al. Identification and characterization of microRNAs in the gonads of Crassostrea hongkongensis using high-throughput sequencing. Comp. Biochem. Physiol.—Part D Genom. Proteom. 2019, 31, 100606. [Google Scholar] [CrossRef]
- He, P.P.; Wei, P.Y.; Chen, X.H.; Lin, Y.; Peng, J.X. Identification and characterization of microRNAs in the gonad of Trachinotus ovatus using Solexa sequencing. Comp. Biochem. Physiol.—Part D Genom. Proteom. 2019, 30, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.W.; Zhu, Y.F.; Wu, Y.; Yuan, C.C.; Chen, K.; Li, M.Y. Identification and expression analysis of microRNAs in medaka gonads. Gene 2018, 646, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Q.W.; Zhao, W.J.; Du, Q.Y.; Chang, Z.J. Effects of short-time exposure to atrazine on miRNA expression profiles in the gonad of common carp (Cyprinus carpio). BMC Genom. 2019, 20, 587. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Fan, S.; Qiu, L. Identification of MicroRNAs and Their Target Genes Associated with Ovarian Development in Black Tiger Shrimp (Penaeus monodon) Using High-Throughput Sequencing. Sci. Rep. 2018, 8, 11602. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, B.Y.; Feng, J.B.; Zhou, L.X.; Qiu, G.F. Identification and profiling of microRNAs during gonadal development in the giant freshwater prawn Macrobrachium rosenbergii. Sci. Rep. 2019, 9, 2406. [Google Scholar] [CrossRef] [PubMed]
- Chong, T.; Yeming, X.; Mei, G.; Wei, Y. AASRA: An anchor alignment-based small RNA annotation pipeline†. Biol. Reprod. 2021, 105, 267–277. [Google Scholar]
- Friedlnder, M.R.; Wei, C.; Adamidi, C.; Maaskola, J.; Rajewsky, N. Discovering MicroRNAs from deep sequencing data using MiRDeep. Nat. Biotechnol. 2008, 26, 407–415. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. MiRanda application: Human microRNA targets. PLoS Biol. 2005, 2, e363. [Google Scholar]
- Vikram, A.; Bell, G.W.; Jin, W.N.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar]
- Mao, X.; Tao, C.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Kang, X.J.; Wang, S.A.; Du, N.S.; Lai, W. Studies on Cytology Spermatogensis and Fertilization of the Marine Prawn, Penaeus chinensis(Osbeck 1765). J. Hebei Univ. 1998, 18, 399–401. [Google Scholar]
- Jiang, Y.H.; Yan, S.F. Histological Studies on the Oogenesis of Penaeus vannamei. Chin. J. Zool. 2004, 39, 59–62. [Google Scholar]
- Chen, D.M. Ontogenetic Development of Gonads and Transcriptome Analysis of the Peppermint Shrimp, Lysmata vittata. Master’s Thesis, Xiamen University, Xiamen, China, May 2019. [Google Scholar]
