Abstract
The estuarine tapertail anchovy Coilia nasus is distributed throughout the Dayang River. However, the life history and habitat use of this fish remain unknown. Here, the microchemistry patterns of Sr and Ca in 23 otoliths collected from the lower reaches of the Dayang River were analyzed using an X-ray electron probe microanalyzer. The anchovies were divided into two patterns: (1) with low Sr/Ca ratios (<3.0) and a single bluish Sr concentration map, indicating that it only experienced a freshwater habitat during its whole life, and (2) with Sr/Ca ratios fluctuating between low and high (>3.0) phases and Sr concentration maps showing various colors, including blue, green, yellow, and red, from the core to the edge of otoliths, whose larvae hatched in freshwater and spent their first winter in brackish or sea waters. The juveniles then stayed in estuarine water areas for further growth and feeding until sexual maturity, when the mature adults returned to the spawning grounds in the river. The co-existence of freshwater residents and anadromous C. nasus in the Dayang River has been studied for the first time, and its possible spawning ground was discovered. These findings provide essential information to effectively protect this species and guide its rational, sustainable utilization.
Keywords:
Coilia nasus; Dayang River; otolith; strontium; calcium; life history; habitat use; anadromy; residency 1. Introduction
The estuarine tapertail anchovy Coilia nasus (junior synonym C. ectenes) is an anadromous engraulid fish with a high commercial and economic value and is widely distributed in the riverine, coastal, and estuarine waters of China, Japan, and Korea [1,2,3,4,5,6]. On reaching sexual maturity, adult individuals migrate upstream annually from the estuaries to spawn in freshwater areas during the spawning season [7]. After hatching, the larvae and juveniles grow in the freshwater habitat for approximately one year and then enter brackish and sea waters, where they continue feeding until they reach sexual maturity [1]. Unfortunately, owing to habitat destruction, overfishing, dam construction, and other anthropogenic impacts, the populations of this small anchovy have decreased sharply in the early 21st century [8,9] and the species has been listed on the IUCN Red List of Threatened Species (Version 2022-1).
Identifying the patterns of the fish’s movement and its critical habitat will help clarify the life-history traits of this species, which is important for effective conservation [10,11]. Otolith microchemistry has received increasing attention as an effective and a convenient tool for understanding the life histories of different species [12,13]. Trace-element deposition in otoliths is affected by physiological and environmental factors [14], where ambient elemental concentration variation is a primary factor that impacts element uptake in fish [15,16]. As otoliths are continuously growing and non-metabolizable, trace-element variation from the core to the edge of otoliths can provide valuable information about the environmental conditions experienced by fish throughout their life stages [12,13]. In addition, trace-element ratios can be used to reconstruct environmental migration patterns, habitat utilization, and other aspects of migration ecology [12,17]. The Sr/Ca ratio in otoliths is usually positively correlated with the salinity of ambient water [18,19], which can accurately reveal the changes in the salinity gradient associated with the life histories of individual migratory fish [1,5,7,11,20,21].
The Dayang River originates from Xiuyan County, Liaoning Province. The basin passes through Xiuyan County, Donggang City, and finally flows into the Yellow Sea at Huangtukan Town [22]. The total length is 202 km with an area of approximately 6504 km2, making it the longest sea-flowing river between the Liaohe and Yalu rivers [23]. Currently, only a certain population level of C. nasus exists in the Dayang River Basin in Liaoning [24]. However, the life history and habitat use patterns of C. nasus in the Dayang River have not yet been studied, although many studies have reported the composition of spawning groups, migratory patterns, phenotypes of individuals, and habitat use for C. nasus from the Yangtze, Qiantang, and Huanghe rivers [1,2,3,25,26,27]. The genetic differences of mtDNA cytochrome b (Cyt b), or the displacement loop (D-loop), between C. nasus from the Yangtze River Basin and from the Dayang or Yalu rivers (adjacent to the Dayang River) [28,29] lead us to hypothesize that the life history of C. nasus in the Dayang River may differ from those in other waters. Mature Yangtze River C. nasus can migrate 800 km upstream from the Yangtze River estuary to Poyang Lake for spawning [26], while the Yellow River C. nasus can migrate to Dongping Lake for spawning [27]. However, the migration distance of the Dayang River C. nasus might be extremely short, as the river flow is obstructed by a dam (30 km upstream from the estuary) [30], which may lead to unique migration patterns and habitat use of the Dayang River C. nasus. In the present study, the Sr and Ca concentrations in the otoliths of C. nasus from the Dayang River were analyzed for the first time using an electron probe microanalyzer to objectively observe the ecological type of C. nasus and understand its life history and habitat use. The findings will act as a reference and theoretical basis for targeted resource conservation of C. nasus.
