Nutritional Profiling of Wild (Pangasius pangasius) and Farmed (Pangasius hypophthalmus) Pangasius Catfish with Implications to Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
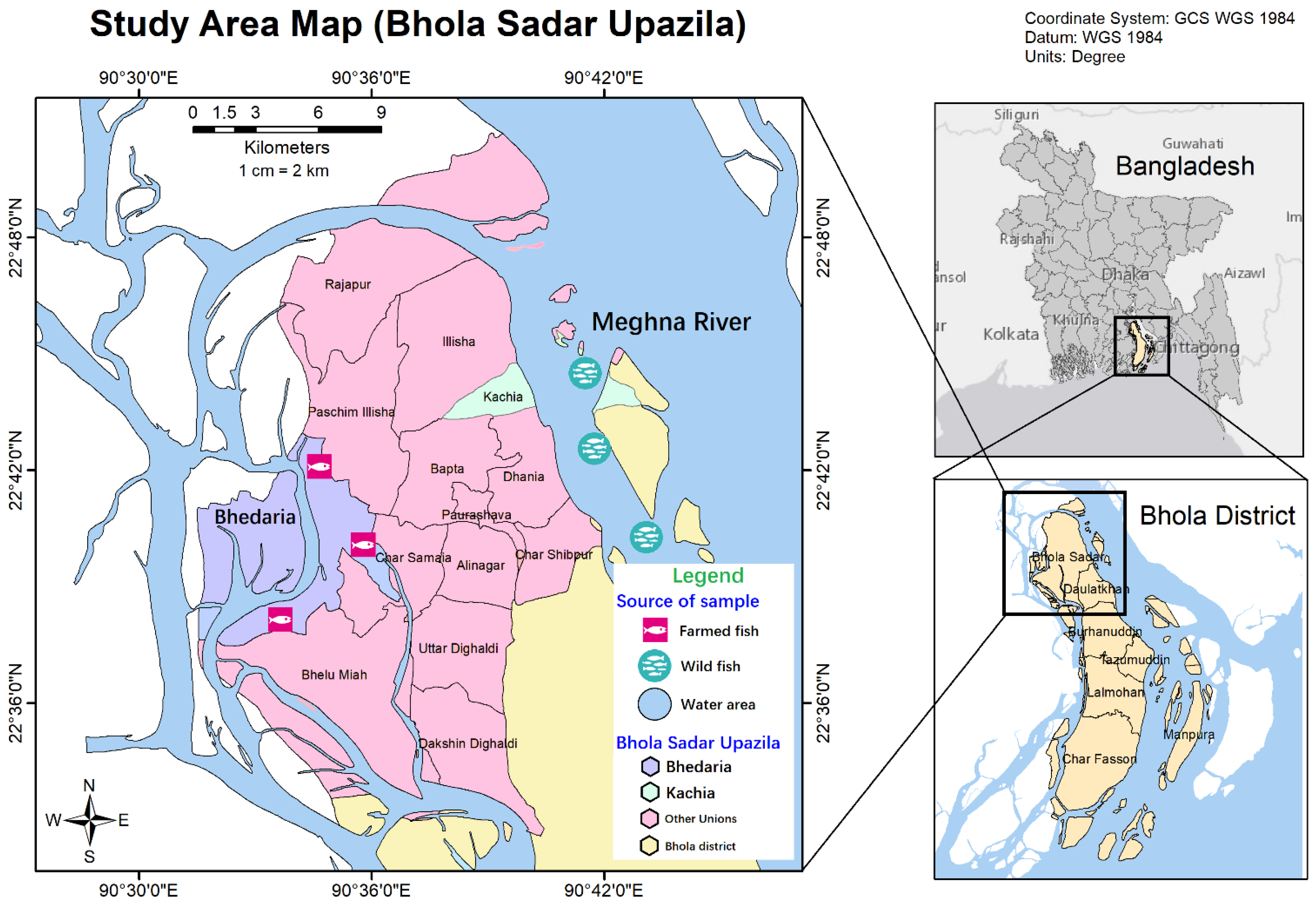
2.2. Collection of Fish and Sample
2.3. Proximate Compositions
2.4. Amino Acid Composition
2.5. Fatty Acid Composition
2.6. Lipid Quality Indices
2.7. Flesh-Lipid Quality (FLQ)
2.8. Nutritional Contribution of LA and ∑EPA + DHA
2.9. Statistical Analysis
3. Results
3.1. Proximate Composition
3.2. Amino Acid Composition
3.3. Fatty Acid Profile
3.4. Nutritional Lipid Quality Indices
3.5. Contribution of LA and ∑EPA + DHA to Human Nutrition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agricultural Organization. World Food and Agriculture—Statistical Yearbook; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Department of Fisheries. Yearbook of fsheries statistics of Bangladesh 2018–19. In Fisheries Resources Survey System (FRSS), 36; Department of Fisheries, Ministry of Fisheries and Livestock: Dhaka, Bangladesh, 2019; pp. 142–146. [Google Scholar]
- Haque, M.M.; Alam, M.M.; Hoque, M.S.; Hasan, N.A.; Nielsen, M.; Hossain, M.I.; Frederiksen, M. Can Bangladeshi pangasius farmers comply with the requirements of aquaculture certification? Aquac. Rep. 2021, 21, 100811. [Google Scholar] [CrossRef]
- Karl, H.; Lehmann, I.; Rehbein, H.; Schubring, R. Composition and quality attributes of conventionally and organically farmed Pangasius fillets (Pangasius hypophthalmus) on the German market. Int. J. Food Sci. Technol. 2010, 45, 56–66. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ullah, M.R.; Kabir, M.A.; Alam, M.A.; Rahman, M.; Hossen, M.F. Artificial propagation of indigenous yellowtail catfish (Pangasius pangasius): Experiences and challenges. Aquaculture 2020, 523, 735215. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of way of cooking on content of essential polyunsaturated fatty acids in muscle tissue of humpback salmon (Oncorhynchus gorbuscha). Food Chem. 2006, 96, 446–451. [Google Scholar] [CrossRef]
- Oluwaniyi, O.O.; Dosumu, O.O.; Awolola, G.V. Effect of local processing methods (boiling, frying and roasting) on the amino acid composition of four marine fishes commonly consumed in Nigeria. Food Chem. 2010, 123, 1000–1006. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Sidhu, K.S. Health benefits and potential risks related to consumption of fish or fish oil. Regul. Toxicol. Pharmacol. 2003, 38, 336–344. [Google Scholar] [CrossRef]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2004, 107, 1–11. [Google Scholar] [CrossRef]
- Mnari, A.; Bouhlel, I.; Chraief, I.; Hammami, M.; Romdhane, M.S.; El Cafsi, M.; Chaouch, A. Fatty acids in muscles and liver of Tunisian wild and farmed gilthead sea bream, Sparus aurata. Food Chem. 2007, 100, 1393–1397. [Google Scholar] [CrossRef]