- Wu, J.W.; Bao, J.Q.; Wang, L.; Hu, Y.Q.; Chen, X. MicroRNA-184 downregulates nuclear receptor corepressor 2 in mouse spermatogenesis. BMC Dev. Biol. 2011, 11, 64. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, X.; Li, J.; Liu, P.J.G. Identification and comparative profiling of ovarian and testicular microRNAs in the swimming crab Portunus trituberculatus. Gene 2018, 640, 6–13. [Google Scholar] [CrossRef]
- Lan, T.; Chen, Y.L.; Gul, Y.; Zhao, B.W.; Gao, Z.X. Comparative expression analysis of let-7 microRNAs during ovary development in Megalobrama amblycephala. Fish Physiol. Biochem. 2019, 45, 1101–1115. [Google Scholar] [CrossRef]
- Shi, L.L. The Isolation of miRNAs from Chinese Mitten Crab (Eriocheir sinensis) and Analysis of Their Expression during the Oocyte Maturation. Master’s Thesis, Shanghai Ocean University, Shanghai, China, November 2011. [Google Scholar]
- Jiang, S.W.; Dai, Z.H.; Chen, L.B.; Xu, Q.H. Screening and identification of highly expressed miRNAs in amphioxus Branchiostoma japonicum testis tissues. J. Shanghai Ocean. Univ. 2012, 21, 671–678. [Google Scholar]
- Li, T.T.; Liu, X.Q.; Gong, X.E.Q.; Zhang, X.Q.; Zhang, X.S. MicroRNA 92b-3p regulates primordial follicle assembly by targeting TSC1 in neonatal mouse ovaries. Cell Cycle 2019, 18, 824–833. [Google Scholar] [CrossRef]
- Xie, L.; Peng, F.L.; Zhai, X.X.; Liu, B.Y. miRNA-133b Promotes the Sertoli Cells Proliferation and Spermatogenesis through TargetingGLI3. Genom. Appl. Biol. 2017, 36, 4469–4474. [Google Scholar]
- Ma, Z.S.; Yang, J.; Zhang, Q.Q.; Xu, C.M.; Wei, J.; Sun, L.; Wang, D.S.; Tao, W.J. miR-133b targets tagln2 and functions in tilapia oogenesis. Comp. Biochem. Physiol.—Part B Biochem. Mol. Biol. 2021, 256, 110637. [Google Scholar] [CrossRef]
- Song, Y.N.; Shi, L.L.; Liu, Z.Q.; Qiu, G.F. Global analysis of the ovarian microRNA transcriptome: Implication for miR-2 and miR-133 regulation of oocyte meiosis in the Chinese mitten crab, Eriocheir sinensis (Crustacea:Decapoda). BMC Genom. 2014, 15, 547. [Google Scholar] [CrossRef]
- Macedo, L.M.; Nunes, F.M.; Freitas, F.C.; Pires, C.V.; Tanaka, E.D.; Martins, J.R.; Piulachs, M.D.; Cristino, A.S.; Pinheiro, D.G.; Simões, Z.L. MicroRNA signatures characterizing caste-independent ovarian activity in queen and worker honeybees (Apis mellifera L.). Insects 2016, 25, 216–226. [Google Scholar]
- Zhou, M.C.; Jia, X.W.; Wan, H.F.; Wang, S.H.; Zhang, X.; Zhang, Z.P.; Wang, Y.L. miR-9 and miR-263 Regulate the Key Genes of the ERK Pathway in the Ovary of Mud Crab Scylla paramamosain. Mar. Biotechnol. 2020, 22, 594–606. [Google Scholar] [CrossRef]
- Monahan, A.J.; Starz-Gaiano, M. Apontic regulates somatic stem cell numbers in Drosophila testes. BMC Dev. Biol. 2016, 16, 5. [Google Scholar] [CrossRef]
- Roush, S.; Slack, F.J. The let-7 family of microRNAs. Trends Cell Biol. 2008, 18, 505–516. [Google Scholar] [CrossRef]