2. Materials and Methods
2.1. Study Sites and Fish Sampling
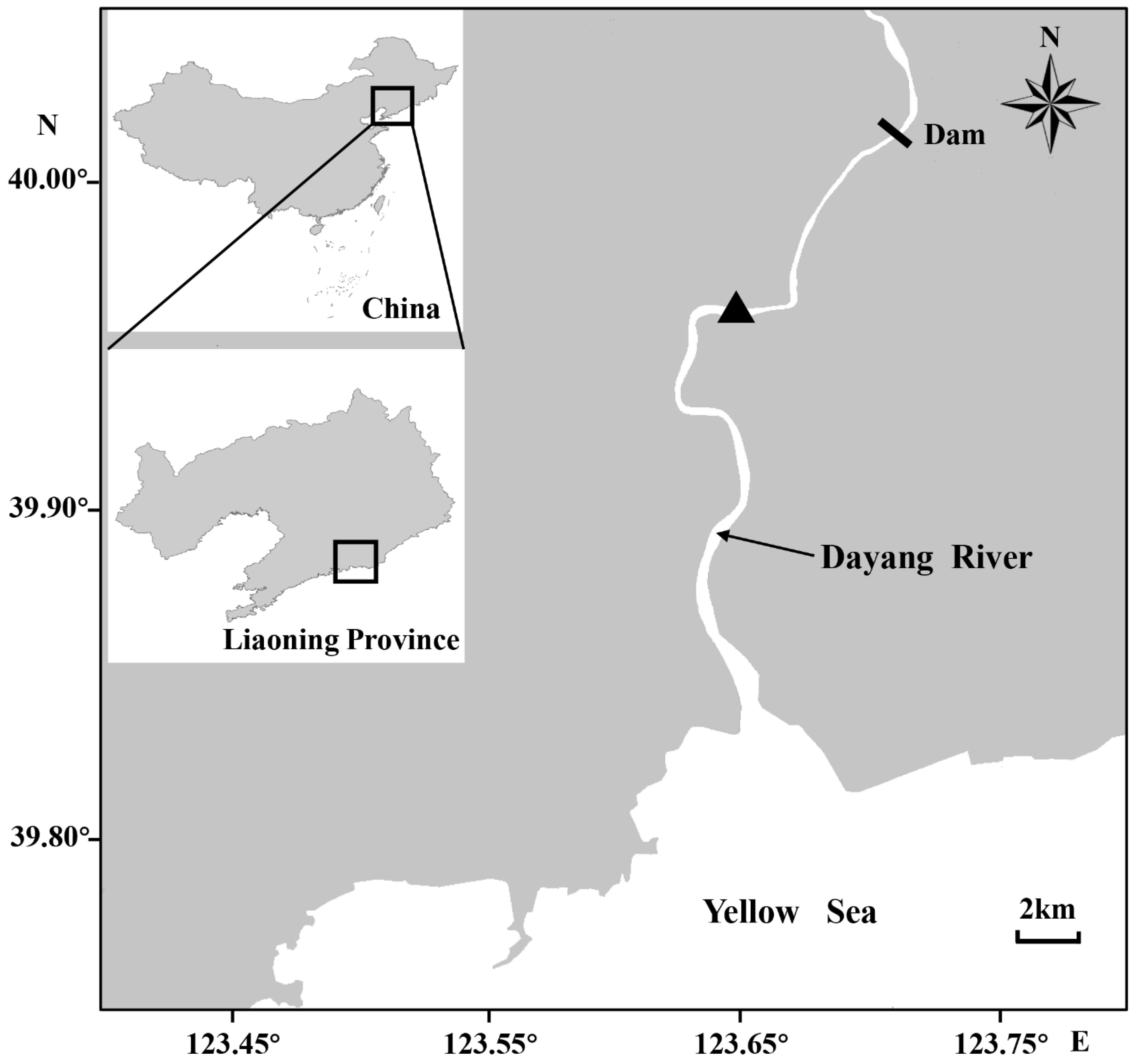
In total, 23 C. nasus individuals were caught with a gill net of 45 mm mesh size, 80 m width, and 1.5 m height from the lower reaches of the Dayang River (DYH, 39°96′ N, 123°66′ E) in southeast Liaoning Province, China, in July 2019 (Figure 1). The body weight was measured using an electronic balance (WPB3K01, AS ONE Crop., Osaka, Japan) having an accuracy of 0.1 g. The body length was measured using a steel scale (Wuhan Tools and Equipment Co., Ltd., Wuhan, China) having an accuracy of 1 mm. To further identify each C. nasus individual up to the long or short upper jaw phenotype along with its ecomorphotypes according to Jiang et al. [31], the upper jaw length and head length were measured in all individuals using Fisherbrand Traceable Digital Calipers (Thermo Fisher Scientific Inc., Waltham, MA, USA) having an accuracy of 0.02 mm; subsequently, the jaw length/head length ratio was calculated (Table 1). The sexuality and sexual maturity stage were determined by visual examination according to the gonad stages of maturation, as described by Xu et al. [32,33]. All fishes were 2–3-year-old mature adults with a mean body length of 257 ± 32 mm and mean weight of 60.1 ± 25.2 g (Table 1). After measurement, the sagittal otoliths were removed from all fish specimens. Subsequently, the otoliths were washed and dried for further experimentation.
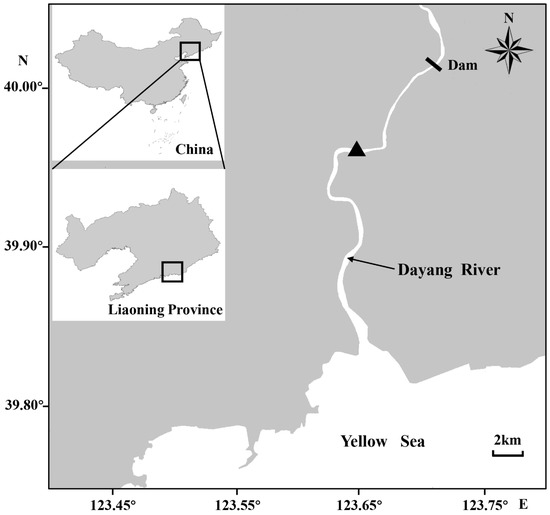
Figure 1.
Sampling site (▲) of Coilia nasus in the Dayang River in southeast Liaoning Province, China.

Table 1.
Sampling details of Coilia nasus.
2.2. Otolith Preparation and Microchemical Analysis
Only the left otolith of each pair of the sagittal otoliths was used in this study. Otoliths were completely embedded in epoxy resin (Epofix; Struers, Copenhagen, Denmark) and ground to expose the core along the sagittal section using a Discoplan-TS grinding machine (Struers, Copenhagen, Denmark). All otoliths were polished (LaboPol-35, Struers, Copenhagen, Demark) with OP-S liquid (Struers, Copenhagen, Denmark), cleaned in an ultrasonic bath, rinsed with Milli-Q water with a resistivity of 18.2 mΩ/cm, and completely dried with a coated carbon plating film (36A, 25S) using a high-vacuum evaporator (JEE-420, JEOL Ltd., Tokyo, Japan) for further analyses.
A wavelength-dispersive X-ray electron probe microanalyzer (JXA-8100 JEOL Ltd., Tokyo, Japan) was used to quantify Sr and Ca concentrations in otoliths, similar to the methods described by Liu et al. [4]. Calcite (CaCO3) and Tausonite (SrTiO3) were used as standards before measuring the Sr and Ca element concentrations along a continuous line down the longest axis of each otolith from the core to the edge in the otoliths. The accelerating voltage was 15 kV, the beam current was 2 × 10−8 A, and the electron beam was focused on a 5 μm diameter point, with measurements spaced at 10 μm intervals. Subsequently, the accelerating voltage was left unchanged, the electron beam current was 5.0 × 10−7 A, the beam spot diameter was 5 μm, the pixel size was 7 μm × 7 μm in diameter, and the counting time was 30 ms to generate the X-ray intensity maps of Sr concentration for the representative otoliths using the same microprobe.
2.3. Otolith Age Determination
Once all the otoliths were analyzed, the carbon coating was removed by polishing and then etched with 5% ethylenediaminetetraacetic acid (EDTA) to reveal the annulus marks for determining the individual ages [3].
2.4. Data Analysis
The data were statistically analyzed using Excel 2016 (Microsoft, Seattle, WA, USA) and IBM SPSS Statistics v. 19.0 (IBM Corp., Armonk, NY, USA). A sequential regime shift algorithm was applied to identify the different phase transition curves of Sr/Ca ratio (customarily using (Sr/Ca) × 1000 throughout the study. According to the parameter settings given by Liu et al. [4], the cut-off length, significance level, and Huber’s weight parameter were 10, 0.1, and 1, respectively. Based on previous studies [2,3,4,26,34] on the microchemical characteristics of some trace elements in C. nasus otoliths, the Sr/Ca ratio in otoliths was found to be related to the salinity of the water where the individual anchovies lived, and 3 and 7 were used as critical values to distinguish low-salinity freshwater, medium-salinity brackish water, and high-salinity seawater. Simultaneously, values corresponding to Sr concentration patterns were represented as bluish (low-salinity), greenish-yellowish (medium-salinity), and reddish (high-salinity) in a 16-color map. These patterns provided comparative references to distinguish anadromous and freshwater resident C. nasus individuals. It also suggested habitat use objectively and intuitively.
