New Report of Zu cristatus (Bonelli, 1819) in the Ionian Sea with an In-Depth Morphometrical Comparison with All Mediterranean Records
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Identification of the Specimen and Data Collection
3. Results
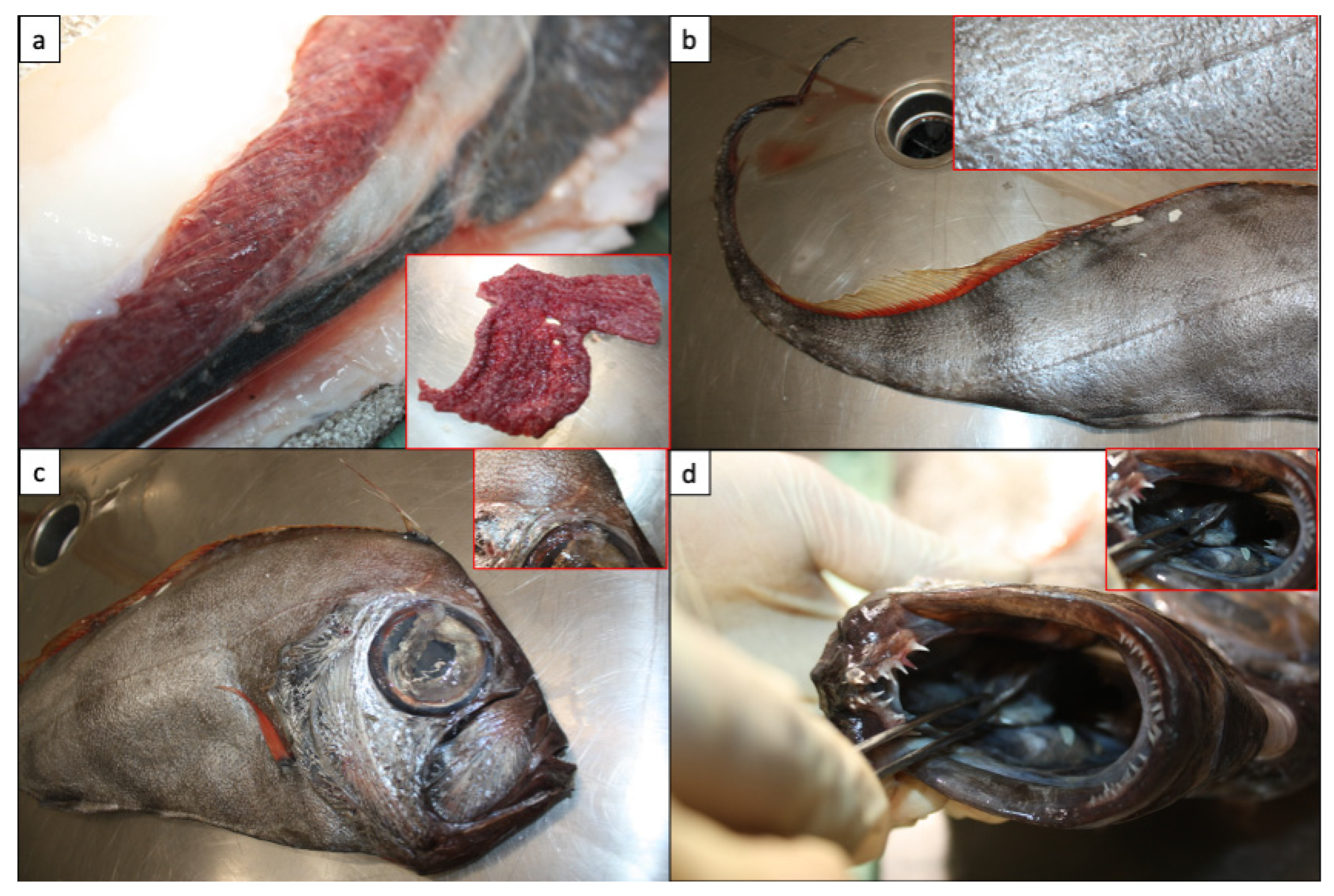
Species Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webb, T.J.; Berghe, E.V.; O’Dor, R. Biodiversity’s big wet secret: The global distribution of marine biological records reveals chronic under-exploration of the deep pelagic ocean. PLoS ONE 2010, 5, e10223. [Google Scholar] [CrossRef]
- Levin, L.A.; Bett, B.J.; Gates, A.R.; Heimbach, P.; Howe, B.M.; Janssen, F.; McCurdy, A.; Ruhl, H.A.; Snelgrove, P.; Stocks, K.I.; et al. Global Observing Needs in the Deep Ocean. Front. Mar. Sci. 2019, 6, 241. [Google Scholar] [CrossRef]
- Yancey, P.H.; Speers-Roesch, B.; Atchinson, S.; Reist, J.D.; Majewski, A.R.; Treberg, J.R. Osmolyte adjustments as a pressure adaptation in deep-sea chondrichthyan fishes: An intraspecific test in arctic skates (Amblyraja hyperborea) along a depth gradient. Physiol. Biochem. Zool. 2018, 91, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Priede, I.G. Deep-Sea Fishes: Biology, Diversity, Ecology and Fisheries; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Lupše, N.; Cortesi, F.; Freese, M.; Marohn, L.; Pohlman, J.-D.; Wysujack, K.; Hanel, R.; Musilova, Z. Visual gene expression reveals a cone to rod developmental progression in deep-sea fishes. Mol. Biol. Evol. 2021, 38, 5664–5677. [Google Scholar] [CrossRef]
- Childress, J.J.; Seibel, B.A. Life at stable low oxygen levels: Adaptations of animals to oceanic oxygen minimum layers. J. Exp. Biol. 1998, 201, 1223–1232. [Google Scholar] [CrossRef]
- Brown, A.; Hauton, C.; Stratmann, T.; Sweetman, A.; van Oevelen, D.; Jones, D.O.B. Metabolic rates are significantly lower in abyssal Holothuroidea than in shallow-water Holothuroidea. R. Soc. Open Sci. 2018, 5, 172162. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shen, Y.; Yang, Y.; Gan, X.; Liu, G.; Hu, K.; Li, Y.; Gao, Z.; Zhu, L.; Yan, G.; et al. Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation. Nat. Ecol. Evol. 2019, 3, 823–833. [Google Scholar] [CrossRef]
- Haedrich, R.L.; Rowe, G.T. Megafaunal biomass in the deep sea. Nature 1977, 269, 141–142. [Google Scholar] [CrossRef]
- Weber, A.A.T.; Hugall, A.F.; O’Hara, T.D. Convergent evolution and structural adaptation to the deep ocean in the protein-folding chaperonin CCTα. Genome Biol. Evol. 2020, 12, 1929–1942. [Google Scholar] [CrossRef]
- Lan, Y.; Sun, J.; Xu, T.; Chen, C.; Tian, R.; Qiu, J.W.; Qian, P.Y. De novo transcriptome assembly and positive selection analysis of an individual deep-sea fish. BMC Genom. 2018, 19, 394. [Google Scholar] [CrossRef]
