Study of the Quality and Nutritional Value of Alosa sapidissima in the Postmortem Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Color Measurement
2.3. Shear Stress Measurement
2.4. Determination of Cooking Loss
2.5. Determination of TVB-N and pH
2.6. Proximate and Fatty Acid Composition
2.7. Amino Acid Composition
2.8. Electronic Tongue
2.9. Statistical Analysis
3. Results
3.1. Color Analysis
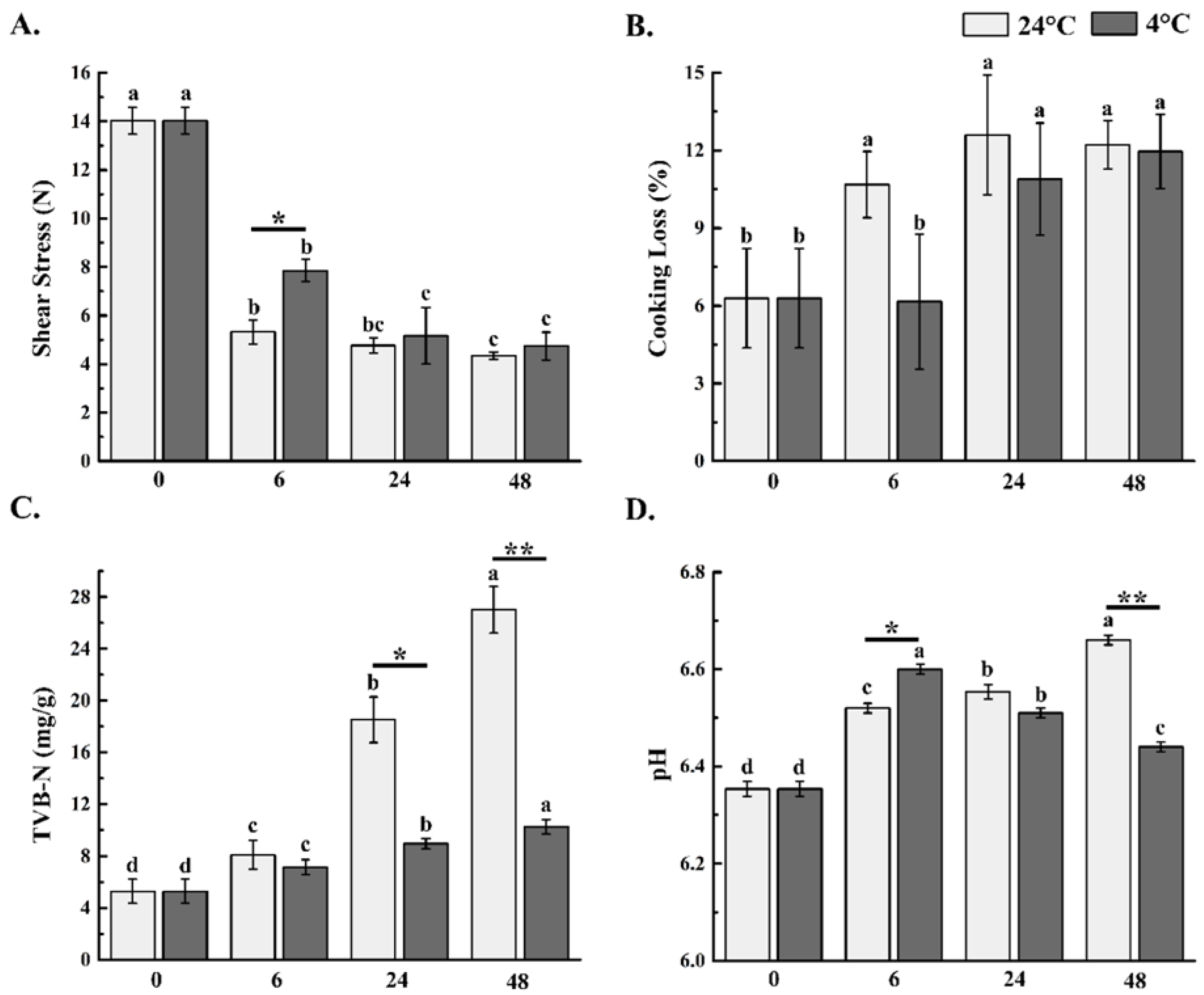
3.2. Change in Shear Stress
3.3. Change in Cooking Loss
3.4. Analysis of pH and TVB-N
3.5. Proximate Composition Analysis
3.6. Fatty Acid Analysis
3.7. Hydrolyzed Amino Acid Analysis
3.8. Free Amino Acid Analysis
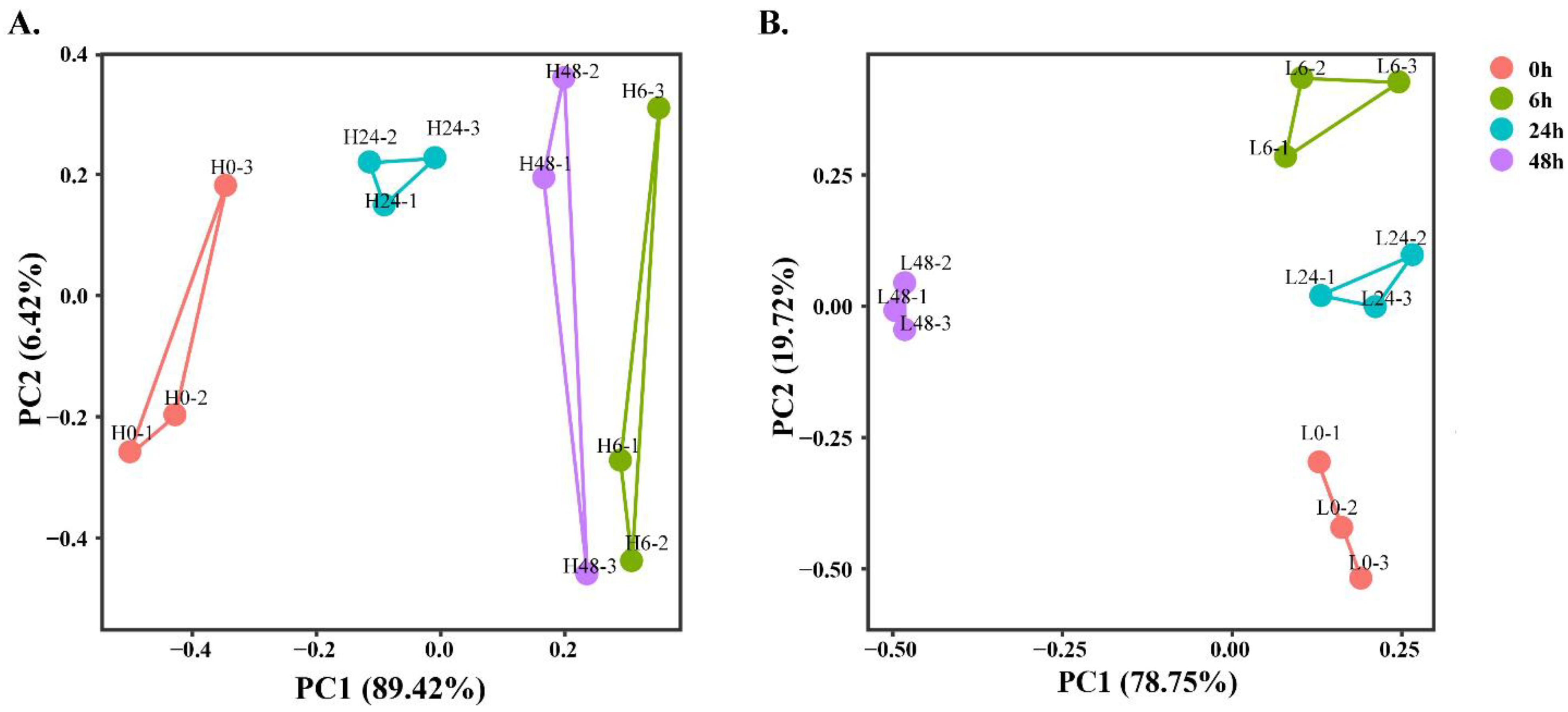
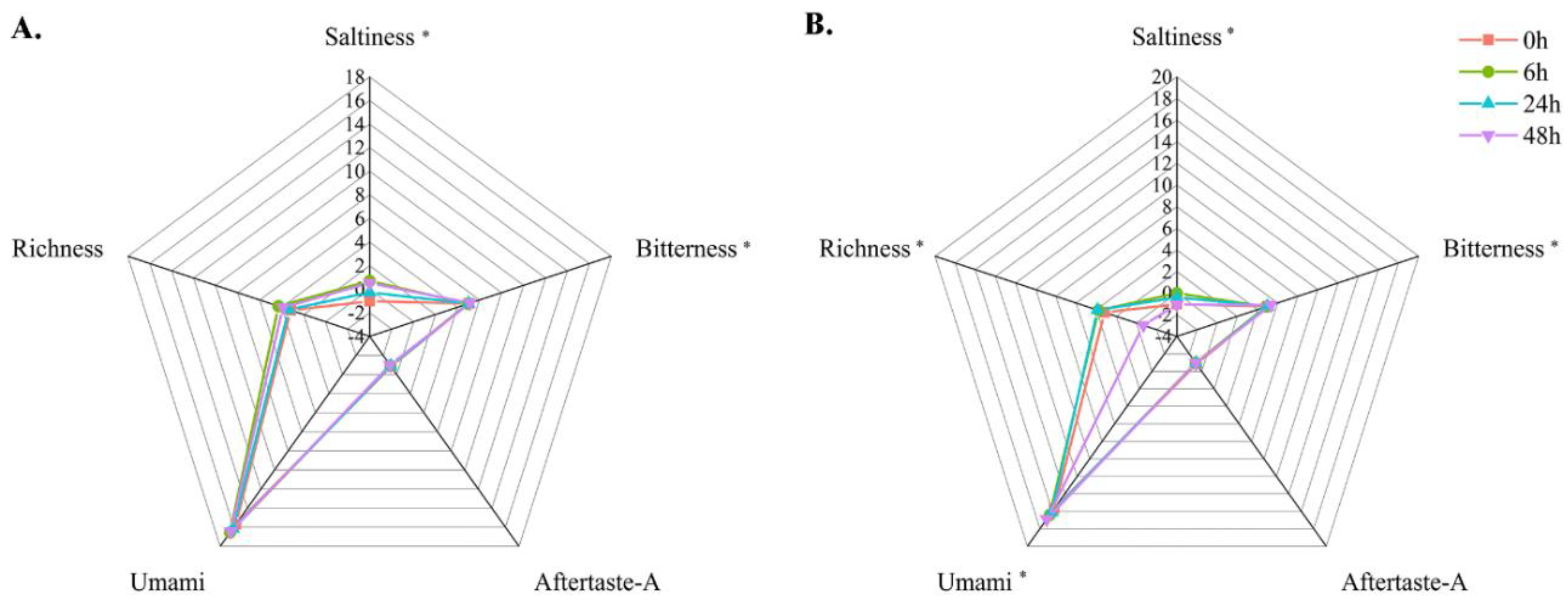
3.9. Electronic Tongue Analysis
4. Discussion
4.1. Color
4.2. Shear Stress and Cooking Loss
4.3. TVB-N and pH
4.4. Proximate and Fatty Acids
4.5. Amino Acid
4.6. Electronic Tongue
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hong, X.; Zhu, X.; Chen, K.; Pan, D.; Li, K. Morphometrics Analysis of Tenualosa ilisha, Alosa sapidissima and Tenualosa reevesii. J. S. China Agric. Univ. 2013, 34, 203–206. [Google Scholar]
- Hong, X.; Xie, W.; Zhu, X.; Chen, K.; Pan, D. Comparison of Muscle Nutrient Composition between Alosa sapidissima and Tenualosa ilisha. Acta Nutr. Sin. 2013, 35, 206–208. [Google Scholar]
- Gu, N.B.; Zhang, C.; Xu, G.C.; Wen, H.B.; Wang, Y.F. Analysis and evaluation of nutritional composition of American shad muscle. J. Fish. 2007, 02, 40–46. [Google Scholar]
- Shui, C.; Yan, Y.; Shi, Y.; Xu, J.; Deng, P. Effects of Different Salinities on Growth, Body Composition, Oxygen Consumption Rate, and Ammonia Excretion Rate in American shad (Alosa sapidissima) Juveniles. Isr. J. Aquac. -Bamidgeh 2019, 71, 20964. [Google Scholar]
- Ocano-Higuera, V.M.; Marquez-Rios, E.; Canizales-Davila, M.; Castillo-Yanez, F.J.; Pacheco-Aguilar, R.; Lugo-Sanchez, M.E. Postmortem changes in cazon fish muscle stored on ice. Food Chem. 2009, 116, 933–938. [Google Scholar] [CrossRef]
- Suarez, M.D.; Martinez, T.F.; Saez, M.I.; Alferez, B.; Garcia-Gallego, M. Changes in muscle properties during postmortem storage of farmed sea bream (Sparus aurata). J. Food Process Eng. 2011, 34, 922–946. [Google Scholar] [CrossRef]
- Li, K.; Luo, Y.; Shen, H. Postmortem Changes of Crucian Carp (Carassius auratus) During Storage in Ice. Int. J. Food Prop. 2015, 18, 205–212. [Google Scholar] [CrossRef]
- Delbarre-Ladrat, C.; Cheret, R.; Taylor, R.; Verrez-Bagnis, V. Trends in postmortem aging in fish: Understanding of proteolysis and disorganization of the myofibrillar structure. Crit. Rev. Food Sci. Nutr. 2006, 46, 409–421. [Google Scholar] [CrossRef]
- Pyz-Lukasik, R.; Paszkiewicz, W. Shelf life of grass carp, bighead carp, Siberian sturgeon, and wels catfish stored under refrigerated conditions. J. Food Saf. 2019, 39, e12607. [Google Scholar] [CrossRef]
- Meng, L.; Feng, J.; Zhou, D.; Jiang, X.; Dai, Z. Changes in Quality and Shelf Life of Pneumatophorus japonicus Detected by Fluorescence Quantitative PCR during Storage. J. Chin. Inst. Food Sci. Technol. 2018, 18, 230–237. [Google Scholar]
- Li, X.; Wang, B.; Xie, T.; Stankovski, S.; Hu, J. Research progress on nondestructive testing technology for aquatic products freshness. J. Food Process Eng. 2022, 45, e14025. [Google Scholar] [CrossRef]
- Shi, C.; Yang, X.; Han, S.; Fan, B.; Zhao, Z.; Wu, X. Nondestructive Prediction of Tilapia Fillet Freshness During Storage at Different Temperatures by Integrating an Electronic Nose and Tongue with Radial Basis Function Neural Networks. Food Bioprocess Technol. 2018, 11, 1840–1852. [Google Scholar] [CrossRef]