- Von Schacky, C. The role of omega-3 fatty acids in cardiovascular disease. Curr. Atheroscler. Rep. 2003, 5, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.S.; Lohar, P.S. Biochemical Composition and Gonadosomatic Index of Three Major Carps in Hatnoor Reservoir, Maharashtra, India. J. Ecobiotechnol. 2011, 3, 1–4. [Google Scholar]
- Azam, K.; Ali, M.Y.; Asaduzzaman, M.; Basher, M.Z.; Hossain, M.M. Biochemical Assessment of Selected Fresh Fish. J. Biol. Sci. 2003, 4, 9–10. [Google Scholar] [CrossRef][Green Version]
- Talwar, P.K.; Jhingran, A.G. Inland Fishes of India Andadjacent Countries, Volumes 1 and 2; Oxford and IBHPublishing Co. Pvt. Ltd.: New Delhi/Bombay/Calcutta, India, 1991; p. 1063. [Google Scholar]
- Bogard, J.R.; Thilsted, S.H.; Marks, G.C.; Wahab, M.A.; Hossain, M.A.R.; Jakobsen, J.; Stangoulis, J. Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J. Food Compos. Anal. 2015, 42, 120–133. [Google Scholar] [CrossRef]
- Sokamte, T.A.; Mbougueng, P.D.; Mohammadou, B.A.; Tatsadjieu, N.L.; Sachindra, N.M. Proximal composition and fatty acid profile of fresh and smoked fillets of Pangasius hypophthalmus. Sci. Afr. 2020, 9, e00534. [Google Scholar] [CrossRef]
- Monalisa, K.; Islam, M.Z.; Khan, T.A.; Abdullah, A.T.M.; Hoque, M.M. Comparative study on nutrient contents of native and hybrid Koi (Anabas testudineus) and Pangas (Pangasius pangasius, Pangasius hypophthalmus) fish in Bangladesh. Int. Food Res. J. 2013, 20, 791–797. [Google Scholar]
- Association of Official Agricultural Chemistry. Official Methods of Analysis of the AOAC, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- James, C.J. The Analytical Chemistry of Foods; Chapman and Hall Press: New York, NY, USA, 1995; p. 86. [Google Scholar]
- Dimova, N. RP-HPLC Analysis of Amino Acids with UV-Detection; Tome 56, No 12; Bulgarian Academy Science: Sofia, Bulgaria, 2003. [Google Scholar]
- Gheshlaghi, R.; Scharer, J.M.; Moo-Young, M.; Douglas, P.L. Application of statistical design for the optimization of amino acid separation by reverse-phase HPLC. Anal. Biochem. 2008, 383, 93–102. [Google Scholar] [CrossRef]
- Basha, S.M.M.; Roberts, R.M. A simple colorimetric method for the determination of tryptophan. Anal. Biochem. 1977, 77, 378–386. [Google Scholar] [CrossRef]
- Food and Agricultural Organization. Dietary Protein Quality Evaluation in Human Nutrition; Report of an FAO Expert Consultation. Food and Nutrition Paper 92; FAO: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
- Method ISO 5509; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization of Standardization: Geneva, Switzerland, 2000. Available online: https://www.iso.org/standard/11560.html (accessed on 25 October 2022).