- Davies, W.L.; Carvalho, L.S.; Tay, B.H.; Brenner, S.; Hunt, D.M.; Venkatesh, B. Into the blue: Gene duplication and loss underlie color vision adaptations in a deep-sea chimaera, the elephant shark Callorhinchus milii. Genome Res. 2009, 19, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Valen, R.; Edvardsen, R.B.; Søviknes, A.M.; Drivenes, Ø.; Helvik, J.V. Molecular evidence that only two opsin subfamilies, the blue light- (SWS2) and green light-sensitive (RH2), drive color vision in Atlantic Cod (Gadus morhua). PLoS ONE 2014, 9, e115436. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. Comparative sequence analysis of myosin heavy chain proteins from congeneric shallow- and deep-living rattail fish (genus Coryphaenoides). J. Exp. Biol. 2008, 211, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Morita, T. Structure-based analysis of high pressure adaptation of α-actin. J. Biol. Chem. 2003, 278, 28060–28066. [Google Scholar] [CrossRef]
- Gage, J.D.; Bett, B.J. Deep-Sea Benthic Sampling. In Methods for the Study of Marine Benthos, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2005; pp. 273–325. [Google Scholar] [CrossRef]
- Consalvey, M.; Clark, M.R.; Rowden, A.A. The Future of Biological Sampling in the Deep Sea. Biol. Sampl. Deep. Sea 2016, 19, 431–436. [Google Scholar] [CrossRef]
- Montagna, P.A.; Baguley, J.G.; Hsiang, C.Y.; Reuscher, M.G. Comparison of sampling methods for deep-sea infauna. Limnol. Oceanogr. Methods 2017, 15, 166–183. [Google Scholar] [CrossRef]
- Falsone, F.; Geraci, M.L.; Scannella, D.; Okpala, C.O.R.; Giusto, G.B.; Bosch-Belmar, M.; Gancitano, S.; Bono, G. Occurrence of two rare species from order lampriformes: Crestfish Lophotus lacepede (Giorna, 1809) and scalloped ribbonfish Zu cristatus (Bonelli, 1819) in the northern coast of sicily, Italy. Acta Adriat. 2017, 58, 137–145. [Google Scholar] [CrossRef]
- Heemstra, P.C.; Kannmeyer, S.X. The families Trachipertidae and Radiicephalidae (Pisces, Lampriformes) and a new species of Zu from South Africa. Ann. South Afr. Mus. 1984, 94, 13–39. [Google Scholar]
- Quigley, D.T. Sherkin Comment; Sherkin Island Marine Station: Cork, Ireland, 2012; p. 12. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fish of the World; Wiley: Hoboken, NJ, USA, 2016; pp. 1–752. [Google Scholar]
- Underkoffler, K.E.; Luers, M.A.; Hyde, J.R.; Craig, M.T. A taxonomic review of Lampris guttatus (Brünnich 1788) (Lampridiformes; Lampridae) with descriptions of three new species. Zootaxa 2018, 4413, 551–565. [Google Scholar] [CrossRef]
- Heemstra, P.C. Family No. 118: Veliferidae. In Smith’s Sea Fishes; Smith, M.M., Heemstra, P.C., Eds.; Macmillan: Johannesburg, South Africa, 1986; pp. 398–399. [Google Scholar]