- Bao, Y.; Zhu, S.; Luo, Y.; Shen, H. Comparison of Postmortem Changes in Blunt-Snout Bream (Megalobrama amblycephala) During Short-Term Storage at Chilled and Partial Freezing Temperatures. J. Aquat. Food Prod. Technol. 2015, 24, 752–761. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Lu, W.; Shen, H.; Luo, Y. Quality predictive models of grass carp (Ctenopharyngodon idellus) at different temperatures during storage. Food Control 2011, 22, 1197–1202. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Zhang, J.; Wang, X.; Shi, W. Study on changes in the quality of grass carp in the process of postmortem. J. Food Biochem. 2018, 42, e12683. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Wei, S.; Sun, Q.; Xia, Q.; Zhang, D. Quality and volatile compound analysis of shrimp heads during different temperature storage. Food Chem. 2021, 12, 100156. [Google Scholar] [CrossRef]
- Chen, J.J.; Chen, J.W.; Tan, L.; Liao, E.; Cheng, S.Y.; Tian, D.C. Change in edible and nutritional qualities of Se-rich rainbow trout during cold storage. J. Wuhan Polytech. Univ. 2021, 40, 133780. [Google Scholar]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- AOAC. International Official Methods of Analysis, 18th ed.; Current through revision 2, 2007; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Smichi, N.; Abdelmalek, B.E.; Kharrat, N.; Sila, A.; Bougatef, A.; Gargouri, Y. The effects of storage on quality and nutritional aspects of farmed and wild sea bass (Dicentrachus labrax) muscle: In vitro oils digestibility evaluation. Fish. Res. 2017, 188, 74–83. [Google Scholar] [CrossRef]
- Brauer, M.; Behrens, J.W.; Christoffersen, M.; Hyldig, G.; Jacobsen, C.; Bjornsdottir, K.H. Seasonal patterns in round goby (Neogobius melanostromus) catch rates, catch composition, and dietary quality. Fish. Res. 2020, 222, 105412. [Google Scholar] [CrossRef]
- Hu, Q.; Gao, P.; Xu, Y.; Xia, W.; Sun, Y.; Jiang, Q. Effect of commercial starter cultures on the quality characteristics of fermented fish-chili paste. Lwt-Food Sci. Technol. 2020, 122, 109016. [Google Scholar] [CrossRef]
- WHO/FAO/UNU. Protein and Amino Acid Requirements in Human Nutrition; WHO Press: Geneva, Switzerland, 2007; p. 276. [Google Scholar]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef] [PubMed]
- Millward, D.J. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr. 2012, 108, S31–S43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Ismail, I.; Joo, S.-T. The Relationship between Muscle Fiber Composition and Pork Taste-traits Assessed by Electronic Tongue System. Korean J. Food Sci. Anim. Resour. 2018, 38, 1305–1314. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, D.; Chen, X.; Li, H. Effect of multiple freeze-thaw cycles on protein and lipid oxidation in rabbit meat. Int. J. Food Sci. Technol. 2021, 56, 3004–3015. [Google Scholar] [CrossRef]
- Calvo, M.M.; Tzamourani, A.; Martinez-Alvarez, O. Halophytes as a potential source of melanosis-inhibiting compounds. Mechanism of inhibition of a characterized polyphenol extract of purslane (Portulaca oleracea). Food Chem. 2021, 355, 129649. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Sun, D.-W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Lan, Y.; Shang, Y.; Song, Y.; Dong, Q. Changes in the quality of superchilled rabbit meat stored at different temperatures. Meat Sci. 2016, 117, 173–181. [Google Scholar] [CrossRef]