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Chungu, P.; Fotedar, R.; Howieson, J. Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch). PLoS ONE 2019, 14, e0215025. [Google Scholar]
- Senso, L.; Suárez, M.D.; Ruiz-Cara, T.; García-Gallego, M. On the possible effects of harvesting season and chilled storage on the fatty acid profile of the fillet of farmed gilthead sea bream (Sparus aurata). Food Chem. 2007, 101, 298–307. [Google Scholar] [CrossRef]
- United States-Environmental Protection Agency. Guidance for assessing chemical contaminant data for use in fish advisories. In Volume 1: Fish Sampling and Analysis, 2nd ed.; EPA 823-R-95-007; Office of Science and Technology, Office of Water: Washington, DC, USA, 1995. [Google Scholar]
- National Health and Medical Research Council. Fats: Total Fats and Fatty Acids. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. Australian National Health and Medical Research Council (NHMRC) and the New Zealand Ministry of Health (MoH), New Zealand. 2006. Available online: https://www.nrv.gov.au/sites/default/files/content/n35-fat_0.pdf (accessed on 25 September 2022).
- European Food Safety Authority. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA); Scientific Opinion on Dietary Reference Values for Fats, including Saturated Fatty Acids, Polyunsaturated Fatty Acids, Monounsaturated Fatty Acids, Trans Fatty Acids, and Cholesterol; EFSA: Parma, Italy, 2010; p. 1461. [Google Scholar]
- Saavedra, M.; Pereira, T.G.; Carvalho, L.M.; Pousão-Ferreira, P.; Grade, A.; Teixeira, B.; Quental-Ferreira, H.; Mendes, R.; Bandarra, N.; Gonçalves, A. Wild and farmed meagre, Argyrosomus regius: A nutritional, sensory and histological assessment of quality differences. J. Food Compos. Anal. 2017, 63, 8–14. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R. Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health and disease resistance to Vibrio harvei in barramundi, Lates calcarifer. Fish Shellfish. Immunol. 2019, 89, 61–70. [Google Scholar]
- Boran, G.; Karaçam, H. Seasonal changes in proximate composition of some fish species from the black sea. Turk. J. Fish. Aquat. Sci. 2011, 11, 1–5. [Google Scholar] [CrossRef]
- Pongpet, J.; Ponchunchoovong, S.; Payooha, K. Partial replacement of fishmeal by brewer’s yeast (Saccharomyces cerevisiae) in the diets of Thai Panga (Pangasianodon hypophthalmus × Pangasius bocourti). Aquac. Nutr. 2016, 22, 575–585. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Serra, J.A.; Barat, J.M. Comparison of wild and cultured sea bass (Dicentrarchus labrax) quality. Food Chem. 2010, 119, 1514–1518. [Google Scholar] [CrossRef]