3. Results
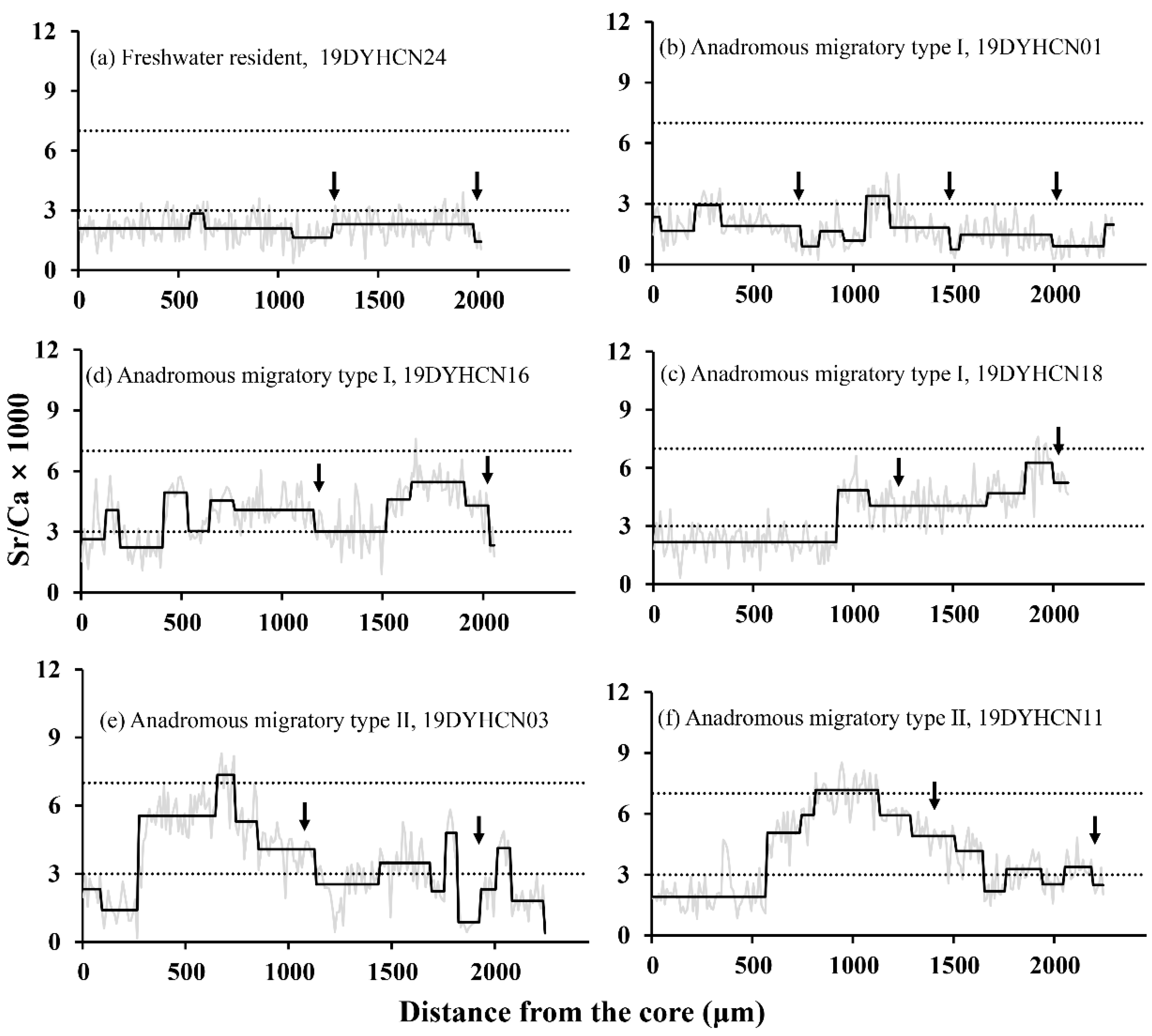
According to Figure 2, Figure 3 and Figure 4, the Sr/Ca ratio of Ca and Sr concentration in otoliths showed the following three types of otolith microchemistry patterns: (1) Freshwater resident (FR): the Sr/Ca ratios from the core to the edge of only one otolith (19DYHCN24, female) remained stable below the Sr/Ca fresh water threshold (1.83 ± 0.84) corresponding to the bluish spectrum of low Sr concentration (Figure 2a and Figure 4FR), indicating that the individual’s entire life cycle was completed in low-salinity fresh water. (2) Anadromous migratory Type I (AM I): The otoliths of 16 individuals (seven females and nine males) had low Sr/Ca ratios (1.52 ± 0.61–2.79 ± 1.23) below the Sr/Ca freshwater threshold for early life stages, while the Sr/Ca ratios went up to medium (3.26 ± 0.90–4.61 ± 1.03) in the later phase. Subsequently, it alternated between low and medium Sr/Ca ratios, and the final edge had a low Sr/Ca ratio of less than three. The otolith of the individual 19DYHCN18 had only two phases of change, while the remaining individuals had three or more phases (Figure 2b–d).
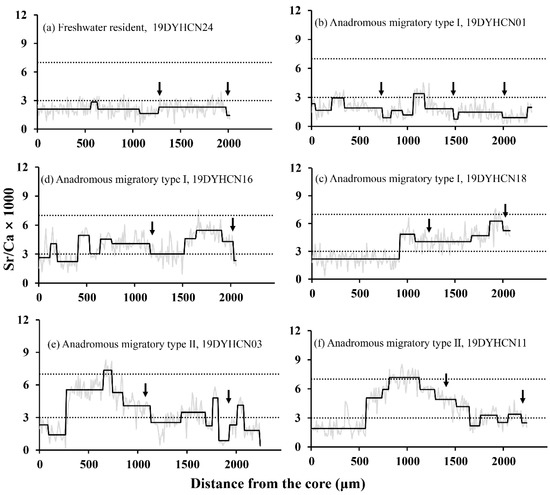
Figure 2.
Representative otolith core-to-edge Sr/Ca patterns of freshwater resident (a), anadromous migratory Type I (b–d), and Type II (e,f) of C. nasus individuals. The gray lines and wide black lines show the fluctuations in the Sr/Ca ratios and shifts, respectively. The two horizontal imaginary lines denote reference boundaries for dividing Sr/Ca ratios as freshwater (≤3), brackish water (3–7), and sea water (>7). The black arrows indicate the positions of the annuli.