- Yu, D.; Regenstein, J.M.; Zang, J.; Xia, W.; Xu, Y.; Jiang, Q. Inhibitory effects of chitosan-based coatings on endogenous enzyme activities, proteolytic degradation and texture softening of grass carp (Ctenopharyngodon idellus) fillets stored at 4 degrees C. Food Chem. 2018, 262, 1–6. [Google Scholar] [CrossRef]
- Taylor, R.G.; Fjaera, S.O.; Skjervold, P.O. Salmon fillet texture is determined by myofiber-myofiber and myofiber-myocommata attachment. J. Food Sci. 2002, 67, 2067–2071. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.A. Texture and Structure Measurements and Analyses for Evaluation of Fish and Fillet Freshness Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Skipnes, D.; Johnsen, S.O.; Skara, T.; Sivertsvik, M.; Lekang, O. Optimization of Heat Processing of Farmed Atlantic Cod (Gadus morhua) Muscle with Respect to Cook Loss, Water Holding Capacity, Color, and Texture. J. Aquat. Food Prod. Technol. 2011, 20, 331–340. [Google Scholar] [CrossRef]
- Yang, F.; Jia, S.; Liu, J.; Gao, P.; Yu, D.; Jiang, Q. The relationship between degradation of myofibrillar structural proteins and texture of superchilled grass carp (Ctenopharyngodon idella) fillet. Food Chem. 2019, 301, 125278. [Google Scholar] [CrossRef]
- Zhuang, S.; Li, Y.; Hong, H.; Liu, Y.; Shu, R.; Luo, Y. Effects of ethyl lauroyl arginate hydrochloride on microbiota, quality and biochemical changes of container-cultured largemouth bass (Micropterus salmonides) fillets during storage at 4 degrees C. Food Chem. 2020, 324, 126886. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.J.E.; Boziaris, I.S. Microbiological spoilage and volatiles production of gutted European sea bass stored under air and commercial modified atmosphere package at 2 degrees C. Food Microbiol. 2015, 50, 44–53. [Google Scholar] [CrossRef]
- Shen, S.; Jiang, Y.; Liu, X.; Luo, Y.; Gao, L. Quality assessment of rainbow trout (Oncorhynchus mykiss) fillets during super chilling and chilled storage. J. Food Sci. Technol. -Mysore 2015, 52, 5204–5211. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Zhang, Y.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Effect of sodium alginate and carboxymethyl cellulose edible coating with epigallocatechin gallate on quality and shelf life of fresh pork. Int. J. Biol. Macromol. 2019, 141, 178–184. [Google Scholar] [CrossRef]
- Ombede, S.N.N.; Kaktcham, P.M.; Seydi, M.; Ngoufack, F.Z. Changes in sensory, physicochemical, and microbiological properties of fresh captured tropical pink shrimps (Penaeus duorarum notialis) inoculated with Lactobacillus plantarum Lp6SH, Lactobacillus rhamnosus Yoba, and their cell-free culture supernatants during storage at 4 degrees C. J. Food Saf. 2019, 39, 453-453. [Google Scholar] [CrossRef]
- Okomoda, V.T.; Tiamiyu, L.O.; Ricketts, A.O.; Oladimeji, S.A.; Agbara, A.; Ikhwanuddin, M. Hydrothermal Processing of Clarias gariepinus (Burchell, 1822) Filets: Insights on the Nutritive Value and Organoleptic Parameters. Vet. Sci. 2020, 7, 133. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; Angon, E.; Gonzalez, M.A.; Tobar, J.M.R.; Capote, C.B.; Martinez, A.R.G. Effect of rearing system and sex on the composition and fatty acid profile of Andinoacara rivulatus meat from Ecuador. Rev. De La Fac. De Cienc. Agrar. 2021, 53, 232–242. [Google Scholar]
- Hedayatifard, M. Comparative Study of Fatty Acid Composition of Golden Mullet Fillet and Roe Oil (Liza aurata Risso, 1810). Asian J. Anim. Vet. Adv. 2009, 4, 209–213. [Google Scholar] [CrossRef]
- Padro, T.; Vilahur, G.; Sanchez-Hernandez, J.; Hernandez, M.; Antonijoan, R.M.; Perez, A. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. J. Lipid Res. 2015, 56, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zou, X.; Yao, Y.; Jin, J.; Xia, Y.; Huang, J. Evaluation of fatty acid composition in commercial infant formulas on the Chinese market: A comparative study based on fat source and stage. Int. Dairy J. 2016, 63, 42–51. [Google Scholar] [CrossRef]