- Zhao, F.; Zhuang, P.; Zhang, L.Z.; Shi, Z.H. 2016Biochemical composition of juvenile cultured vs. wild silver pomfret, Pampus argenteus: Determining the diet for cultured fish. Fish Physiol. Biochem. 2010, 36, 1105–1111. [Google Scholar] [CrossRef]
- Rodríguez-Barreto, D.; Jerez, S.; Cejas, J.R.; Martin, M.V.; Acosta, N.G.; Bolaños, A.; Lorenzo, A. Comparative study of lipid and fatty acid composition in different tissues of wild and cultured female broodstock of greater amberjack (Seriola dumerili). Aquaculture 2012, 360–361, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yu, S.L.; Ma, G.J.; Chen, S.B.; Shi, Y.; Yang, Y.H. Comparative study of proximate composition and amino acid in farmed and wild Pseudobagrus ussuriensis muscles. Int. J. Food. Sci. Technol. 2014, 49, 983–989. [Google Scholar] [CrossRef]
- Cardinal, M.; Cornet, J.; Donnay-Moreno, C.; Gouygou, J.P.; Bergé, J.P.; Rocha, E.; Soares, S.; Escórcio, C.; Borges, P.; Valente, L.M.P. Seasonal variation of physical, chemical and sensory characteristics of sea bream (Sparus aurata) reared under intensive conditions in Southern Europe. Food Control 2011, 22, 574–585. [Google Scholar] [CrossRef]
- Nurnadia, A.A.; Azrina, A.; Amin, I.; Mohd, Y.A.S.; Mohd, I.E.H. Mineral contents of selected marine fish and shellfish from the west coast of Peninsular Malaysia. Food Res. Int. 2013, 20, 431–437. [Google Scholar]
- Stansby, M. Chemical characteristics of fish caught in the northeast Pacific Ocean. Mar. Fish. Rev. 1976, 38, 9. [Google Scholar]
- Khan, M.S.; Ang, K.J.; Ambak, M.A.; Saad, C.R. Optimum dietary protein requirement of a Malaysian freshwater catfish, Mystus nemurus. Aquaculture 1993, 112, 227–235. [Google Scholar] [CrossRef]
- Oztekin, A.; Yigit, M.; Kizilkaya, B.; Ucyol, N.; Yilmaz, S.; Tan, E.; Bulut, M.; Ergün, S.; Ayaz, A. Fatty Acid Profiles in Wild Axillary Seabream (Pagellus acarne) versus Cage-Aggregated and Cage-Farmed Fish with Reference to Nutritional Contribution for Human Consumers. Aquac. Stud. 2018, 18, 29–38. [Google Scholar] [CrossRef]
- Islam, M.N.; Joadder, M.A.R. Seasonal variation of the proximate composition of freshwater Gobi, Glossoqibius giuris (Hamilton) for river Padma. Pak. J. Biol. Sci. 2005, 8, 532–536. [Google Scholar]
- Ali, M.Z.; Hossain, M.A.; Mazid, M.A. Effect of mixed feeding schedules with varying dietary protein levels on the growth of sutchi catfish, Pangasius hypophthalmus (Sauvage) with silver carp, Hypophthalmichthys molitrix (Valenciennes) in ponds. Aquac. Res. 2005, 36, 627–634. [Google Scholar] [CrossRef]
- Özden, Ö.; Erkan, N. Comparison of biochemical composition of three aqua cultured fishes (Dicentrarchus labrax, Sparus aurata, Dentex dentex). Int. J. Food Sci. Nutr. 2008, 59, 545–557. [Google Scholar] [CrossRef]