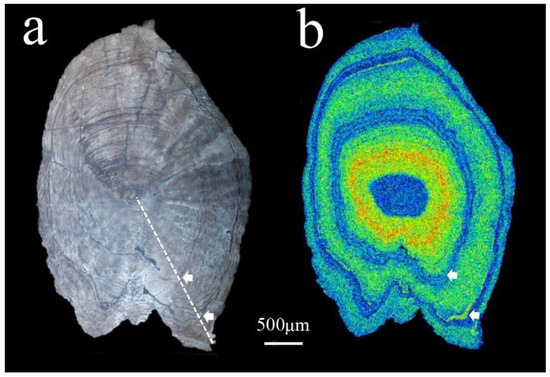
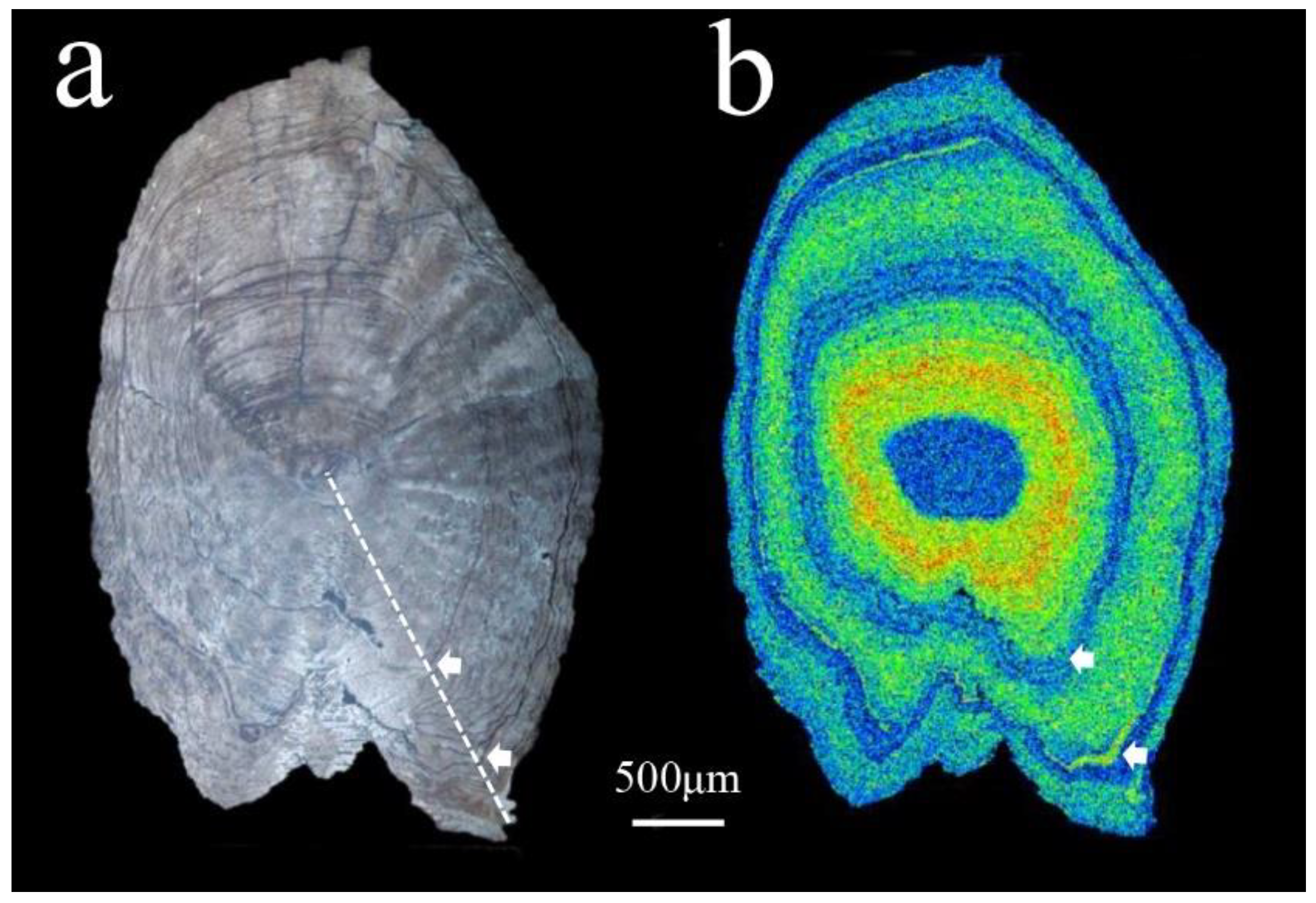
Figure 3.
Sagittal plane of the otolith etched with EDTA. (a) The white dashed line denotes the transect direction of the Sr/Ca ratio, and the arrows show the annuli. (b) Two-dimensional X-ray intensity map of the Sr concentrations.
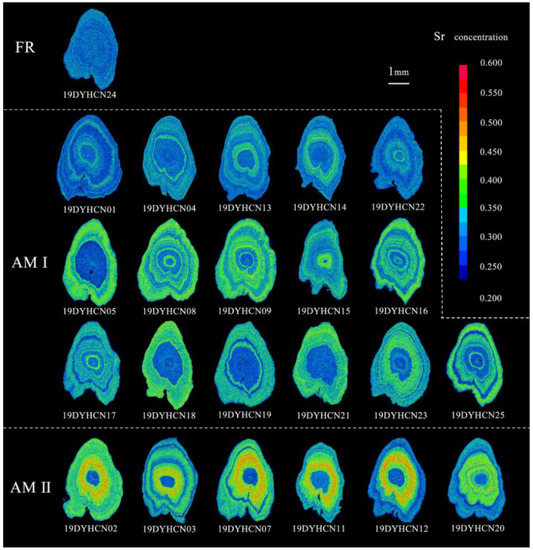
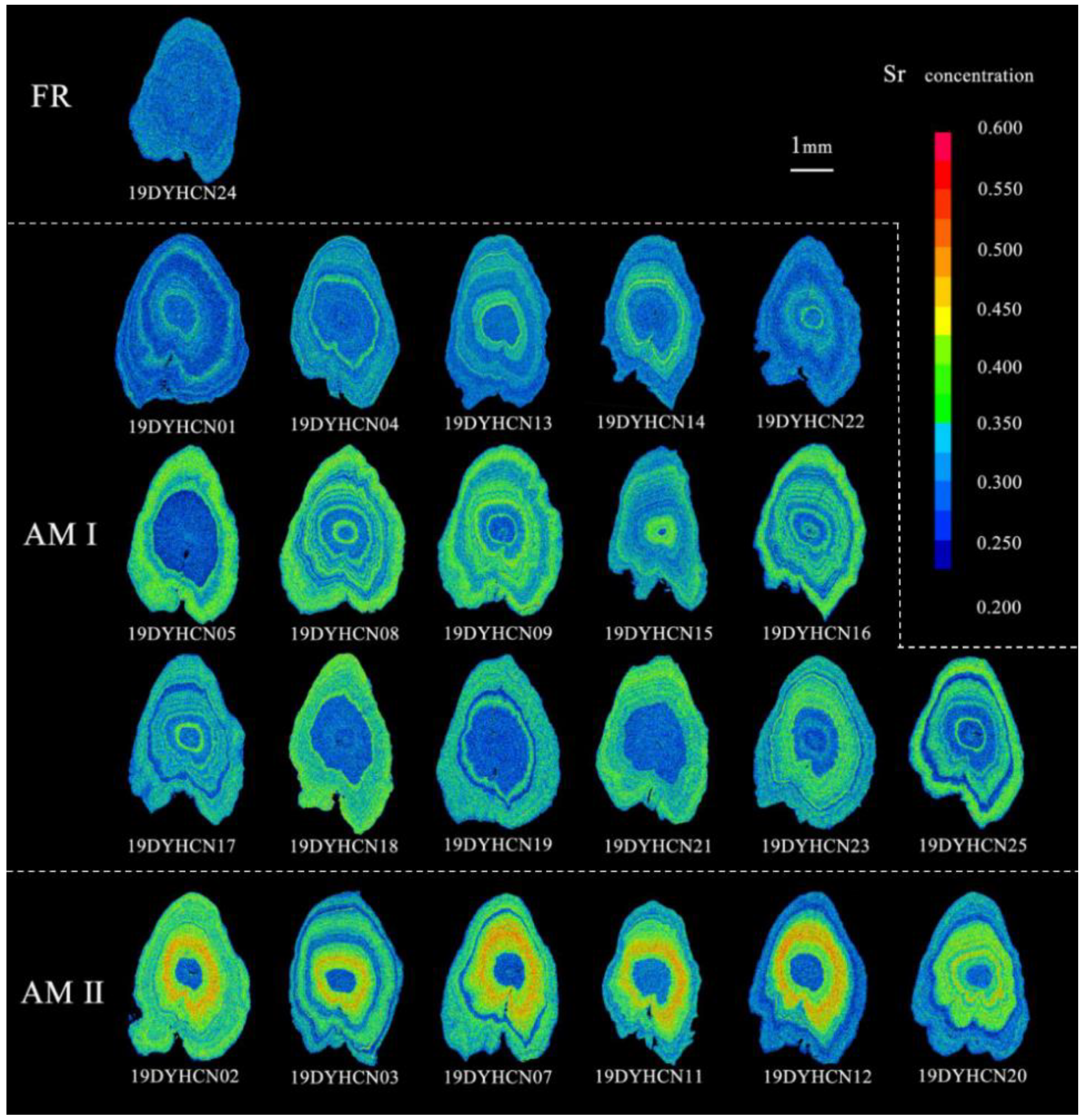
Figure 4.