- Sun, T.T.; Xiao, C.; Deng, J.; Yang, Z.L.; Zou, L.Q.; Du, W.Y.; Li, S.X.; Huo, X.Q.; Zeng, L.H.; Yang, X.R. Transcriptome analysis reveals key genes and pathways associated with egg production in Nandan-Yao domestic chicken. Comp. Biochem. Physiol.—Part D Genom. Proteom. 2021, 40, 100889. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, J.L.; Meng, S.L.; Chen, J.Z.; Gu, Z.M. Testicular transcriptome alterations in zebrafish (Danio rerio) exposure to 17β-estradiol. Chemosphere 2019, 218, 14–25. [Google Scholar] [CrossRef]
- Xu, R.Y.; Pan, L.Q.; Yang, Y.Y.; Zhou, Y.Y. Characterizing transcriptome in female scallop Chlamys farreri provides new insights into the molecular mechanisms of reproductive regulation during ovarian development and spawn. Gene 2020, 758, 144967. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, C.; Zhao, Y. Hedgehog in the Drosophila testis niche: What does it do there? Protein Cell 2013, 4, 650–655. [Google Scholar] [CrossRef]
- Franco, H.L.; Yao, H.H. Sex and hedgehog: Roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res. 2012, 20, 247–258. [Google Scholar] [CrossRef]
- Russell, M.C.; Cowan, R.G.; Harman, R.M.; Walker, A.L.; Quirk, S.M. The Hedgehog Signaling Pathway in the Mouse Ovary. Biol. Reprod. 2007, 77, 226–236. [Google Scholar] [CrossRef]
- Asiabi, P.; David, C.; Camboni, A.; Marbaix, E.; Dolmans, M.M.; Amorim, C.A.O. New insights into the GDF9-Hedgehog-GLI signaling pathway in human ovaries: From fetus to postmenopause. Assist. Reprod. Genet. 2021, 38, 1387–1403. [Google Scholar] [CrossRef]
- Liu, M.; Pan, J.; Dong, Z.; Cheng, Y.; Gon, J.; Wu, X.A.O. Comparative transcriptome reveals the potential modulation mechanisms of estradiol affecting ovarian development of female Portunus trituberculatus. PLoS ONE 2019, 14, e0226698. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Sun, X.W.; Guo, F.Y.; Zhao, Y.J.; Zhang, J.H.; Zhao, Z.Q. Transcriptome analysis of the uniparous and multiparous goats ovaries. Reprod. Domest. Anim. 2016, 51, 877–885. [Google Scholar] [CrossRef]
- Guo, Y.P.; Li, E.Z.; Zhang, Y.J.; Wang, A.L. Aerobic exercise improves spermatogenesis of male rats: Results of iTRAQ-based proteomic analysis of the testis tissue. Natl. J. Androl. 2017, 23, 776–781. [Google Scholar]
- Cai, J.; Li, L.; Song, L.Y.; Xie, L.; Luo, F.; Sun, S.H.; Chakraborty, T.; Zhou, L.Y.; Wang, D.S. Effects of long term antiprogestine mifepristone (RU486) exposure on sexually dimorphic lncRNA expression and gonadal masculinization in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2019, 215, 105289. [Google Scholar] [CrossRef]

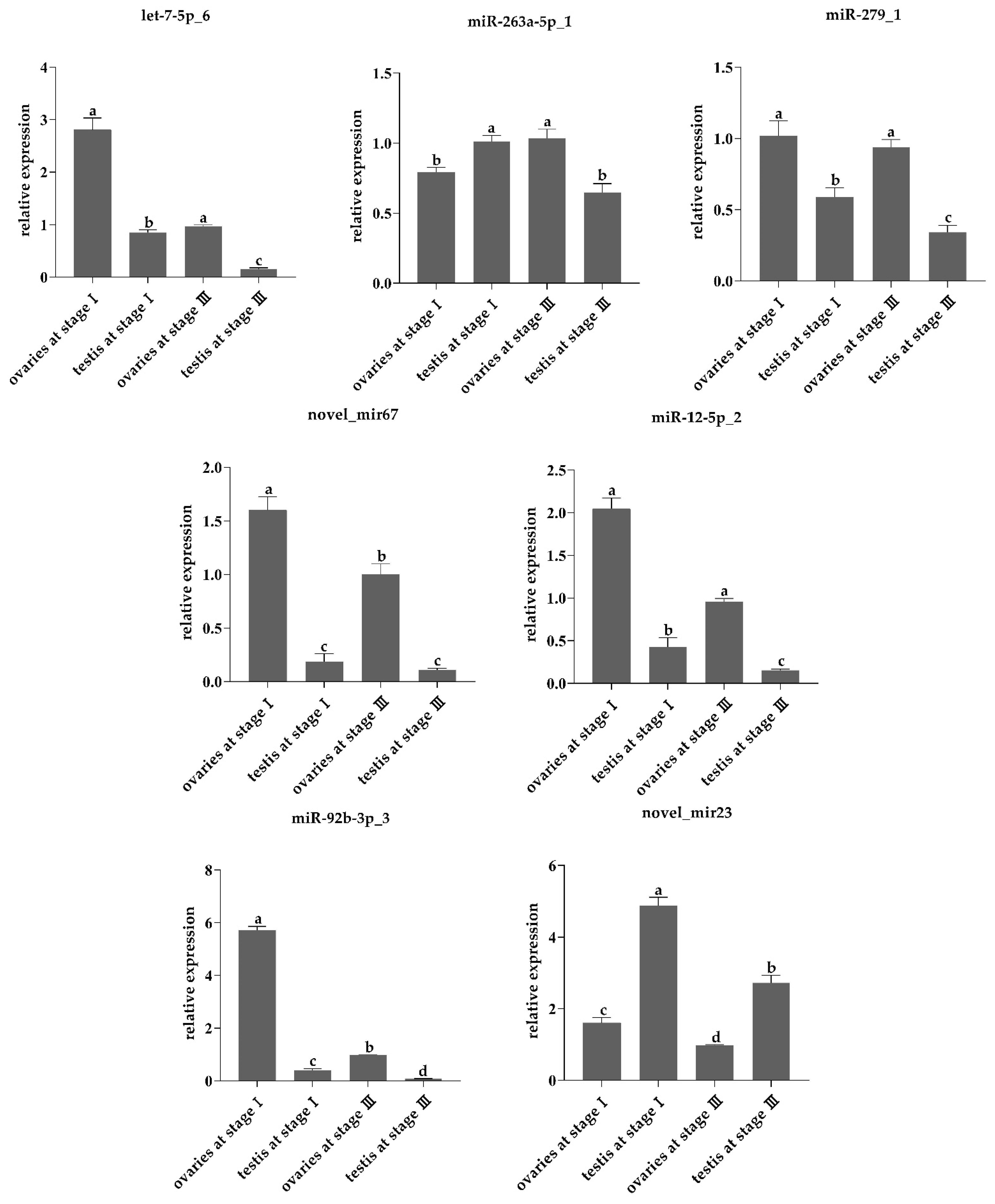

| miRNA id | Expression (Testis) | Expression (Ovary) | log2FoldChange (Ovary/Testis) |
|---|---|---|---|
| novel_mir23 | 6437.118799 | 0.508025321 | −13.62922707 |