- Furuya, W.M.; Graciano, T.S.; Vidal, L.V.O.; Xavier, T.O.; Gongora, L.D.; Righetti, J.S.; Furuya, V.R.B. Digestible lysine requirement of Nile tilapia fingerlings fed arginine-tolysine-balanced diets. Rev. Bras. Zootec. 2012, 41, 485–490. [Google Scholar] [CrossRef]
- Imura, K.; Okada, A. Amino acid metabolism in pediatric patients. Nutrition 1998, 14, 143–148. [Google Scholar] [CrossRef]
- Jais, A.M.M.; McCulloch, R.; Croft, K. Fatty acid and amino acid composition in haruan as a potential role in wound healing. Gen. Pharmacol. 1994, 25, 947–950. [Google Scholar] [CrossRef]
- Swendseid, M.E.; Villalobos, J.; Friedrich, B. Ratios of Essential-to-Nonessential Amino Acids in Plasma from Rats Fed Different Kinds and Amounts of Proteins and Amino Acids. J. Nutr. 1963, 80, 99–102. [Google Scholar] [CrossRef]
- Pinto, J.F.; Nunes, M.L.; Cardoso, C. Feeding interruption and quality of cultured gilthead sea bream. Food Chem. 2007, 100, 1504–1510. [Google Scholar] [CrossRef]
- Alam, M.D.; Karim, S.H.M.; Chakrabortty, A.; Amin, R.; Hasan, S. Nutritional Characterization of the Long-Whiskered Catfish Sperata aor: A Commercially Important Freshwater Fish of Bangladesh. Int. J. Food Sci. Nutr. Eng. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Ackman, R.G. Nutritional composition of fats in seafoods. Prog. Food Nutr. Sci. 1989, 13, 161–289. [Google Scholar] [PubMed]
- Islam, R.; Paul, D.K.; Rahman, A.; Parvin, T.; Islam, D.; Sattar, A. Comparative Characterization of Lipids and Nutrient Contents of Pangasius Pangasius and Pangasius sutchi Available in Bangladesh. J. Nutr. Food Sci. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Weber, P.C.; Sellmayer, A. S-3-4 Cardiovascular Effects of N-3 Fatty Acids. J. Nutr. Sci. Vitaminol. 1992, 38, 144–147. [Google Scholar] [CrossRef]
- Özyurt, G.; Polat, S.; Özkütük, S. Seasonal changes in the fatty acids of gilthead sea bream (Sparus aurata) and white sea bream (Diplodus sargus) captured in Iskenderun Bay, eastern Mediterranean coast of Turkey. Eur. Food Res. Technol. 2005, 220, 120–124. [Google Scholar] [CrossRef]
- Bang, H.O.; Dyerberg, J.; Sinclair, H.M. The composition of the Eskimo food in north Western Greenland. Am. J. Clin. Nutr. 1980, 33, 2657–2661. [Google Scholar] [CrossRef]
- Thammapat, P.; Raviyan, P.; Siriamornpun, S. Proximate and fatty acids composition of the muscles and viscera of Asian catfish (Pangasius bocourti). Food Chem. 2010, 122, 223–227. [Google Scholar] [CrossRef]