Two-dimensional X-ray intensity maps of the Sr concentrations in the sagittal plane of the otoliths for the three types (FR: freshwater resident, AM I: anadromous migratory Type I, AM II: anadromous migratory Type II). The values corresponding to Sr concentrations are represented by 16 colors from blue (lowest) to red (highest).
The bluish and greenish-yellowish spectrums can be clearly seen in the corresponding two-dimensional X-ray intensity maps of Sr concentration, which exhibit the same pattern as the results of transect analysis (Figure 2 and Figure 4AM I). These individuals hatched in freshwater while the larvae and juveniles grew and fed in freshwater and estuarine environments and then finally migrated to the freshwater environment to spawn. (3) Anadromous migratory Type II (AM II): The Sr/Ca ratios of the remaining six specimens (four females and two males) increased from a low value (1.65 ± 0.54–2.15 ± 0.58) of the core regions to a medium value (5.02 ± 1.66–6.29 ± 1.06) in the second phase and continued to increase to a high value (7.11 ± 0.79–7.94 ± 0.73) in the third phase. After a certain distance, it descended to a medium ratio (4.50 ± 0.95–5.59 ± 1.40) in the fourth stage, and the Sr/Ca ratio of the final edge was below the Sr/Ca freshwater threshold. The otoliths of individuals 19DYHCN03 and 19DYHCN11 exhibited alternating low and medium ratios many times after the fourth stage (Figure 2e, f). Moreover, the two-dimensional X-ray intensity maps of Sr concentration clearly show the variation trend of bluish (central region), greenish-yellowish, reddish, greenish-yellowish, and bluish (edge) spectrums (Figure 4AM II). The life-history patterns of most individuals were of anadromous migratory Type I. The migratory type individuals moved to seawater for growth, feeding, and overwintering in addition to living in freshwater and brackish environments.
All individuals were aged 2–3 years. For example, only two annuli were obtained by etching the sagittal otolith plane of a two-year-old individual (19DYHCN03) (Figure 3a) to EDTA. Combined with its two-dimensional X-ray intensity map of Sr concentrations (Figure 3b), the greenish-yellowish spectrums representing brackish water are found within the first annulus, which means this fish moved from fresh water to brackish water before it was one-year-old. The small fraction of the bluish spectrums at the edge outside the second annulus suggested that C. nasus moved quickly upstream to the sampling site from brackish water when it reached sexual maturity after turning two years old.
4. Discussion
Coilianasus can be divided into two phenotypes by the ratio between their upper jaw and head lengths. The long-jaw C. nasus (upper jaw length/head length >1) are traditionally believed to be anadromous individuals with high economic value, while short-jaw C. nasus (upper jaw length/head length <1) are traditionally believed to be freshwater resident individuals with low economic value [31]. The jaw lengths of all C. nasus from the Dayang River were longer than the head length of a typical long-jaw phenotype anchovy (Table 1). Notably, C. nasus could be divided into three ecotypes: i.e., anadromous migratory type, freshwater resident type, and landlocked type [2,3,34,35], and further into five ecomorphotypes (i.e., anadromous migratory, freshwater resident long-jaw C. nasus, anadromous migratory, freshwater resident long-jaw C. nasus, and landlocked C. nasus) [31]. The freshwater resident long-jaw phenotype C. nasus is relatively rare [1,2,3,4]. In the present study, the otolith of only one fish (19DYHCN24) exhibited low Sr/Ca ratios and a bluish map of Sr concentration. Its microchemical characteristics were comparable to those of freshwater resident C. nasus described by Chen et al. [2] and landlocked C. nasus described by Sokta et al. [35], which suggested that its entire life cycle occurred in freshwater (Figure 5A). The individual 19DYHCN24 was sampled in the main stream of the Dayang River, and hence, its ecomorphotype was not landlocked but freshwater resident long-jaw C. nasus.
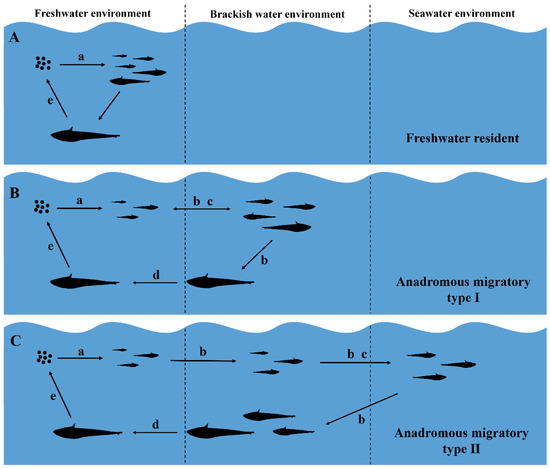
Figure 5.
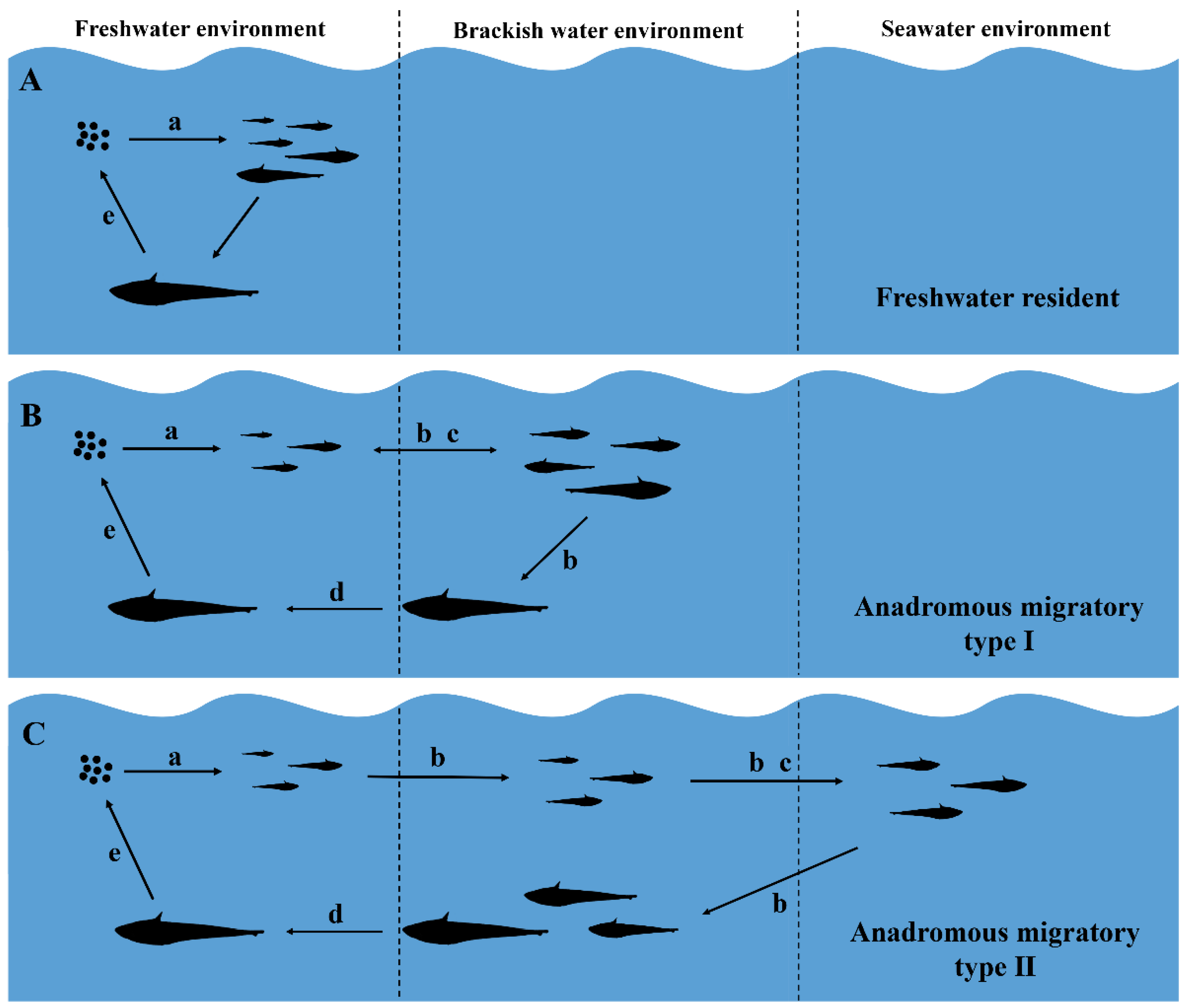
Life-history and habitat-use patterns observed for C. nasus from the Dayang River, China. (A) Freshwater resident, i.e., lived in freshwater throughout its entire life. (B) Anadromous migratory Type I, i.e., spawned in freshwater and moved at least once to a brackish environment (freshwater–brackish). (C) Anadromous migratory Type II, i.e., spawned in freshwater, moved to seawater, and then to brackish environment (freshwater–brackish–seawater). Notes: a: hatching; b: growth and feeding; c: overwintering; d: migrating; e: spawning.