- Martin, J.; Hilton, E.J. A taxonomic review of the family Trachipteridae (Lampridiformes), with an emphasis on taxa distributed in the Western Pacific Ocean. Zootaxa 2021, 5039, 301–351. [Google Scholar] [CrossRef]
- Olney, J.E. Order Lampriformes. In FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific. Volume 3: Batoid Fishes, Chimaeras and Bony Fishes Part 1 (Elopidae to Linophrynidae); Carpenter, K.E., Niem, V.H., Eds.; FAO: Rome, Italy, 1999; pp. 952–959. ISBN 92-5-104302-7. [Google Scholar]
- Martín-Cuadrado, A.B.; López-García, P.; Alba, J.C.; Moreira, D.; Monticelli, L.; Strittmatter, A.; Gottschalk, G.; Rodríguez-Valera, F. Metagenomics of the deep Mediterranean, a warm bathypelagic habitat. PLoS ONE 2007, 2, e914. [Google Scholar] [CrossRef] [PubMed]
- D’iglio, C.; Albano, M.; Tiralongo, F.; Famulari, S.; Rinelli, P.; Savoca, S.; Spanò, N.; Capillo, G. Biological and ecological aspects of the blackmouth catshark (Galeus melastomus, Rafinesque, 1810) in the southern tyrrhenian sea. J. Mar. Sci. Eng. 2021, 9, 967. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spanò, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef]
- Moullec, F.; Velez, L.; Verley, P.; Barrier, N.; Ulses, C.; Carbonara, P.; Esteban, A.; Follesa, C.; Gristina, M.; Jadaud, A.; et al. Capturing the big picture of Mediterranean marine biodiversity with an end-to-end model of climate and fishing impacts. Prog. Oceanogr. 2019, 178, 102179. [Google Scholar] [CrossRef]
- Angulo, A.; Lopez-Sanchez, M.I. New records of lampriform fishes (Teleostei: Lampriformes) from the Pacific coast of lower Central America, with comments on the diversity, taxonomy and distribution of the Lampriformes in the eastern Pacific Ocean. Zootaxa 2017, 4236, 573–591. [Google Scholar] [CrossRef]
- Savinykh, V.F.; Baitalyuk, A.A. Taxonomic status of ribbonfishes of the genus Trachypterus (Trachipteridae) from the northern part of the Pacific Ocean. J. Ichthyol. 2011, 51, 581–589. [Google Scholar] [CrossRef]
- Augee, M.L. Proceedings of the Linnean Society of New South Wales: Editorial; Linnean Society of New South Wales: Sydney, Australia, 2003; Volume 124. [Google Scholar]
- Hamilton, H. Notes on the occurrence of the genus Trachipterus in New Zealand. Trans. Proc. Roy. Soc. N. Z. 1915, 48, 370–382. [Google Scholar]
- Palmer, G. The Dealfishes (Trachipteridae) of the Mediterranean and north east Atlantic. Bull. Br. Mus. Nat. Hist. Zool. 1961, 7, 335–351. [Google Scholar] [CrossRef]
- Fitch, J.E. The unicornfish, Eumecichthys fiski (Gunther), in the eastern tropical Pacific. Calif. Fish Game 1966, 52, 208–210. [Google Scholar]
- Scott, E.O.G. Observations on some Tasmanian fishes: Part XXIX. Pap. Proc.-R. Soc. Tasman. 1983, 117, 167–202. [Google Scholar] [CrossRef]
- Hayashi, M. Trachipteridae. In Fishes of Japan with Pictorial Keys to the Species; Nakabo, T., Ed.; Tokai Univ. Press: Tokyo, Japan, 2002; pp. 404–406. [Google Scholar]