- Erkkilä, A.; De Mello, V.D.F.; Risérus, U.; Laaksonen, D.E. Dietary fatty acids and cardiovascular disease: An epidemiological approach. Prog. Lipid Res. 2008, 47, 172–187. [Google Scholar] [CrossRef]
- Kulawik, P.; Migdał, W.; Tkaczewska, J.; Gambu, F.; Szczurowska, K.; Özoğul, F. Nutritional composition of frozen fillets from Pangasius catfish (Pangasius hypophthalmus) and Nile tilapia (Oreochromis nilotcus) imported to European countries. Ann. Anim. Sci. 2016, 16, 931–950. [Google Scholar] [CrossRef][Green Version]
- Diraman, H.; Dibeklioglu, H. Chemometric characterization and classification of selected freshwater and marine fishes from turkey based on their fatty acid profiles. J. Am. Oil Chem. Soc. 2009, 86, 235–246. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Çiçek, E.; Polat, A.; Kuley, E. Fat content and fatty acid compositions of 34 marine water fish species from the Mediterranean Sea. Int. J. Food Sci. Nutr. 2009, 60, 464–475. [Google Scholar] [CrossRef]
- Usydus, Z.; Szlinder-Richert, J.; Adamczyk, M.; Szatkowska, U. Marine and farmed fish in the Polish market: Comparison of the nutritional value. Food Chem. 2011, 126, 78–84. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.B.; Visentainer, J.V.; De Souza, N.E.; Matsushita, M. Fatty acids profile and cholesterol contents of three Brazilian Brycon freshwater fishes. J. Food Compos. Anal. 2001, 14, 565–574. [Google Scholar] [CrossRef]
- Orban, E.; Nevigato, T.; Di Lena, G.; Masci, M.; Casini, I.; Gambelli, L.; Caproni, R. New trends in the seafood market. Sutchi catfish (Pangasius hypophthalmus) fillets from Vietnam: Nutritional quality and safety aspects. Food Chem. 2008, 110, 383–389. [Google Scholar] [CrossRef]
- Turan, H. Fatty acid profile and proximate composition of the thornback ray (Raja clavata L. 1758) from the Sinop coast in the Black Sea. J. Fish. Sci. 2007, 1, 97–103. [Google Scholar] [CrossRef]
- Tonial, I.B.; Oliveira, D.F.; Coelho, A.R.; Matsushita, M.; Coró, F.A.G.; De Souza, N.E.; Visentainer, J.V. Quantification of Essential Fatty Acids and Assessment of the Nutritional Quality Indexes of Lipids in Tilapia Alevins and Juvenile Tilapia Fish (Oreochromis niloticus). J. Food Res. 2014, 3, 105. [Google Scholar] [CrossRef]
- Abrami, G.; Natiello, F.; Bronzi, P.; McKenzie, D.; Bolis, L.; Agradi, E. A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla Anguilla). Comp. Biochem. Physiol.-B 1992, 101, 79–81. [Google Scholar] [CrossRef]
- Stancheva, M.; Merdzhanova, A.; Dobreva, D.A.; Makedonski, L. Common Carp (Cyprinus caprio) and European Catfish (Sillurus glanis) from the Danube River as Sources of Fat Soluble Vitamins and Fatty Acids. Czech J. Food Sci. 2014, 32, 16–24. [Google Scholar] [CrossRef]