In addition, another life-history pattern was as follows: hatching and feeding of larvae occurred in a low-salinity environment; the juveniles entered medium (estuary) or high-saline (northern Yellow Sea) environments for overwintering, grew in the waters near the estuary, and migrated upstream to the spawning grounds for spawning after reaching initial sexual maturity (Figure 5B,C). Each of the otoliths had a distinct blue core region that varied in size, which indicated that these individuals had spawned eggs and hatched in a low-salinity environment and that the spawning grounds of C. nasus exist in the freshwater reaches of the Dayang River. A survey showed that the dam 30 km above the estuary did not necessarily affect the upward migration of ayu (Plecoglossus altivelis), but no C. nasus were found in the middle reaches of the river above the dam [23]. Zhang et al. [30] also indicated that the dam could obstruct the migration of C. nasus. Therefore, it is reasonable to believe that the dam would most likely block the upward migration of C. nasus, leading to the formation of spawning grounds below the dam. The anchovies’ stage IV, V, or Ⅵ gonads were defined as fully mature [36], and the waters in which the fully mature females were caught were spawning areas [37,38]. We captured some fully mature individuals with stage IV and especially stage V gonads from the area below the dam. The bottom of the river near the sampling site was mostly muddy, and the reach was a typical “S” shape (Figure 1), which was consistent with Xue et al.’s [39] description of the spawning habitat characteristics. This suggested that the water near the sampling site was a spawning area for C. nasus. The larvae hatched from the fertilized eggs are weak and drift passively with the water current [40,41], which suggests that the duration that C. nasus lived in freshwater in its early days reflects the distance between spawning grounds and the estuary [42]. In this study, we observed that most individuals took only a few months after hatching to first enter the estuary, whose two-dimensional maps of Sr concentrations in otoliths had a small proportion of blue core areas representing freshwater habitats. Such individuals with an early short-term use of freshwater also existed in the Qiantang, Oujiang, and Chikugo rivers [3,4,42]. These rivers are shorter in length compared with the spawning ground of C. nasus near the estuary [3,5,39,42]. This supports the existence of the spawning ground of anchovy C. nasus below the dam in the Dayang River.
Li et al. [43] found that the annulus on the otolith of C. nasus usually forms after winter between April and June. Interestingly, the Sr/Ca ratios of these individuals increased well before the first annulus, which means that the individual entered the estuary long before the following spring. The Dayang River was different from the low-latitude rivers, such as the Yangtze and Qiantang rivers. The frozen period of its estuarine section lasts for four months starting at the end of November every winter; the thickness of the ice was 30–50 cm and the water temperature reached as low as 0.4 °C [44]. Notably, Li et al. [45] found that a mass mortality of C. nasus fries occurred in outdoor ponds, as they could not overwinter when the water temperature decreased to approximately 8 °C. Therefore, the fries and juveniles of the fish might migrate from freshwater to the estuary and even to the north shore of the Yellow Sea for overwintering before the Dayang River freezes. Zhang et al. [30] also found that Dayang River C. nasus started entering the sea by the end of October, which was consistent with the observations of this study. Therefore, overwintering conditions (e.g., water temperature) could also affect the utilization of freshwater in the early life stage. However, a few individuals (19DYHCN04, -05, and -19) chose to overwinter in freshwater and began to enter the estuary in the next spring. Owing to the freezing and shallow depth in the river [44], the numbers of individuals overwintering in freshwater were relatively small.
After the first overwintering, fish mainly completed their next growth, feeding, and overwintering in the brackish and freshwater environments near the mouth of the Dayang River. Until they reached sexual maturity at the age of 2 or 3 years, the mature C. nasus started moving upstream from the estuary in April [30]. Furthermore, many otoliths had distinct alternating blue and yellow-green phases along their edges, indicating more than one anadromous migration. There are two possible reasons for this case: First, as the Dayang River is a mountain river, it experiences rapid water flow during the rainy season. Thus, upstream adults are carried passively into the estuary by fast-flow and returned to the freshwater spawning grounds after the flood ends. The anadromous individuals usually resume food intake when they reach spawning grounds [46] so that they acquire the energy needed for the final maturation of gonads and reproductive activities [47,48]. Rainwater brings a large amount of sediment from the upper stream, resulting in a sharp decline of food resources in the freshwater that consequently causes a deprivation and fails to meet the energy requirements. Thus, fish actively enter brackish water having rich forage to ensure the maturation of their gonads, suggesting that the selection and utilization of habitat by this anchovy are quite flexible. Some individuals (19DYHCN07 and 19DYHCN18) did not show the bluish stage at the otolith edge. Although Sr has a time-lag effect in the biomineralization process from water to body to otolith [49], the Sr/Ca ratios in the otolith could not quickly drop to the freshwater level after C. nasus rapidly migrated to the spawning grounds in freshwater. The otoliths of the Yangtze River C. nasus also showed this pattern [26].
Compared with the habitat-use patterns of the C. nasus population from the Yangtze River [1,2,26], the anadromous migratory long-jaw ecomorphotype C. nasus from the Dayang River generally has a short initial riverine life history, and its use of freshwater, estuarine, and seawater habitats is more flexible. The life-history pattern of most individuals was AM I, which shows that the habitat use of the Dayang River C. nasus has an estuarine dependency. Females and males had similar anadromous migratory life-history patterns. Additionally, one female showed freshwater resident patterns, which suggests that females probably have a relatively higher ability to adapt to complex habitats than males. In recent years, overfishing, water pollution, or other human impacts have substantially decreased C. nasus populations in the Dayang River, and this species has been included in List of Wildlife under Special Protection of Liaoning Province. Therefore, to restore the Dayang River basin to ensure sustainable recovery of natural C. nasus resources, we suggest that future efforts should focus on the strict control of fishing intensity and the discharge of wastewater, and fisheries’ enforcement should be strengthened in the river section from the dam to the estuary. As a typical river–sea migratory fish species, the river study of the recovery of C. nasus will provide reference for the recovery of other migratory fish resources.