- Ji, H.W.; Yoon, S.C.; Kim, J.K. Taxonomic review of the family Trachipteridae (Lampridiformes) from Korea. Korean J. Ichthyol. 2009, 21, 273–282. [Google Scholar]
- Garibaldi, F. By-catch in the mesoplagic swordfish longline fishery in the ligurian sea (Western Mediterranean). ICCAT 2015, 71, 1495–1498. [Google Scholar]
- Olney, J.E.; Johnson, G.D.; Baldwin, C.C. Phylogeny of lampridiform fishes. Bull. Mar. Sci. 1993, 52, 137–169. [Google Scholar]
- Miya, M.; Takeshima, H.; Endo, H.; Ishiguro, N.B.; Inoue, J.G.; Mukai, T.; Satoh, T.P.; Yamaguchi, M.; Kawaguchi, A.; Mabuchi, K.; et al. Major patterns of higher teleostean phylogenies: A new perspective based on 100 complete mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2003, 26, 121–138. [Google Scholar] [CrossRef]
- Martin, J.M. Phylogeny, Ontogeny and Distribution of the Ribbonfishes (Lampridiformes: Trachipteridae). Master’s Thesis, Virginia Institute of Marine Science, Gloucester Point, VA, USA, 2015. Paper 1539616922. [Google Scholar] [CrossRef]
- Albano, M.; D’Iglio, C.; Spanò, N.; Fernandes, J.M.O.; Savoca, S.; Capillo, G. Distribution of the Order Lampriformes in the Mediterranean Sea with Notes on Their Biology, Morphology, and Taxonomy. Biology 2022, 11, 1534. [Google Scholar] [CrossRef]
- Dulčić, J. First record of scalloped ribbon fish, Zu cristatus (Pisces: Trachipteridae), eggs in the Adriatic Sea. J. Plankton Res. 2002, 24, 1245–1246. [Google Scholar] [CrossRef]
- Bianco, P.G.; Zupo, V.; Ketmaier, V. Occurrence of the scalloped ribbonfish Zu cristatus (Lampridiformes) in coastal waters of the central Tyrrhenian Sea, Italy. J. Fish Biol. 2006, 68, 150–155. [Google Scholar] [CrossRef]
- Bradai, M.N.; El Ouaer, A. New record of the scalloped ribbon fish, Zu cristatus (Osteichthyes: Trachipteridae) in Tunisian waters (central Mediterranean). ANZIAM J. 2014, 5, e59. [Google Scholar] [CrossRef]
- Psomadakis, P.N.; Bottaro, M.; Vacchi, M. On Two Large Specimens of Zu Cristatus (Trachipteridae) from the Gulf of Genoa (NW Mediterranean); Cybium: Paris, France, 2007; Volume 31, pp. 480–482. [Google Scholar]
- Tiralongo, F.; Lillo, A.O.; Tibullo, D.; Tondo, E.; Martire, C.L.; D’Agnese, R.; Macali, A.; Mancini, E.; Giovos, I.; Coco, S.; et al. Monitoring uncommon and non-indigenous fishes in Italian waters: One year of results for the AlienFish project. Reg. Stud. Mar. Sci. 2019, 28, 100606. [Google Scholar] [CrossRef]
- Macali, A.; Semenov, A.; de Mendoza, F.P.; Dinoi, A.; Bergami, E.; Tiralongo, F. Relative influence of environmental factors on biodiversity and behavioural traits of a rare mesopelagic fish, Trachipterus trachypterus (gmelin, 1789), in a continental shelf front of the Mediterranean Sea. J. Mar. Sci. Eng. 2020, 8, 581. [Google Scholar] [CrossRef]