| miR-92b-3p_3 | 22,983.4716 | 1102.313603 | −4.381990104 |
| miR-12-5p_2 | 969.0430681 | 217.7167985 | −2.154108059 |
| novel_mir67 | 0.172733727 | 1287.990965 | 12.86428505 |
| miR-279_1 | 418.747114 | 4246.721485 | 3.342198342 |
| let-7-5p_6 | 414.5091587 | 28,964.08332 | 6.126717217 |
| miR-263a-5p_1 | 5.9993158 | 156.8735167 | 4.708660031 |
| MiRNAs | Mature Sequence (5′-3′) | Primer Sequences (5′-3′) | |
|---|---|---|---|
| novel_mir23 | GAGGACGUGGUAG CCUAGUGGU | Fwd | GCGGAGGACGTGGTAGCCT |
| Rev | AGTGCAGGGTCCGAGGTATT | ||
| Stem | GTCGTATCCAGTGCAGGGTC | ||
| CGAGGTATTCGCACTGGATACGACACCACT | |||
| miR-92b-3p_3 | AATTGCACTAGTC CCGGCCTGC | Fwd | GCGCGAAGCACAGCCC |
| Rev | AGTGCAGGGTCCGAGGTATT | ||
| Stem | GTCGTATCCAGTGCAGG | ||
| GTCCGAGGTATTCGCACTGGATACGACGCGGCC | |||
| miR-12-5p_2 | TGAGTATTACATC AGGTACTGGT | Fwd | GCGCGCGGAGAACACA |
| Rev | AGTGCAGGGTCCGAGGTATT | ||
| Stem | GTCGTATCCAGTGCAGG | ||
| GTCCGAGGTATTCGCACTGGATACGACCCGTCC | |||
| novel_mir67 | UGAUGAGGUCUU GUCGUGAGGAGU | Fwd | GCGTGATGAGGTCTTGTCGTG |
| Rev | AGTGCAGGGTCCGAGGTATT | ||
| Stem | GTCGTATCCAGTGCAGGGTCC | ||
| GAGGTATTCGCACTGGATACGACACTCCT | |||
| miR-279_1 | TGACTAGATCCAC ACTCATCCA | Fwd | CGCGCGGACAGACCACA |
| Rev | AGTGCAGGGTCCGAGGTATT | ||
| Stem | GTCGTATCCAGTGCAGGG | ||
| TCCGAGGTATTCGCACTGGATACGACTGGTGG | |||
| let-7-5p_6 | TGAGGTAGTAGGT TGTATAGTT | Fwd | GCGCGCGCGGAGGAGA |
| Rev | AGTGCAGGGTCCGAGGTATT | ||
| Stem | GTCGTATCCAGTGCAGG | ||
| GTCCGAGGTATTCGCACTGGATACGACCTTCCC | |||
| miR-263a-5p_1 | AATGGCACTGGAA GAATTCACGG | Fwd | CGCGAAGGCACGGAAGA |
| Rev | AGTGCAGGGTCCGAGGTATT | ||
| Stem | GTCGTATCCAGTGCAGGG | ||
| TCCGAGGTATTCGCACTGGATACGACCCGTGT | |||
| U6 | Fwd | CTCGCTTCGGCAGCACA | |
| Rev | AACGCTTCACGAATTTGCGT |
| miRNA_id | Source of Variance | Sum of Squares | Degrees of Freedom | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|---|
| let-7-5p_6 | Regression | 11.54 | 3 | 3.846 | 275.5 | 0.000 |
| Residual | 0.1117 | 8 | 0.01396 | |||
| Total | 11.65 | 11 | ||||
| miR-263a-5p_1 | Regression | 0.3094 | 3 | 0.1031 | 36.11 | 0.000 |
| Residual | 0.02285 | 8 | 0.002856 | |||
| Total | 0.3322 | 11 | ||||
| miR-279_1 | Regression | 0.8922 | 3 | 0.2974 | 58.52 | 0.000 |
| Residual | 0.04065 | 8 | 0.005082 | |||
| Total | 0.9328 | 11 | ||||
| novel_mir67 | Regression | 4.534 | 3 | 1.511 | 197.1 | 0.000 |
| Residual | 0.06134 | 8 | 0.007667 | |||
| Total | 4.596 | 11 | ||||
| miR-12-5p_2 | Regression | 6.314 | 3 | 2.105 | 282.7 | 0.000 |
| Residual | 0.05956 | 8 | 0.007445 | |||
| Total | 6.374 | 11 | ||||
| miR-92b-3p_3 | Regression | 63.06 | 3 | 21.02 | 3057 | 0.000 |
| Residual | 0.05501 | 8 | 0.006877 | |||
| Total | 63.11 | 11 | ||||
| novel_mir23 | Regression | 26.51 | 3 | 8.837 | 280.5 | 0.000 |