| Parameters (%) | Wild Pangasius | Farmed Pangasius | p-Value |
|---|---|---|---|
| Moisture | 79.49 ± 0.09 | 75.05 ± 0.09 | <0.0001 |
| Protein | 14.36 ± 0.06 | 20.19 ± 0.06 | <0.0001 |
| Lipid | 3.74 ± 0.09 | 2.11 ± 0.4 | <0.0001 |
| Ash | 0.73 ± 0.02 | 0.52 ± 0.3 | <0.001 |
| Carbohydrate | 1.05 ± 0.04 | 1.23 ± 0.07 | 0.017 |
| Parameters | Wild Pangasius | Farmed Pangasius | p-Value |
|---|---|---|---|
| Methionine | 0.52 ± 0.02 | 0.38 ± 0.01 | <0.0001 |
| Threonine | 0.81 ± 0.03 | 0.59 ± 0.02 | <0.0001 |
| Valine | 0.88 ± 0.03 | 0.62 ± 0.02 | <0.0001 |
| Isoleucine | 0.79 ± 0.02 | 0.58 ± 0.02 | <0.0001 |
| Histidine | 0.49 ± 0.05 | 0.34 ± 0.01 | 0.005 |
| Lysine | 1.48 ± 0.03 | 1.07 ± 0.02 | <0.0001 |
| Leucine | 1.20 ± 0.02 | 0.87 ± 0.01 | <0.0001 |
| Arginine | 0.89 ± 0.03 | 0.65 ± 0.02 | <0.0001 |
| Glycine | 1.85 ± 0.02 | 1.36 ± 0.03 | <0.0001 |
| Tyrosine | 0.63 ± 0.02 | 0.45 ± 0.03 | <0.001 |
| Alanine | 1.10 ± 0.03 | 0.79 ± 0.03 | <0.0001 |
| Aspartic acid | 1.38 ± 0.03 | 0.98 ± 0.02 | <0.0001 |
| Serine | 0.76 ± 0.02 | 0.51 ± 0.03 | <0.0001 |
| Glutamic acid | 0.86 ± 0.03 | 0.60 ± 0.03 | <0.0001 |
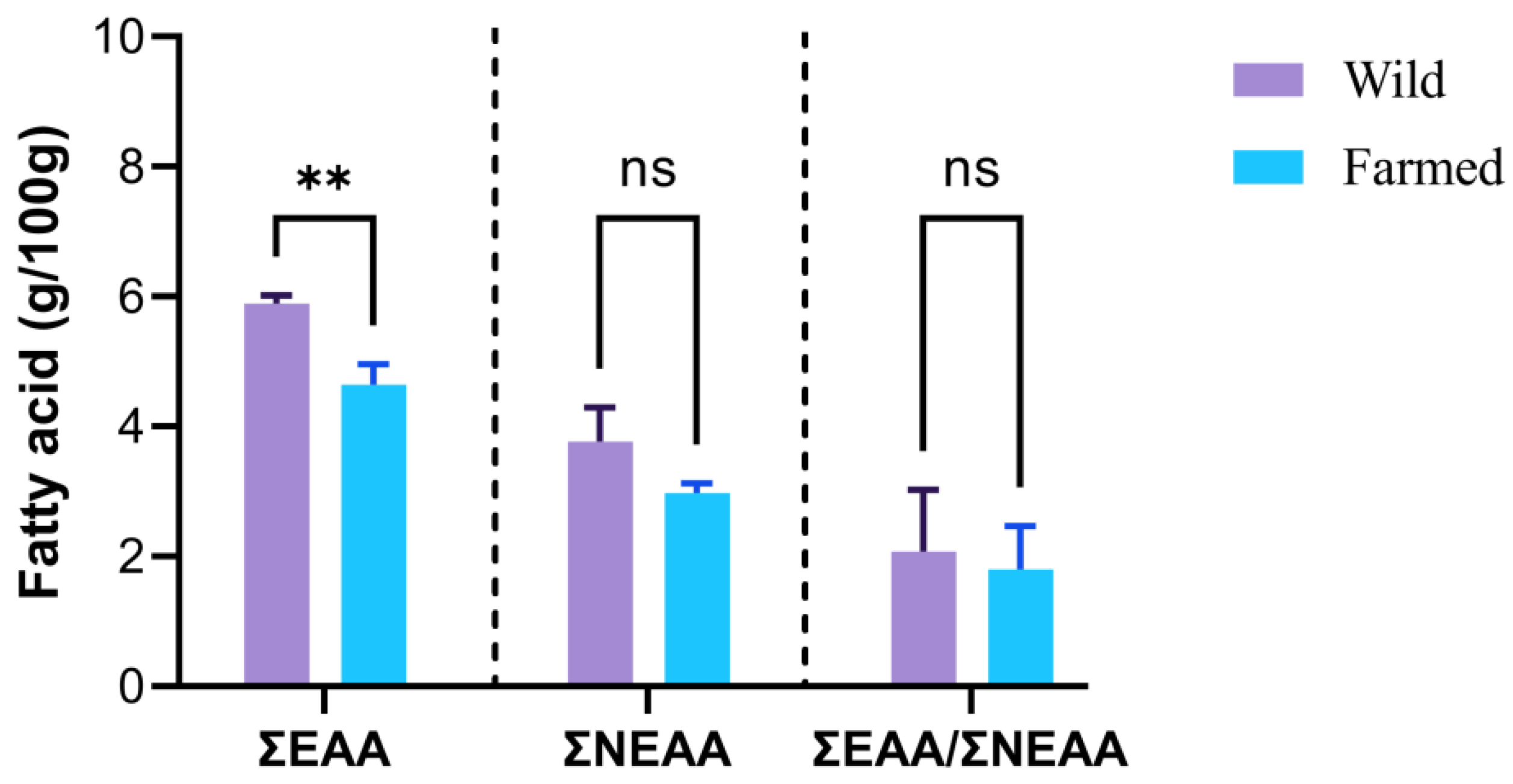
| Amino Acids (mg/g) | Infant (0–6 Month) | Child (6 Month–3 Years) | Old Child, Adolescent, Adult | Wild Pangasius | Farmed Pangasius | p-Value |
|---|---|---|---|---|---|---|
| His. | 21 | 20 | 16 | 4.92 ± 0.45 | 3.42 ± 0.10 | 0.0050 |
| Ile. | 55 | 32 | 30 | 7.93 ± 0.19 | 5.80 ± 0.17 | <0.0001 |
| Leu. | 96 | 66 | 61 | 12.03 ± 0.22 | 8.73 ± 0.06 | <0.0001 |
| Lys. | 69 | 57 | 48 | 14.81 ± 0.25 | 10.72 ± 0.24 | <0.0001 |
| Met. + Cys. * | 33 | 27 | 23 | 5.21 ± 0.19 | 3.77 ± 0.12 | <0.0001 |
| Phe. + Tyr. * | 94 | 52 | 41 | 6.24 ± 0.07 | 4.37 ± 0.12 | <0.0001 |
| Thr. | 44 | 31 | 25 | 8.11 ± 0.25 | 5.90 ± 0.17 | <0.0001 |
| Trp. * | 17 | 8.5 | 6.6 | - | - | - |
| Val. | 55 | 43 | 40 | 8.82 ± 0.31 | 6.20 ± 0.17 | <0.0001 |
| Nutrients | Fatty Acids | Wild Pangasius | Farmed Pangasius | p-Value |
|---|---|---|---|---|
| Saturated fatty acid (SFA) | C14:0, Myristic acid | 5.68 ± 0.06 | 2.79 ± 0.04 | <0.0001 |
| C16:0, Palmitic Acid | 29.17 ± 0.09 | 34.46 ± 0.16 | <0.0001 | |
| C18:0, Stearic Acid | 6.47 ± 0.04 | 5.90 ± 0.04 | <0.0001 | |
| C20:0, Arachidic Acid | ND | 0.55 ± 0.05 | <0.0001 | |
| C24:0, Lignoseric Acid | 2.50 ± 0.04 | 0.40 ± 0.03 | <0.0001 | |
| Monounsaturated fatty acid (MUFA) | C16:1, Palmitoleic acid | 18.36 ± 0.04 | ND | <0.0001 |
| C18:1, Oleic acid | 27.35 ± 0.08 | 43.27 ± 0.09 | <0.0001 | |
| C20:1, Eicosenoic acid | 1.85 ± 0.03 | 1.15 ± 0.05 | <0.0001 | |
| Polyunsaturated fatty acid (PUFA) | C18:2, Linoleic Acid | 0.57 ± 0.04 | 10.06 ± 0.03 | <0.0001 |