- Topuz, O.K.; Yerlikaya, P.; Yatmaz, H.A.; Kaya, A.; Alp, A.C. Polyunstaurated fatty acid (PUFA) contents of meat and egg of rainbow trout fish (Oncorhynchus mykiss). Sci. Pap. -Ser. D-Anim. Sci. 2017, 60, 312–315. [Google Scholar]
- Pirestani, S.; Sahari, M.A.; Barzegar, M. Fatty Acids Changes during Frozen Storage in Several Fish Species from South Caspian Sea. J. Agric. Sci. Technol. 2010, 12, 321–329. [Google Scholar]
- Rincon, L.; Luis Castro, P.; Alvarez, B.; Dolores Hernandez, M.; Alvarez, A.; Claret, A. Differences in proximal and fatty acid profiles, sensory characteristics, texture, colour and muscle cellularity between wild and farmed blackspot seabream (Pagellus bogaraveo). Aquaculture 2016, 451, 195–204. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Gilani, G.S. Amino acid analysis. Curr. Protoc. Protein Sci. 2009, 11, 9-9. [Google Scholar] [CrossRef]
- Usydus, Z.; Szlinder-Richert, J.; Adamczyk, M. Protein quality and amino acid profiles of fish products available in Poland. Food Chem. 2009, 112, 139–145. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Wang, W.; Liu, J. Comparison of proximate composition, amino acid and fatty acid profiles in wild, pond- and cage-cultured longsnout catfish (Leiocassis longirostris). Int. J. Food Sci. Technol. 2012, 47, 1772–1776. [Google Scholar] [CrossRef]
- Baki, B.; Gonener, S.; Kaya, D. Comparison of Food, Amino Acid and Fatty Acid Compositions of Wild and Cultivated Sea Bass (Dicentrarchus labrax L., 1758). Turk. J. Fish. Aquat. Sci. 2015, 15, 175–179. [Google Scholar] [CrossRef]
- Hwang, D.F.; Chen, T.Y.; Shiau, C.Y.; Jeng, S.S. Seasonal variations of free amino acids and nucleotide-related compounds in the muscle of cultured Taiwanese puffer Takifugu rubripes. Fish. Sci. 2000, 66, 1123–1129. [Google Scholar] [CrossRef]
- Raithore, S.; Bai, J.; Plotto, A.; Manthey, J.; Irey, M.; Baldwin, E. Electronic Tongue Response to Chemicals in Orange Juice that Change Concentration in Relation to Harvest Maturity and Citrus Greening or Huanglongbing (HLB) Disease. Sensors 2015, 15, 30062–30075. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shi, W.; Qu, Y.; Qin, J.; Wang, Z. Research on quality changes of grass carp (Ctenopharyngodon idellus) during short-term starvation. Food Sci. Nutr. 2020, 8, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ye, C.; Li, P. Molecular mechanisms of taste receptors. Chem. Life 2010, 30, 810–814. [Google Scholar]
| Essential Amino Acid | WHO/FAO/UNN 2007 | FAO Parttern 1984 |
|---|---|---|
| Ile | 30 | 40 |
| Leu | 59 | 70 |
| Lys | 45 | 55 |
| Thr | 23 | 40 |
| Val | 39 | 50 |
| Met + Cys | 22 | 35 |
| Phe + Tyr | 38 | 60 |
| Temperature | Time (h) | L* | a* | b* |
|---|---|---|---|---|
| 24 °C | 0 | 55.56 ± 0.49 a | 5.90 ± 0.36 a | 6.44 ± 0.56 d |
| 6 | 53.33 ± 0.44 b | 4.66 ± 0.06 b | 7.38 ± 0.28 bc | |
| 24 | 51.43 ± 0.56 c | 3.07 ± 0.34 c | 10.48 ± 0.32 b** | |
| 48 | 49.60 ± 1.02 d | 2.95 ± 0.71 c | 11.77 ± 0.29 a** | |
| 4 °C | 0 | 55.56 ± 0.49 a | 5.90 ± 0.36 a | 6.44 ± 0.57 c |
| 6 | 54.17 ± 0.23 b | 4.93 ± 0.10 a | 6.82 ± 0.59 bc | |
| 24 | 52.50 ± 0.31 c | 3.75 ± 0.08 b | 7.56 ± 0.32 b** | |
| 48 | 51.54 ± 0.52 d | 3.32 ± 0.10 c | 8.59 ± 0.24 a** |
| Temperature | Time (h) | Moisture (%) | Ash (%) | Crude Protein (%) | Crude Fat (%) |
|---|---|---|---|---|---|
| 24 °C | 0 | 65.74 ± 3.90 b | 1.41 ± 0.03 a | 15.06 ± 1.41 a | 15.43 ± 0.094 a |