- Bianchi, G.; Carpenter, K.E.; Roux, J.-P.; Molloy, F.J.; Boyer, D.; Boyer, H.J. Species identification guide for fishery purposes. In Field Guide to the Living Marine Resources of Namibia; FAO: Windhoek, Namibia, 1999; ISBN 9251043450. [Google Scholar]
- Whitehead, P.J.P.; Bauchot, M.L.; Hureau, J.C.; Nielsen, J.; Tortonese, E. Fishes of the North-Eastern Atlantic and the Mediterranean; UNESCO Headquarters: Paris, France, 1984; Volume 1. [Google Scholar]
- Kaminas, A.; Minasidis, V.; Doumpas, N.; Aga-Spyridopoulou, R.N. Occurrence of Trachipterus trachypterus and Zu cristatus in the Greek Seas: Contribution of citizen science projects in rare species monitoring. In Proceedings of the HydroMedit 2021; University of Thessaly: Wallos, Greece, 2021. [Google Scholar]
- Sparta’, A. Trachipteridae. Fauna e flora del golfo di Napoli. Monografia della Stazione zoologica di Napoli; Stazione Zoologica di Napoli: Napoli, Italy, 1956. [Google Scholar]
- Fisher, W.; Bauchot, M.L.; Schneider, M. Fiches FAO d’Identification des Especes Pour les Besoins de la Pêche. Méditerranée et Mer Noire (Zone de Pêche 37); FAO: Rome, Italy, 1987; Révision 1; Volume 2, Available online: http://www.fao.org/3/x0170f/x0170f00.htm (accessed on 6 March 2022).
- Bottaro, M.; Ferrando, S.; Ferrando, T.; Psomadakis, P.N.; Vacchi, M. Melanism in the gastric mucosa of the scalloped ribbonfish from the Ligurian Sea. J. Fish Biol. 2005, 66, 1489–1492. [Google Scholar] [CrossRef]
- Zenetos, A.; Akel, E.H.K.; Apostolidis, C.; Bilecenoglu, M.; Bitar, G.; Buchet, V.; Chalari, N.; Corsini-Foka, M.; Crocetta, F.; Dogrammatzi, A.; et al. New mediterranean biodiversity records (April 2015). Mediterr. Mar. Sci. 2015, 16, 266–284. [Google Scholar] [CrossRef]
- Quignard, J.P.; Tomasini, J.A. Mediterranean fish biodiversity. Biol. Mar. Mediterr. 2000, 7, 1–66. [Google Scholar]
- García-Barcelona, S.; García-Cancela, R.; Cayuela, M.J.; Fernández-Peralta, L.; de Carlos, A.; Bañón, R.; Macías, D.; Báez, J.C. Descripción de dos ejemplares de Zu cristatus (Bonelli, 1820) capturados accidentalmente con un palangre semipelágico en el Mediterráneo occidental. Arx. Miscel·Lània Zoològica 2014, 14, 91–98. [Google Scholar] [CrossRef]
- Roig, A.; Demestre, M. Nota sobre la captura de dos Zu cristatus (Bonelli, 1820) en aguas del litoral catalán (Pisces, Trachipterigidae). Misc. Zool. 1982, 6, 152–154. [Google Scholar]
- Ibáñez, M.; Gállego, L. A New Record of a Zu Cristatus (Trachipteridae Pisces) Off the Coast of Blanes (Spain). Vie Milieu 1974, 523–526. [Google Scholar]
- Papaconstantinou, C. Fauna Graeciae, IV Pisces. Check-list Mar. Fishes Greece. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2016062731 (accessed on 5 October 2022).