| C18:3, Linolenic Acid | 0.45 ± 0.02 | 0.34 ± 0.04 | 0.013 | |
| C20:5, EPA | 2.72 ± 0.03 | ND | <0.0001 | |
| C22:6, DHA | 4.89 ± 0.03 | 1.07 ± 0.01 | <0.0001 |
| Fatty Acids | Wild Pangasius | Farmed Pangasius | p-Value |
|---|---|---|---|
| ΣSFA | 43.82 ± 0.03 | 44.10 ± 0.30 | 0.181 |
| ΣUFA | 56.18 ± 0.14 | 55.89 ± 0.15 | 0.065 |
| ΣMUFA | 47.56 ± 0.04 | 44.42 ± 0.13 | <0.0001 |
| ΣPUFA | 8.62 ± 0.11 | 11.47 ± 0.03 | <0.0001 |
| ω-3 | 8.06 ± 0.07 | 1.41 ± 0.05 | <0.0001 |
| ω-6 | 0.57 ± 0.04 | 10.06 ± 0.03 | <0.0001 |
| ΣPUFA/ΣSFA | 0.20 ± 0.002 | 0.26 ± 0.001 | <0.0001 |
| ω-6/ω-3 | 0.07 ± 0.00 | 7.14 ± 0.29 | <0.0001 |
| ω-3/ω-6 | 14.14 ± 0.925 | 0.14 ± 0.006 | <0.0001 |
| EPA + DHA | 7.61 ± 0.06 | 1.07 ± 0.01 | <0.0001 |
| DHA/EPA | 1.79 ± 0.009 | - | - |
| EPA/DHA | 0.56 ± 0.003 | - | <0.0001 |
| Fatty Acids | Wild Pangasius | Farmed Pangasius | p-Value |
|---|---|---|---|
| Dietary levels (mg/100 g wet) | |||
| LA | 566.67 ± 23.33 | 10056.67 ± 17.64 | <0.0001 |
| EPA | 2723.33 ± 17.64 | ND | - |
| DHA | 4886.67 ± 18.56 | 1073.33 ± 6.67 | <0.0001 |
| DHA/EPA | 1.79 ± 0.01 | 0.00 | <0.0001 |
| Σ EPA + DHA | 7610.00 ± 36.06 | 1073.33 ± 6.67 | <0.0001 |
| Dietary reference intake (mg/day) | |||
| LA | 1300 | 1300 | - |
| ΣEPA + DHA | 500 | 500 | - |
| Nutritional contribution for human consumption | |||
| LA (%) | 98.95 ± 4.07 | 1756.05 ± 3.08 | <0.0001 |
| ∑EPA + DHA (%) | 345.49 ± 1.64 | 48.73 ± 0.30 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakma, S.; Rahman, M.A.; Siddik, M.A.B.; Hoque, M.S.; Islam, S.M.; Vatsos, I.N. Nutritional Profiling of Wild (Pangasius pangasius) and Farmed (Pangasius hypophthalmus) Pangasius Catfish with Implications to Human Health. Fishes 2022, 7, 309. https://doi.org/10.3390/fishes7060309
Chakma S, Rahman MA, Siddik MAB, Hoque MS, Islam SM, Vatsos IN. Nutritional Profiling of Wild (Pangasius pangasius) and Farmed (Pangasius hypophthalmus) Pangasius Catfish with Implications to Human Health. Fishes. 2022; 7(6):309. https://doi.org/10.3390/fishes7060309
Chicago/Turabian StyleChakma, Suprakash, Md. Arifur Rahman, Muhammad A. B. Siddik, Md. Sazedul Hoque, SM Majharul Islam, and Ioannis N. Vatsos. 2022. "Nutritional Profiling of Wild (Pangasius pangasius) and Farmed (Pangasius hypophthalmus) Pangasius Catfish with Implications to Human Health" Fishes 7, no. 6: 309. https://doi.org/10.3390/fishes7060309
APA StyleChakma, S., Rahman, M. A., Siddik, M. A. B., Hoque, M. S., Islam, S. M., & Vatsos, I. N. (2022). Nutritional Profiling of Wild (Pangasius pangasius) and Farmed (Pangasius hypophthalmus) Pangasius Catfish with Implications to Human Health. Fishes, 7(6), 309. https://doi.org/10.3390/fishes7060309