| 6 | 71.96 ± 4.72 a | 1.52 ± 0.07 a | 13.62 ± 0.82 a | 12.14 ± 2.54 b | |
| 24 | 73.94 ± 2.29 a | 1.57 ± 0.01 a | 13.73 ± 1.37 a | 9.01 ± 3.42 c | |
| 48 | 74.15 ± 2.49 a* | 1.53 ± 0.26 a | 13.62 ± 0.31 a | 8.63 ± 1.11 c | |
| 4 °C | 0 | 65.74 ± 3.90 a | 1.41 ± 0.03 a | 15.06 ± 1.41 a | 15.43 ± 0.94 a |
| 6 | 70.14 ± 4.63 a | 1.48 ± 0.26 a | 14.69 ± 1.00 a | 13.90 ± 5.00 ab | |
| 24 | 69.68 ± 1.66 a | 1.56 ± 0.10 a | 13.92 ± 0.43 a | 10.49 ± 1.28 b | |
| 48 | 64.91 ± 1.23 a* | 1.53 ± 0.12 a | 13.71 ± 0.29 a | 10.18 ± 2.10 b |
| FA (%) | 0 | 6 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|---|---|
| 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | |
| C12:0 | 0.05 ± 0.00 b | 0.05 ± 0.00 c | 0.06 ± 0.00 a | 0.06 ± 0.01 a | 0.05 ± 0.01 ab | 0.05 ± 0.00 bc | 0.05 ± 0.00 ab | 0.06 ± 0.00 ab |
| C14:0 | 2.03 ± 0.14 a | 2.03 ± 0.14 b | 2.17 ± 0.05 a | 2.19 ± 0.03 b | 2.14 ± 0.11 a | 2.14 ± 0.02 b | 2.20 ± 0.07 a* | 3.44 ± 0.02 a* |
| C15:0 | 0.27 ± 0.01 b | 0.27 ± 0.01 b | 0.30 ± 0.01 ab | 0.28 ± 0.01 b | 0.29 ± 0.02 ab | 0.28 ± 0.00 b | 0.30 ± 0.01 a* | 0.36 ± 0.00 a* |
| C16:0 | 18.92 ± 0.22 b | 18.92 ± 0.22 b | 19.94 ± 0.15 a | 19.42 ± 0.61 b | 20.50 ± 0.76 a | 19.09 ± 0.20 b | 19.70 ± 0.71 ab* | 22.47 ± 0.24 a* |
| C17:0 | 0.39 ± 0.03 a | 0.39 ± 0.03 ab | 0.41 ± 0.02 a | 0.38 ± 0.04 ab | 0.41 ± 0.02 a | 0.38 ± 0.01 a | 0.42 ± 0.02 a* | 0.29 ± 0.00 b* |
| C18:0 | 6.54 ± 0.50 a | 6.54 ± 0.50 a | 6.36 ± 0.18 a | 6.06 ± 0.11 a | 6.46 ± 0.31 a | 6.08 ± 0.07 a | 6.37 ± 0.41 a* | 3.32 ± 0.08 b* |
| C20:0 | 0.30 ± 0.06 a | 0.30 ± 0.06 a | 0.25 ± 0.01 a | 0.25 ± 0.01 ab | 0.30 ± 0.01 a | 0.23 ± 0.04 b | 0.27 ± 0.04 a | 0.16 ± 0.01 c |
| C22:0 | 0.18 ± 0.07 a | 0.18 ± 0.07 a | 0.12 ± 0.00 a | 0.12 ± 0.02 a | 0.17 ± 0.02 a | 0.12 ± 0.02 a | 0.13 ± 0.03 a | 0.09 ± 0.02 a |
| C14:1 | 0.03 ± 0.00 a | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.03 ± 0.01 b | 0.03 ± 0.00 ab | 0.04 ± 0.01 b | 0.03 ± 0.00 b* | 0.06 ± 0.00 a* |
| C16:1 | 2.59 ± 0.14 a | 2.59 ± 0.14 bc | 2.71 ± 0.02 a | 2.67 ± 0.05 b | 2.50 ± 0.31 a | 2.36 ± 0.24 c | 2.63 ± 0.11 a* | 6.11 ± 0.04 a |
| C17:1 | 0.30 ± 0.01 a | 0.30 ± 0.01 b | 0.31 ± 0.02 a | 0.30 ± 0.01 b | 0.29 ± 0.01 a | 0.29 ± 0.02 b | 0.31 ± 0.00 a* | 0.37 ± 0.01 a* |
| C18:1 | 30.46 ± 1.57 a | 30.46 ± 1.57 ab | 28.91 ± 0.37 a | 29.29 ± 0.23 a | 26.75 ± 2.07 a | 29.15 ± 1.18 ab | 27.82 ± 0.46 a* | 25.02 ± 0.19 b* |
| C20:1 | 1.95 ± 0.22 a | 1.95 ± 0.22 a | 1.64 ± 0.11 ab | 1.58 ± 0.11 b | 1.46 ± 0.22 b | 1.43 ± 0.17 b | 1.61 ± 0.14 ab* | 0.79 ± 0.06 c* |
| C18:2 | 23.17 ± 1.66 a | 23.17 ± 1.66 a | 24.72 ± 0.51 a | 23.98 ± 0.36 a | 24.27 ± 1.37 a | 24.63 ± 0.20 a | 25.09 ± 0.54 a | 24.30 ± 0.59 a |
| C18:3n6 | 1.69 ± 0.07 a | 1.69 ± 0.07 a | 1.42 ± 0.05 b | 1.98 ± 0.12 a | 1.69 ± 0.08 a | 1.65 ± 0.26 a | 1.33 ± 0.19 b* | 0.13 ± 0.03 b |
| C18:3n3 | 2.33 ± 0.24 a | 2.33 ± 0.24 a | 2.57 ± 0.11 a | 2.57 ± 0.10 a | 2.41 ± 0.17 a | 2.56 ± 0.11 a | 2.58 ± 0.09 a | 2.57 ± 0.09 a |
| C20:2 | 0.89 ± 0.09 ab | 0.89 ± 0.09 a | 0.81 ± 0.03 b | 0.82 ± 0.05 a | 0.89 ± 0.04 ab | 0.81 ± 0.04 a | 0.94 ± 0.02 a | 0.41 ± 0.04 b* |
| C20:3n6 | 0.97 ± 0.05 ab | 0.97 ± 0.05 a | 0.77 ± 0.03 c | 1.01 ± 0.09 a | 1.07 ± 0.05 a | 0.97 ± 0.15 ab | 0.83 ± 0.14 bc* | 0.13 ± 0.01 b* |
| C20:4 | 0.58 ± 0.04 b | 0.58 ± 0.04 a | 0.57 ± 0.03 b | 0.55 ± 0.02 a | 0.70 ± 0.02 a | 0.65 ± 0.11 a | 0.63 ± 0.03 b* | 0.43 ± 0.04 b* |
| C20:3n3 | 0.15 ± 0.01 a | 0.15 ± 0.01 b | 0.14 ± 0.01 a | 0.15 ± 0.01 b | 0.14 ± 0.01 a | 0.14 ± 0.02 b | 0.15 ± 0.01 a | 0.19 ± 0.00 a |