- Golani, D.; Ozturk, B.; Basusta, N. Fishes of the Eastern Mediterranean. Isr. J. Aquac.-Bamidgeh 2007, 59, 260. [Google Scholar] [CrossRef]
- Prakash Maurya, S.; Ohri, A.; Mishra, S. Open Source GIS: A Review. In Proceedings of the National Conference on Open Source GIS: Opportunities and Challenges Department of Civil Engineering, Varanasi, India, 9–10 October 2015; pp. 150–155. [Google Scholar]
- Orsi Relini, L.; Palandri, G.; Garibaldi, F.; Cima, C. Longline sworfish fishery in the Ligurian Sea: Eight years of observations on target and by-catch species. Collect. Vol. Sci. Pap. Comm. Conserv. Atl. TUNAS 1998, 49, 146–150. [Google Scholar]
- Sanfilippo, M.; Albano, M.; Manganaro, A.; Capillo, G.; Spanò, N.; Savoca, S. Spatiotemporal Organic Carbon Distribution in the Capo Peloro Lagoon (Sicily, Italy) in Relation to Environmentally Sustainable Approaches. Water 2022, 14, 108. [Google Scholar] [CrossRef]
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2021, 14, 398. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Spanò, N.; Rinelli, P.; Savoca, S.; Capillo, G. Basic Intersexuality (Abnormal Hermaphroditism) in the Blackmouth Catshark, Galeus melastomus, (Rafinesque, 1810), from the Southern Tyrrhenian Sea (Central Mediterranean Sea). Fishes 2022, 7, 120. [Google Scholar] [CrossRef]
- D’Iglio, C.; Famulari, S.; Albano, M.; Giordano, D.; Rinelli, P.; Capillo, G.; Spanò, N.; Savoca, S. Time-Scale Analysis of Prey Preferences and Ontogenetic Shift in the Diet of European Hake Merluccius merluccius (Linnaeus, 1758) in Southern and Central Tyrrhenian Sea. Fishes 2022, 7, 167. [Google Scholar] [CrossRef]
- Dragicevic, B.; Pallaoro, A.; Grgicevic, R.; Lipej, L.; Dulcic, J. On the occurrence of early life stage of the king of herrings, Regalecus glesne (Actinopterygii: Lampriformes: Regalecidae), in the Adriatic Sea. Acta Ichthyol. Piscat. 2011, 41, 251. [Google Scholar] [CrossRef]
- Francour, P.; Cottalorda, J.-M.; Aubert, M.; Bava, S.; Colombey, M.; Gilles, P.; Kara, H.; Lelong, P.; Mangialajo, L.; Miniconi, R. Recent occurrences of opah, Lampris guttatus (actinopterygii, lampriformes, lampridae), in the western mediterranean sea. Acta Ichthyol. Piscat. 2010, 40, 91–98. [Google Scholar] [CrossRef]
- Sang, H.M.; Lam, H.S.; Hy, L.H.K.; Ky, P.X.; Minh-Thu, P. Changes in plasma and ovarian steroid hormone level in wild female blue tang fish Paracanthurus hepatus during a reproductive cycle. Animals 2019, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Walters, V.; Fitch, J.E. The families and genera of the Lampridiform (Allotriognath) suborder Trachipteroidei. Calif. Fish Game 1960, 46, 441–451. [Google Scholar]
- Tortonese, E. Cattura di Trachypterus cristatus Bon. e note sui Trachypteridae del Mar Ligure. Doriana 1958, 2, 1–5. [Google Scholar]
- Olney, J.E. Lampridiformes: Development and relationships. Ontog. Syst. Fishes 1984, 368–379. [Google Scholar]
- Borme, D.; Voltolina, F. On the occurrence of ribbon fish Trachipterus trachypterus (Gmelin, 1789) in the Gulf of Trieste (Northern Adriatic Sea). Ann. Ser. Hist. Nat. 2006, 16, 181–188. [Google Scholar]