| Residual | 0.2521 | 8 | 0.03151 | |||
| Total | 26.76 | 11 |
| Pathway-Term | p-Value | q-Value | Pathway ID |
|---|---|---|---|
| IL-17 signaling pathway | 8.68 × 10−16 | 2.98 × 10−13 | ko04657 |
| Hepatitis B | 3.42 × 10−7 | 5.87 × 10−5 | ko05161 |
| Endocrine resistance | 8.08 × 10−7 | 9.24 × 10−5 | ko01522 |
| Relaxin signaling pathway | 3.666 × 10−6 | 0.0003144 | ko04926 |
| AGE-RAGE signaling pathway in diabetic complications | 4.594 × 10−6 | 0.0003152 | ko04933 |
| Parathyroid hormone synthesis, secretion and action | 6.455 × 10−6 | 0.000369 | ko04928 |
| Other glycan degradation | 1.177 × 10−5 | 0.0005062 | ko00511 |
| Phospholipase D signaling pathway | 1.181 × 10−5 | 0.0005062 | ko04072 |
| MicroRNAs in cancer | 1.905 × 10−5 | 0.0007261 | ko05206 |
| Insulin resistance | 2.48 × 10−5 | 0.0008508 | ko04931 |
| ECM-receptor interaction | 3.992 × 10−5 | 0.0012447 | ko04512 |
| Human papillomavirus infection | 0.0001299 | 0.0034973 | ko05165 |
| Amoebiasis | 0.0001325 | 0.0034973 | ko05146 |
| Hedgehog signaling pathway | 0.0003282 | 0.0080415 | ko04340 |
| Glycosaminoglycan biosynthesis—keratan sulfate | 0.0006459 | 0.0147702 | ko00533 |
| Prostate cancer | 0.0011405 | 0.0241681 | ko05215 |
| Protein digestion and absorption | 0.0012572 | 0.0241681 | ko04974 |
| Cell adhesion molecules (CAMs) | 0.0012683 | 0.0241681 | ko04514 |
| Insect hormone biosynthesis | 0.001342 | 0.0242271 | ko00981 |
| Hepatitis C | 0.0014316 | 0.0245517 | ko05160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; He, P.; Zhang, X.; Guan, J.; Chen, Y.; Zhang, L.; Zhang, B.; Zheng, Y.; Li, X.; He, Q.; et al. Identification and Characterization of microRNAs in the Gonads of Litopenaeus vannamei Using High-Throughput Sequencing. Fishes 2022, 7, 308. https://doi.org/10.3390/fishes7060308
Li W, He P, Zhang X, Guan J, Chen Y, Zhang L, Zhang B, Zheng Y, Li X, He Q, et al. Identification and Characterization of microRNAs in the Gonads of Litopenaeus vannamei Using High-Throughput Sequencing. Fishes. 2022; 7(6):308. https://doi.org/10.3390/fishes7060308
Chicago/Turabian StyleLi, Wei, Pingping He, Xingzhi Zhang, Junliang Guan, Yongxian Chen, Li Zhang, Bin Zhang, Yusi Zheng, Xin Li, Qingsong He, and et al. 2022. "Identification and Characterization of microRNAs in the Gonads of Litopenaeus vannamei Using High-Throughput Sequencing" Fishes 7, no. 6: 308. https://doi.org/10.3390/fishes7060308
APA StyleLi, W., He, P., Zhang, X., Guan, J., Chen, Y., Zhang, L., Zhang, B., Zheng, Y., Li, X., He, Q., Liu, L., Yuan, C., Wei, P., & Peng, J. (2022). Identification and Characterization of microRNAs in the Gonads of Litopenaeus vannamei Using High-Throughput Sequencing. Fishes, 7(6), 308. https://doi.org/10.3390/fishes7060308