| C20:5 | 0.95 ± 0.14 a | 0.95 ± 0.14 ab | 1.06 ± 0.03 a | 0.99 ± 0.00 b | 1.00 ± 0.04 a | 0.96 ± 0.15 ab | 0.98 ± 0.04 a* | 1.40 ± 0.05 a* |
| C22:6 | 6.23 ± 0.45 bc | 6.23 ± 0.45 b | 5.79 ± 0.23 c | 6.30 ± 0.31 b | 7.48 ± 0.33 a | 6.97 ± 1.05 ab | 6.61 ± 0.28 b* | 9.30 ± 0.11 a* |
| ∑SFA | 28.68 ± 0.73 b | 28.68 ± 0.73 b | 29.60 ± 0.29 ab | 28.76 ± 0.75 b | 30.32 ± 0.75 a | 28.36 ± 0.32 b | 30.19 ± 0.35 ab | 29.43 ± 0.44 a |
| ∑MUFA | 35.32 ± 1.64 a | 35.32 ± 1.64 ab | 33.61 ± 0.44 a | 33.87 ± 0.34 a | 31.03 ± 1.98 a | 33.27 ± 1.56 ab | 32.36 ± 0.24 a | 32.40 ± 0.44 b |
| ∑PUFA | 36.00 ± 1.71 b | 36.00 ± 1.71 b | 36.79 ± 0.71 ab | 37.37 ± 0.44 ab | 38.65 ± 1.58 a | 38.36 ± 1.25 a | 37.45 ± 0.58 ab | 38.17 ± 0.17 ab |
| ∑PUFA/∑SFA | 1.26 ± 0.08 a | 1.26 ± 0.08 b | 1.24 ± 0.04 a | 1.30 ± 0.05 ab | 1.27 ± 0.05 a | 1.35 ± 0.03 a | 1.30 ± 0.02 a | 1.24 ± 0.03 b |
| Amino Acids (g/100 g) | 0 h | 6 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|---|---|
| 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | |
| Asp | 7.96 ± 0.38 b | 7.96 ± 0.38 a | 7.07 ± 0.59 a | 8.45 ± 0.38 a | 6.68 ± 0.30 a* | 8.59 ± 0.10 a* | 7.10 ± 0.91 a | 7.14 ± 0.87 a |
| Glu | 12.03 ± 0.53 b | 12.03 ± 0.53 a | 10.73 ± 0.96 a | 12.9 ± 0.75 a | 10.19 ± 0.46 a* | 13.30 ± 0.12 a* | 10.96 ± 1.41 a | 11.10 ± 1.27 a |
| Ser | 2.59 ± 0.13 b | 2.59 ± 0.13 bc | 2.28 ± 0.18 ab | 2.81 ± 0.12 ab | 2.17 ± 0.10 b | 2.92 ± 0.04 a | 2.51 ± 0.20 a | 2.49 ± 0.25 c |
| His | 2.37 ± 0.12 a | 2.37 ± 0.12 a | 2.04 ± 0.04 b | 1.60 ± 0.40 b | 1.90 ± 0.13 b | 1.88 ± 0.04 b | 1.99 ± 0.23 b | 1.82 ± 0.10 b |
| Gly | 3.94 ± 0.16 b | 3.94 ± 0.16 ab | 3.49 ± 0.33 a | 4.02 ± 0.22 ab | 3.37 ± 0.15 a* | 4.30 ± 0.13 a* | 3.45 ± 0.39 a | 3.66 ± 0.43 b |
| Thr | 3.09 ± 0.15 b | 3.09 ± 0.15 ab | 2.72 ± 0.23 ab | 3.23 ± 0.27 ab | 2.59 ± 0.12 ab* | 3.43 ± 0.03 a* | 2.89 ± 0.32 b | 2.90 ± 0.30 b |
| Arg | 4.24 ± 0.17 b | 4.24 ± 0.17 ab | 3.71 ± 0.29 ab | 4.41 ± 0.37 ab | 3.52 ± 0.17 b* | 4.70 ± 0.07 b* | 3.91 ± 0.39 ab | 3.98 ± 0.42 a |
| Ala | 4.48 ± 0.19 b | 4.48 ± 0.19 a | 3.99 ± 0.34 ab | 4.72 ± 0.24 a | 3.79 ± 0.15 b* | 4.91 ± 0.06 a* | 4.03 ± 0.46 ab | 4.15 ± 0.46 a |
| Tyr | 2.40 ± 0.11 a | 2.40 ± 0.11 a | 1.97 ± 0.12 bc | 2.34 ± 0.26 ab | 1.83 ± 0.14 c | 2.33 ± 0.16 ab | 2.09 ± 0.01 b | 1.98 ± 0.22 b |
| Cys-s | 0.24 ± 0.01 a | 0.24 ± 0.01 a | 0.18 ± 0.02 ab | 0.13 ± 0.08 ab | 0.17 ± 0.02 b | 0.19 ± 0.01 b | 0.16 ± 0.05 b | 0.15 ± 0.04 ab |
| Val | 4.22 ± 0.19 b | 4.22 ± 0.19 ab | 3.69 ± 0.37 ab | 4.26 ± 0.31 ab | 3.55 ± 0.15 b* | 4.41 ± 0.06 a* | 3.82 ± 0.31 ab | 3.93 ± 0.27 b |
| Met | 2.37 ± 0.09 a | 2.37 ± 0.09 a | 1.89 ± 0.07 b | 2.29 ± 0.25 ab | 1.81 ± 0.15 b | 2.30 ± 0.02 ab | 1.99 ± 0.07 b | 1.92 ± 0.36 b |
| Phe | 3.29 ± 0.18 b | 3.29 ± 0.18 a | 2.88 ± 0.24 ab | 3.37 ± 0.17 a | 2.73 ± 0.12 b* | 3.44 ± 0.02 a* | 2.87 ± 0.33 ab | 2.91 ± 0.32 a |
| Ile | 3.69 ± 0.16 b | 3.69 ± 0.16 ab | 3.29 ± 0.29 ab | 3.87 ± 0.26 a | 3.13 ± 0.12 b* | 3.99 ± 0.04 a* | 3.35 ± 0.38 ab | 3.40 ± 0.35 b |
| Leu | 5.92 ± 0.27 b | 5.92 ± 0.27 a | 5.26 ± 0.46 a | 6.37 ± 0.33 a | 5.01 ± 0.21 a* | 6.50 ± 0.05 a* | 5.43 ± 0.63 a | 5.49 ± 0.59 a |
| Lys | 7.41 ± 0.38 a | 7.41 ± 0.38 a | 6.60 ± 0.62 a | 6.26 ± 2.81 a | 6.26 ± 0.25 a* | 8.00 ± 0.11 a* | 6.38 ± 1.00 a | 6.68 ± 0.74 a |
| Pro | 1.99 ± 0.27 a | 1.99 ± 0.27 a | 1.69 ± 0.18 a | 1.92 ± 0.38 a | 1.96 ± 0.58 a | 2.00 ± 0.14 a | 2.16 ± 0.33 a | 2.14 ± 0.56 a |
| TAA | 72.22 ± 3.30 a | 72.22 ± 3.30 ab | 63.47 ± 5.04 b | 72.94 ± 6.48 ab | 60.68 ± 2.23 b* | 77.19 ± 0.96 a* | 65.09 ± 5.99 ab | 65.85 ± 7.31 b |
| EAA | 32.35 ± 1.51 a | 32.35 ± 1.51 a | 28.38 ± 2.25 b | 31.24 ± 4.39 a | 26.99 ± 1.15 b* | 33.95 ± 0.30 a* | 28.72 ± 2.59 ab | 29.05 ± 2.83 a |