5. Conclusions
Using the advantages of fish otolith microchemical techniques, this is the first study in which we observed the existence of both freshwater resident and anadromous migratory C. nasus in the Dayang River and reconstructed its life-history pattern and habitat use. This study also provides reference materials and a theoretical basis for the conservation of the targeted and local anchovy resources. In future studies, we will expand the scope of investigation, increase the number of samples, and combine otolith microchemistry with biomolecule technology to accurately confirm the population distribution, nursery grounds, feeding grounds, wintering grounds, and spawning grounds of migratory C. nasus in the Dayang River basin and its surrounding areas. Moreover, as the economic values of freshwater resident and anadromous migratory C. nasus are completely different, the resources of the two C. nasus ecotypes in the Dayang River basin should be monitored to promote its sustainable utilization in the future.
Author Contributions
Conceptualization, Y.H., J.Y., T.J., H.L. and X.C.; methodology, Y.H., T.J., H.L. and J.Y.; investigation, Y.H., T.J., H.L. and X.C.; resources, J.Y.; funding acquisition, T.J.; writing—original draft, Y.H., T.J., H.L. and X.C.; writing—review and editing, J.Y.; supervision, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Central Public-interest Scientific Institution Basal Research Fund, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences; grant number 2021JBFM14.
Institutional Review Board Statement
All Coilia nasus samples were dead individuals acquired from legal commercial fisheries. Therefore, approval by the Animal Ethics Committee was not required.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Jilong Wang of Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences for his support in Coilia nasus sampling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, T.; Yang, J.; Liu, H.B.; Shen, X. Life history of Coilia nasus from the Yellow Sea inferred from otolith Sr:Ca ratios. Environ. Biol. Fish. 2012, 95, 503–508. [Google Scholar] [CrossRef]
- Chen, T.T.; Jiang, T.; Liu, H.B.; Li, M.M.; Yang, J. Do all long supermaxilla-type estuarine tapertail anchovies (Coilia nasus Temminck et Schlegel, 1846) migrate anadromously? J. Appl. Ichthyol. 2017, 33, 270–273. [Google Scholar] [CrossRef]
- Khumbanyiwa, D.D.; Li, M.M.; Jiang, T.; Liu, H.B.; Yang, J. Unraveling habitat use of Coilia nasus from Qiantang River of China by otolith microchemistry. Reg. Stud. Mar. Sci. 2018, 18, 122–128. [Google Scholar] [CrossRef]
- Liu, H.B.; Jiang, T.; Yang, J. Unravelling habitat use of Coilia nasus from the Rokkaku River and Chikugo River estuaries of Japan by otolith strontium and calcium. Acta Oceanol. Sin. 2018, 37, 52–60. [Google Scholar] [CrossRef]
- Itakura, H.; Yokouchi, K.; Kanazawa, T.; Matsumoto, M.; Matoba, T.; Wakiya, R.; Shirai, K.; Ishimatsu, A. Diverse downstream migration patterns of the anadromous Japanese grenadier anchovy Coilia nasus in the Chikugo River estuary and Ariake Sea, Japan. Reg. Stud. Mar. Sci. 2020, 39, 101436. [Google Scholar] [CrossRef]
- Jo, S.G.; Yoon, J.M. Genetic distances between tailfin anchovy (Coilia nasus) populations analyzed by PCR. Dev. Reprod. 2021, 25, 59–65. [Google Scholar] [CrossRef]
- Dou, S.Z.; Yokouchi, K.; Yu, X.; Cao, L.; Kuroki, M.; Otake, T.; Tsukamotoet, K. The migratory history of anadromous and non-anadromous tapertail anchovy Coilia nasus in the Yangtze River Estuary revealed by the otolith Sr:Ca ratio. Environ. Bio. Fish. 2012, 95, 481–490. [Google Scholar] [CrossRef]
- Tian, S.Q.; Tian, Z.Q.; Gao, C.X.; Dai, X.J. Analyzing of annual changes for the stock status of Coilia nasus in fishing season in Yangtze River estuary. J. Shanghai Ocean Univ. 2014, 23, 245–250. [Google Scholar]
- Guo, H.Y.; Zhang, X.G.; Tang, W.Q.; Li, H.H.; Shen, L.H.; Zhou, T.S.; Liu, D. Temporal variations of Coilia nasus catches at Jingjiang section of the Yangtze River in fishing season in relation to environmental factors. Resour. Environ. Yangtze Basin. 2016, 25, 1850–1859. [Google Scholar] [CrossRef]
- Beger, M.; Grantham, H.S.; Pressey, R.L.; Wilson, K.A.; Peterson, E.L.; Dorfman, D.; Mumby, P.J.; Lourival, R.; Brumbaugh, D.R.; Possingham, H.P. Conservation planning for connectivity across marine, freshwater, and terrestrial realms. Biol. Conserv. 2010, 143, 565–575. [Google Scholar] [CrossRef]
- Bounket, B.; Tabouret, H.; Gibert, P.; Bareille, G.; Pecheyran, C.; Carrel, G.; Argillier, C.; Morat, F. Spawning areas and migration patterns in the early life history of Squalius cephalus (Linnaeus, 1758): Use of otolith microchemistry for conservation and sustainable management. Aquat. Conserv. 2021, 31, 2772–2787. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Wells, B.K.; Campana, S.E.; Gillanders, B.M.; Jones, C.M.; Limburg, K.E.; Secor, D.H.; Thorrold, S.R.; Walther, B.D. Otolith chemistry to describe movement and life-history parameters of fishes: Hypotheses assumptions, limitation and inferences. Oceanogr. Mar. Biol. 2008, 46, 297–330. [Google Scholar] [CrossRef]
- Carlson, A.K.; Phelps, Q.E.; Graeb, B.D.S. Chemistry to conservation: Using otoliths to advance recreational and commercial fisheries management. J. Fish Biol. 2017, 90, 505–527. [Google Scholar] [CrossRef]
- Hussy, K.; Limburg, K.E.; Pontual, H.D.; Thomas, O.R.B.; Cook, P.K.; Heimbrand, Y.; Blass, M.; Sturrock, A.M. Trace element patterns in otoliths: The role of biomineralization. Rev. Fish. Sci. Aquac. 2020, 29, 445–477. [Google Scholar] [CrossRef]
- Izzo, C.; Reis-Santos, P.; Gillanders, B.M. Otolith chemistry does not just reflect environmental conditions: A meta-analytic evaluation. Fish Fish. 2018, 19, 441–454. [Google Scholar] [CrossRef]
- Tian, H.L.; Liu, J.H.; Cao, L.; Dou, S.Z. Interactive effects of strontium and barium water concentration on otolith incorporation in juvenile flounder Paralichthys olivaceus. PLoS ONE 2019, 14, e0218446. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Arai, T.; Chino, N. Influence of water salinity on the strontium: Calcium ratios in otoliths of the giant mottled eel, Anguilla marmorata. Environ. Biol. Fish. 2016, 100, 281–286. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, T.; Liu, H. Are there habitat salinity markers of the Sr:Ca ratio in the otolith of wild diadromous fishes? A literature survey. Ichthyol. Res. 2011, 58, 291–294. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, J.; Jiang, T.; Liu, H.B.; Zhong, X.M.; Tang, J.H. Early life history of the small yellow croaker (Larimichthys polyactis) in sandy ridges of the South Yellow Sea. Mar. Biol. Res. 2017, 13, 993–1002. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Huang, H.H.; Yang, J. Migration patterns and habitat use of the tapertail anchovy Coilia mystus in the Oujiang River Estuary and the Zhujiang River Estuary, China. Acta Oceanol. Sin. 2019, 38, 35–40. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, D.Z.; Zhang, F.S.; Wei, B.Q. Changes of wetland landscape pattern in Dayang River Estuary based on high-resolution remote sensing image. Chin. J. Appl. Ecol. 2011, 22, 1833–1840. [Google Scholar] [CrossRef]