- Moritz, T.; Stümer, D.; Jakobsen, K.; Jakobsen, J. Observations on two live specimens of Trachipterus arcticus (Lampriformes: Trachipteridae) from the Azores. Cybium 2015, 39, 78–80. [Google Scholar]
- Rosenblatt, R.H.; Butler, J.L. The ribbonfish genus Desmodema, with the description of a new species (Pisces, Trachipteridae). Fish. Bull. 1977, 75, 843–855. [Google Scholar]
- Charter, S.R. Lampridiformes, Lophotidae, Radiicephalidae, Trachipteridae. In The Early Stages of Fishes in the California Current Region; California Cooperative Oceanic Fisheries Investigations Atlas, Ed.; Allen Press, Inc.: Lawrence, KS, USA, 1996; pp. 659–677. [Google Scholar]
- Robins, C.R.; Ray, G.C.; Douglass, J. A Field Guide to Atlantic Coast Fishes of North America; Houghton Mifflin Harcourt: Boston, MA, USA, 1986; ISBN 0395975158. [Google Scholar]
- Bolin, R.L. New Fish Records from Southern California. Copeia 1933, 1933, 35. [Google Scholar] [CrossRef]
- Tucker, A.S.; Fraser, G.J. Evolution and developmental diversity of tooth regeneration. In Proceedings of the Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 25, pp. 71–80. [Google Scholar]
- Bei, M. Molecular genetics of tooth development. Curr. Opin. Genet. Dev. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, J. Molecular genetics of supernumerary tooth formation. Genesis 2011, 49, 261–277. [Google Scholar] [CrossRef] [PubMed]



| Biometric (mm) | Present Study (Ionian Sea) | 1 Southern Tyrrhenian Sea | 2 Ligurian Sea * | 3 Ligurian Sea | 4 Iberian Sea * | 5 Central Tyrrhenian Sea | 6 Iberian Sea | 7 Balearic Sea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total length | 1210 | 876 | 1031–1219 | 1105 | 1115–700 | 875 | 878 | |||||||||
| Fork length | 1090 | |||||||||||||||
| % TL | % TL | % TL | % TL | % TL | % TL | % TL | % TL | |||||||||
| Standard length | 1060 | 87.6 | 733 | 83.7 | 926–1105 | 89.8–90.6 | 980 | 88.7 | 1000 | 89.7 | 180 | 785 | 89.7 | 803 | 91.5 | |
| % SL | % SL | % SL | % SL | % SL | % SL | % SL | % SL | |||||||||
| Head length | 181 | 17.1 | 128 | 17.5 | 153–191 | 16.5–17.3 | 160 | 16.3 | 165–85 | 38 | 21 | 175 | 22.3 | 165 | 20.5 | |
| Pre-orbital length | 50 | 4.7 | 39.6 | 5.4 | 5.6 | 5.6 | 5–3 | 55 | 7 | |||||||
| Post-orbital length | 58 | 5.5 | 5.5–2.7 | |||||||||||||
| Eye diameter | 62 | 5.8 | 47.8 | 6.5 | 6.2 | 5.8 | 66 | 8.4 | 54 | 6.7 | ||||||
| Opeculum height | 198 | 18.7 | 146.2 | 19.9 | ||||||||||||
| Upper jaw length | 75 | 7.1 | 53.7 | 7.3 | 68 | 8.5 | ||||||||||
| Lower jaw length | 107 | 10.1 | 75.3 | 10.3 | 9.2 | 93 | 11.6 | |||||||||
| Pre-pectoral length | 161 | 15.2 | ||||||||||||||
| Pectoral fin length | 74 | 7.0 | 47.7 | 6.5 | 6.1–6.5 | 6.6 | 7–3.5 | 65 | 8.3 | 61 | 7.6 | |||||
| Width of pectoral fin base | 13 | 1.2 | 10 | 1.4 | ||||||||||||
| Maximum height of dorsal fin | 67 | 6.3 | 91.3 | 12.5 | 6.6 | 7.1 | ||||||||||
| Dorsal fin length | 950 | 89.6 | 840 | 104.6 | ||||||||||||
| Caudal fin length | 128 | 12.1 | 130.2 | 17.3 | 10.3 | 12.8 | 11.5–11 | 90 | 11.5 | 66 | 8.2 | |||||