| NEAA | 39.87 ± 1.80 a | 39.87 ± 1.80 ab | 35.10 ± 2.82 ab | 41.69 ± 2.17 a | 33.69 ± 1.08 b* | 43.24 ± 0.77 a* | 36.36 ± 3.42 ab | 36.80 ± 4.49 b |
| E/T | 0.45 ± 0.00 a | 0.45 ± 0.00 a | 0.45 ± 0.00 a | 0.43 ± 0.02 a | 0.44 ± 0.00 a | 0.44 ± 0.00 a | 0.44 ± 0.00 a | 0.44 ± 0.00 a |
| N/T | 0.55 ± 0.00 a | 0.55 ± 0.00 a | 0.55 ± 0.00 a | 0.57 ± 0.02 a | 0.56 ± 0.00 a | 0.56 ± 0.00 a | 0.56 ± 0.00 a | 0.56 ± 0.00 a |
| E/N | 0.81 ± 0.01 a | 0.81 ± 0.01 a | 0.81 ± 0.01 a | 0.75 ± 0.07 a | 0.80 ± 0.01 a | 0.79 ± 0.01 a | 0.79 ± 0.01 a | 0.79 ± 0.02 a |
| AAs | 0 h | 6 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|---|---|
| 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | |
| Ile | 84.05 ± 8.96 a | 84.05 ± 8.96 a | 67.44 ± 9.69 a | 78.86 ± 13.38 a | 59.46 ± 2.79 a** | 86.92 ± 2.87 a** | 64.23 ± 14.66 a | 87.16 ± 11.8 a |
| Leu | 134.91 ± 14.46 a | 134.91 ± 14.46 a | 107.95 ± 15.95 a | 129.62 ± 21.42 a | 95.28 ± 4.75 a** | 141.53 ± 4.4 a** | 104.08 ± 24.19 a | 140.97 ± 19.85 a |
| Lys | 168.82 ± 19.48 a | 168.82 ± 19.48 a | 135.29 ± 19.16 a | 125.96 ± 57.97 a | 118.97 ± 5.6 a** | 174.06 ± 3.89 a** | 122.46 ± 32.15 a | 171.51 ± 24.36 a |
| Thr | 70.28 ± 7.68 a | 70.28 ± 7.68 a | 55.97 ± 9.02 a | 65.73 ± 11.86 a | 49.24 ± 2.57 a** | 74.61 ± 2.89 a** | 55.38 ± 12.52 a | 74.33 ± 10.02 a |
| Val | 96.04 ± 10.25 a | 96.04 ± 10.25 a | 75.41 ± 8.94 b | 86.76 ± 15.44 a | 67.37 ± 3.45 b** | 95.95 ± 2.17 a** | 73.06 ± 14.33 b | 100.75 ± 10.3 a |
| Met + Cys | 108.44 ± 8.23 a | 108.44 ± 8.23 a | 42.79 ± 9.13 b | 48.79 ± 4.95 b | 37.77 ± 3.75 b** | 54.2 ± 1.82 b** | 40.88 ± 3.46 b | 53.17 ± 11.52 b |
| Phe + Tyr | 129.56 ± 14.79 a | 129.56 ± 14.79 a | 100.07 ± 18.65 b | 116.07 ± 18.19 a | 86.86 ± 8.73 b** | 125.72 ± 6.36 a** | 94.65 ± 16.89 b | 125.27 ± 17.15 a |
| Total content | 792.11 ± 83.63 a | 792.11 ± 83.63 a | 584.93 ± 90.01 b | 651.8 ± 119.16 a | 514.96 ± 28.43 b** | 753 ± 23.83 a** | 554.74 ± 117.31 b | 753.17 ± 104.39 a |
| AAs (mg/g pro) | EAAS | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 6 h | 24 h | 48 h | |||||
| 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | |
| Ile | 280.18 ± 29.85 a | 280.18 ± 29.85 a | 224.8 ± 32.31 a | 262.87 ± 44.6 a | 198.2 ± 9.29 a** | 289.73 ± 9.57 a** | 198.2 ± 9.29 a* | 289.73 ± 9.57 a* |
| Leu | 228.67 ± 24.52 a | 228.67 ± 24.52 a | 182.96 ± 27.03 a | 219.69 ± 36.3 a | 161.5 ± 8.05 a** | 239.88 ± 7.46 a** | 161.5 ± 8.05 a | 239.88 ± 7.46 a |
| Lys | 375.15 ± 43.29 a | 375.15 ± 43.29 a | 300.65 ± 42.57 a | 279.92 ± 128.83 a | 264.38 ± 12.43 a** | 386.81 ± 8.63 a** | 264.38 ± 12.43 a | 386.81 ± 8.63 a |
| Thr | 305.57 ± 33.37 a | 305.57 ± 33.37 a | 243.36 ± 39.23 a | 285.8 ± 51.55 a | 214.09 ± 11.19 a** | 324.39 ± 12.58 a** | 214.09 ± 11.19 a* | 324.39 ± 12.58 a* |
| Val | 246.26 ± 26.27 a | 246.26 ± 26.27 a | 179.55 ± 21.29 b | 222.45 ± 39.59 a | 172.75 ± 8.84 b** | 246.03 ± 5.56 a** | 172.75 ± 8.84 b** | 246.03 ± 5.56 a** |
| Met + Cys | 492.9 ± 37.42 a | 492.9 ± 37.42 a | 194.49 ± 41.51 b | 221.78 ± 22.52 b | 171.7 ± 17.04 b** | 246.37 ± 8.27 b** | 171.7 ± 17.04 b | 246.37 ± 8.27 b |
| Phe + Tyr | 340.95 ± 38.91 a | 340.95 ± 38.91 a | 263.35 ± 49.08 b | 305.46 ± 47.87 a | 228.59 ± 22.96 b** | 330.85 ± 16.73 a** | 228.59 ± 22.96 b* | 330.85 ± 16.73 a* |
| EAAI | 223.73 ± 23.42 a | 223.73 ± 23.42 a | 160.5 ± 25.31 b | 179.53 ± 31.87 a | 141.4 ± 7.79 b** | 196.11 ± 3.57 a** | 153.04 ± 30.58 b | 206.84 ± 29.55 a |
| Amino Acids (g/kg) | Flavor Contribution | T0 | T6 | T24 | T48 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | 24 °C | 4 °C | ||