- Shi, W.L. Fish fauna characteristics of Dayang River and its adjacent rivers. Fish. Sci. 1985, 4, 53 57+52. (In Chinese) [Google Scholar] [CrossRef]
- Wei, H.X.; Yang, P.M.; Jiang, X.H.; Kou, L.X. Study on community characteristics of plankton in Coilia nasus migratory section of Dayang river. J. Aquac. 2021, 42, 29–36. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.H.; Feng, G.P.; Yang, J.; Zhao, F.; Shen, C.C.; Song, C.; Jiang, T. Otolith microchemistry assessment: Evidence of migratory Coilia nasus of Yangtze River living in the Shengsi Sea area. Fishes 2022, 7, 172. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, X.Q.; Liu, H.B.; Liu, H.Z.; Yang, J. Two microchemistry patterns in otoliths of Coilia nasus from Poyang Lake, China. J. Fish. China 2013, 37, 239–244. [Google Scholar] [CrossRef]
- Cong, X.R.; Li, Q.X.; Dong, G.C.; Yang, J.; Jiang, T. Anadromous tapertail anchovy Coilia nasus is still found in Dongping Lake. Chin. J. Fish. 2019, 32, 55–59. [Google Scholar]
- Ma, C.Y. A Study on Molecular Phylogeny of Engraulidae and Population Genetic Diversity of C. mystus and C. ectenes. Ph.D. Thesis, East China Normal University, Shanghai, China, 2010. [Google Scholar]
- Zhang, S.S. The Genetic Diversity Study of Coilia nasus of Liaoning Province in China. Master’s Thesis, Dalian Ocean University, Dalian, China, 2016. [Google Scholar]
- Zhang, J.; Yang, P.M.; Hu, Z.Y.; Jiang, X.H.; Wang, J.J. Reproductive biology of Coilia nasus in Dayang River. Freshw. Fish. 2021, 51, 91–96. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Xuan, Z.Y.; Chen, X.B.; Yang, J. Classification of ecomorphotypes of Coilia nasus from the middle and lower reaches of the Yangtze River Basin. J. Lake Sci. 2020, 32, 518–527. [Google Scholar] [CrossRef]
- Xu, G.C.; Nie, Z.J.; Zhang, C.X.; Wei, G.L.; Xu, P.; Gu, R.B. Histological studies on testis development of Coilia nasus under artificial farming conditions. J. Huazhong Agric. Univ. 2012, 31, 247–252. [Google Scholar] [CrossRef]
- Xu, G.C.; Wan, J.J.; Gu, R.B.; Zhang, C.X.; Xu, P. Morphological and histological studies on ovary development of Coilia nasus under artificial farming conditions. J. Fish. Sci. China 2011, 18, 537–546. [Google Scholar] [CrossRef]
- Yang, J.; Arai, T.; Liu, H.; Miyazaki, N.; Tsukamoto, K. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. J. Fish Biol. 2006, 69, 1120–1135. [Google Scholar] [CrossRef]
- Sokta, L.; Jiang, T.; Liu, H.B.; Xuan, Z.Y.; Qiu, C.; Chen, X.B.; Yang, J. Loss of Coilia nasus habitats in Chinese freshwater lakes: An otolith microchemistry assessment. Heliyon 2020, 6, e04571. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Xie, S.G.; Li, Z.J.; Cong, W.B.; He, W.P. Gonad development of an anadromous fish Coilia ectenes (Engraulidae) in lower reach of Yangtze River, China. Fish. Sci. 2007, 73, 1224–1230. [Google Scholar] [CrossRef]
- Diana, J.S.; Hanchin, P.; Popoff, N. Movement patterns and spawning sites of muskellunge Esox masquinongy in the Antrim chain of lakes, Michigan. Environ. Biol. Fish. 2014, 98, 833–844. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Lu, M.J.; Liu, H.B.; Chen, T.T.; Gao, Y.W. Discovery of a spawning area for anadromous Coilia nasus Temminck et Schlegel, 1846 in Poyang Lake, China. J. Appl. Ichthyol. 2017, 33, 189–192. [Google Scholar] [CrossRef]
- Xue, X.P.; Peng, Y.X.; Fang, D.A.; Xu, D.P.; Wang, X.H.; Ren, K.C. Preliminary study of Coilia nasus spawning grounds at Sutong section in the lower reaches of the Yangtze River. J. Fish. China 2022, 46, 1377–1388. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, P.; Yang, X.W.; Li, X.F.; Xu, D.P.; Fang, D.A. Spatial and temporal distribution of larvae and juveniles Coilia nasus in the lower reaches of the Yangtze River. J. Lake Sci. 2020, 32, 506–517. [Google Scholar] [CrossRef]
- Rao, Y.Y.; Zhong, J.S.; Liu, H.; Li, L.F.; Chen, W. Annual variation of the resources of Coilia nasus larvae and juveniles in the southern branch of the Yangtze River estuary. J. Shanghai Ocean Univ. 2021, 30, 828–836. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.; Shen, X.; Shimasaki, Y.; Oshima, Y.; Yang, J. Life history variations among different populations of Coilia nasus along the Chinese coast inferred from otolith microchemistry. J. Fac. Agric. Kyushu Univ. 2014, 59, 383–389. [Google Scholar] [CrossRef]
- Li, Y.X.; He, W.P.; Liu, J.S.; Li, Z.J.; Xie, S.G. Annulus validation and age and growth estimation of anadromous Coilia ectenes in the Yangtze Estuary. Acta Hydrobiol. Sin. 2010, 34, 787–793. [Google Scholar] [CrossRef]
- Wang, H.F.; Li, Z.Y.; Tian, J.; Sun, L.; Tan, X.Q.; Holi, J. Transplant of Oncorhynchus in Dayang River of the Yellow Sea northern shore. J. Fish. China 2004, 3, 347–351. [Google Scholar]
- Li, Z.H.; Li, G.; Li, S.; Jiang, Z.Q.; Sun, S.H. Study on short-distance transportation and overwintering technologies of Colia nasus fries in artificial breeding from Dayang River. J. Anhui Agric. Sci. 2018, 46, 96 97+108. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.B.; Li, M.M.; Yang, J. Investigation on shrimp feeding of Coilia nasus during its anadromous migration along the Yangtze River. J. Lake Sci. 2018, 30, 458–463. [Google Scholar] [CrossRef][Green Version]
- Ma, F.J.; Yin, D.H.; Fang, D.A.; Yang, Y.P.; Jiang, M.; You, L.; Tian, J.L.; Xu, P.; Liu, K. Insights into response to food intake in anadromous Coilia nasus through stomach transcriptome analysis. Aquac. Res. 2020, 51, 2799–2812. [Google Scholar] [CrossRef]
- McBride, R.S.; Somarakis, S.; Fitzhugh, G.R.; Albert, A.; Yaragina, N.A.; Wuenschel, M.J.; Alonso-Fernández, A.; Basilone, G. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish. 2015, 16, 23–57. [Google Scholar] [CrossRef]
- Yokouchi, K.; Fukuda, N.; Shirai, K.; Aoyama, J.; Daverat, F.; Tsukamoto, K. Time lag of the response on the otolith strontium/calcium ratios of the Japanese eel, Anguilla japonica to changes in strontium/calcium ratios of ambient water. Environ. Biol. Fish. 2011, 92, 469–478. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).