| Lateral line length | 1000 | 94.3 | ||||||||||||||
| Spiny lateral line length | 520 | 49.1 | ||||||||||||||
| Maximum height of the body | 230 | 21.7 | 19.7–21.3 | 21.4 | 20.5–11 | 41.4 | 22.8 | 195 | 24.8 | 125 | 15.6 | |||||
| % HL | % HL | % HL | % HL | % HL | % HL | % HL | % HL | |||||||||
| Pre-orbital length | 50 | 27.6 | 39.6 | 30.9 | 29.4–32.5 | 34.4 | 55 | 31.4 | ||||||||
| Post-orbital length | 58 | 32.0 | 46.5 | 36.3 | ||||||||||||
| Eye diameter | 62 | 34.3 | 47.8 | 37.3 | 34.6–35.6 | 35.6 | 2.1 | 5.6 | 66 | 37.7 | 54 | 32.7 | ||||
| Opeculum height | 198 | 109.4 | 146.2 | 114,2 | ||||||||||||
| Upper jaw length | 75 | 41.4 | 53.7 | 41.9 | 68 | 41.2 | ||||||||||
| Lower jaw length | 107 | 59.1 | 75.3 | 58.8 | 93 | 56.4 | ||||||||||
| Total weight (g) | 4000 | 1301 | 4400 | 2800 | 2160–500 | |||||||||||
| Meristic (Counts) | Present Study (Ionian Sea) | 1 Southern Tyrrhenian Sea | 2 Ligurian Sea * | 3 Ligurian Sea | 4 Iberian Sea * | 5 Central Tyrrhenian Sea | 6 Iberian Sea | 7 Balearic Sea |
|---|---|---|---|---|---|---|---|---|
| Lateral line scales | 101 | 102–96 | 107 | 96 | ||||
| Last spine | 47 | 38 | ||||||
| Pectoral fin rays | 10 | 11 | 10–11 | 10 | 11–11 | 12 | 11 | 11 |
| Dorsal fin spines | 6 | |||||||
| Dorsal fin rays | 122 | 126 | 125–130 | 125 | 117 (6–111)–132 (6–126) | 120 | 120 | 119 |
| Caudal fin rays | 9 + 3 | 9 | 9–9 | 9 + 1 | 9 + 4–9 + 4 | 9 + 3 | ||
| Caudal fin spines | 9 | |||||||
| Upper jaw teeth | 18 | 18 | 14–21 | 16 | 8 | |||
| Lower jaw teeth | 12 | 12 | 10–10 | 10 | 12 | |||
| Palatine teeth | 4 | 4 | 4–4 | 2 | 4 | |||
| Vomerine teeth | 4 | 3 | 3–4 | 2 | 3 | |||
| Gill rakes total | 11 | 11 | 10–11 | 11 | 10–10 | 11 | 11 | |
| Gill rakes epibranchial | 3 | 3 | 3–3 | 2 | 2–2 | 3 | 3 | |
| Gill rakes ceratobranchial | 8 | 8 | 7–8 | 9 | 8–8 | 8 | 8 | |
| Vertebrae | 62 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, M.; D’Iglio, C.; Spanò, N.; Di Paola, D.; Alesci, A.; Savoca, S.; Capillo, G. New Report of Zu cristatus (Bonelli, 1819) in the Ionian Sea with an In-Depth Morphometrical Comparison with All Mediterranean Records. Fishes 2022, 7, 305. https://doi.org/10.3390/fishes7060305
Albano M, D’Iglio C, Spanò N, Di Paola D, Alesci A, Savoca S, Capillo G. New Report of Zu cristatus (Bonelli, 1819) in the Ionian Sea with an In-Depth Morphometrical Comparison with All Mediterranean Records. Fishes. 2022; 7(6):305. https://doi.org/10.3390/fishes7060305
Chicago/Turabian StyleAlbano, Marco, Claudio D’Iglio, Nunziacarla Spanò, Davide Di Paola, Alessio Alesci, Serena Savoca, and Gioele Capillo. 2022. "New Report of Zu cristatus (Bonelli, 1819) in the Ionian Sea with an In-Depth Morphometrical Comparison with All Mediterranean Records" Fishes 7, no. 6: 305. https://doi.org/10.3390/fishes7060305
APA StyleAlbano, M., D’Iglio, C., Spanò, N., Di Paola, D., Alesci, A., Savoca, S., & Capillo, G. (2022). New Report of Zu cristatus (Bonelli, 1819) in the Ionian Sea with an In-Depth Morphometrical Comparison with All Mediterranean Records. Fishes, 7(6), 305. https://doi.org/10.3390/fishes7060305