| Asp | Fresh/Acid (+) | 0.15 ± 0.01 a | 0.15 ± 0.01 a | 0.16 ± 0.01 a** | 0.13 ± 0.01 b** | 0.15 ± 0.01 a | 0.14 ± 0.00 bc | 0.16 ± 0.01 a* | 0.14 ± 0.00 c* |
| Glu | Fresh/Acid (+) | 0.28 ± 0.03 b | 0.28 ± 0.03 b | 0.78 ± 0.10 a** | 0.31 ± 0.04 a** | 0.52 ± 0.14 ab | 0.32 ± 0.04 a | 0.34 ± 0.01 ab** | 0.22 ± 0.03 a** |
| Ser | Sweet (+) | 0.08 ± 0.02 a | 0.08 ± 0.02 a | 0.07 ± 0.03 a | 0.04 ± 0.00 b* | 0.07 ± 0.01 a | 0.04 ± 0.01 b | 0.08 ± 0.00 a* | 0.04 ± 0.02 b* |
| His | Bitter (-) | 5.48 ± 0.58 a | 5.48 ± 0.58 a | 5.49 ± 1.37 a | 6.23 ± 1.31 b | 6.13 ± 1.28 a | 6.63 ± 1.25 b | 7.68 ± 0.35 a | 8.94 ± 0.70 a |
| Gly | Sweet (+) | 0.42 ± 0.06 b | 0.42 ± 0.06 b | 0.68 ± 0.15 a | 0.46 ± 0.15 a | 0.51 ± 0.14 ab | 0.42 ± 0.09 a | 0.61 ± 0.03 ab** | 0.45 ± 0.03 a** |
| Thr | Sweet (+) | 0.27 ± 0.05 a | 0.27 ± 0.05 a | 0.57 ± 0.13 ab* | 0.26 ± 0.04 b* | 0.32 ± 0.04 a | 0.29 ± 0.04 ab | 0.48 ± 0.03 b** | 0.34 ± 0.02 a** |
| Arg | Sweet/Bitter (+) | 0.18 ± 0.02 a | 0.18 ± 0.02 a | 0.19 ± 0.04 ab | 0.16 ± 0.03 a | 0.16 ± 0.05 ab | 0.16 ± 0.00 a | 0.26 ± 0.01 b | 0.19 ± 0.07 a |
| Ala | Sweet (+) | 0.84 ± 0.15 ab | 0.84 ± 0.15 ab | 1.75 ± 0.40 ab* | 0.98 ± 0.21 a* | 0.98 ± 0.09 b | 1.05 ± 0.08 a | 1.51 ± 0.05 a* | 1.38 ± 0.02 a* |
| Tyr | Bitter (-) | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.22 ± 0.05 a** | 0.04 ± 0.01 b** | 0.06 ± 0.04 b | 0.06 ± 0.01 b | 0.12 ± 0.01 | 0.09 ± 0.01 a |
| Cys-s | 0.03 ± 0.04 a | 0.03 ± 0.04 a | 0.10 ± 0.03 a | 0.14 ± 0.03 b | 0.07 ± 0.07 a | 0.15 ± 0.03 b | 0.03 ± 0.03 a** | 0.21 ± 0.01 b** | |
| Val | Sweet/Bitter (-) | 0.13 ± 0.02 a | 0.13 ± 0.02 a | 0.54 ± 0.13 ab** | 0.18 ± 0.03 c** | 0.7 ± 0.04 ab | 0.23 ± 0.03 bc | 0.31 ± 0.03 b | 0.30 ± 0.01 a |
| Met | Bitter/Sweet/Sulfur (-) | 0.08 ± 0.02 c | 0.08 ± 0.02 c | 0.27 ± 0.06 a* | 0.11 ± 0.03 bc* | 0.13 ± 0.03 bc | 0.13 ± 0.01 b | 0.16 ± 0.03 b | 0.19 ± 0.01 a |
| Phe | Bitter (-) | 0.06 ± 0.02 a | 0.06 ± 0.02 a | 0.27 ± 0.08 a** | 0.04 ± 0.01 b** | 0.11 ± 0.02 a* | 0.05 ± 0.00 bc* | 0.12 ± 0.01 a** | 0.08 ± 0.01 a** |
| Ile | Bitter (-) | 0.07 ± 0.01 a | 0.07 ± 0.01 a | 0.23 ± 0.06 ab* | 0.07 ± 0.01 a* | 0.10 ± 0.0 a | 0.08 ± 0.01 a | 0.16 ± 0.00 b** | 0.12 ± 0.00 b** |
| Leu | Bitter (-) | 0.12 ± 0.02 a | 0.12 ± 0.02 a | 0.50 ± 0.13 ac** | 0.12 ± 0.02 a** | 0.21 ± 0.01 c* | 0.14 ± 0.01 a* | 0.28 ± 0.00 ab* | 0.08 ± 0.12 a* |
| Lys | Sweet/Bitter (-) | 1.43 ± 0.16 b | 1.43 ± 0.16 b | 2.03 ± 0.72 ab | 1.61 ± 0.14 a* | 1.41 ± 0.68 ab | 1.77 ± 0.31 a | 2.43 ± 0.04 a* | 1.58 ± 0.62 a* |
| Pro | Sweet/Bitter (+) | 0.66 ± 0.15 ab | 0.66 ± 0.15 ab | 0.39 ± 0.07 c | 0.36 ± 0.08 b | 0.43 ± 0.13 bc | 0.36 ± 0.01 b | 0.72 ± 0.15 a | 0.51 ± 0.18 ab |
| Fresh, sweet free amino acids | 2.70 ± 0.43 d | 2.70 ± 0.43 d | 4.40 ± 0.80 a* | 2.54 ± 0.08 a* | 2.99 ± 0.41 cd | 2.62 ± 0.25 a | 3.90 ± 0.15 abc** | 3.08 ± 0.20 a** | |
| Bitter free amino acids | 7.60 ± 0.83 b | 7.60 ± 0.83 b | 9.75 ± 2.28 ab* | 8.56 ± 1.30 b* | 8.58 ± 1.16 ab | 9.25 ± 1.63 b | 11.52 ± 0.43 a | 11.57 ± 0.27 a | |
| TAA | 10.33 ± 1.22 c | 10.33 ± 1.22 a | 14.25 ± 3.11 ab | 11.24 ± 1.72 b | 11.64 ± 2.04 bc | 12.02 ± 1.90 b | 15.45 ± 0.32 a* | 14.85 ± 0.19 a* | |
| Temperature | Time | Saltiness | Bitterness | Aftertaste-A | Umami | Richness |
|---|---|---|---|---|---|---|
| 24 °C | 0 h | −1.02 ± 0.08 d | 5.02 ± 0.07 ab | −0.85 ± 0.01 b | 15.72 ± 0.21 ab | 3.18 ± 0.08 a |
| 6 h | 0.70 ± 0.04 a | 4.94 ± 0.08 b | −0.93 ± 0.06 ab | 16.55 ± 0.06 a | 4.29 ± 0.40 a | |
| 24 h | −0.27 ± 0.08 c | 5.01 ± 0.05 ab | −0.91 ± 0.04 b | 16.12 ± 0.04 b | 3.36 ± 0.10 a | |
| 48 h | 0.53 ± 0.03 b | 5.13 ± 0.02 a | −1.00 ± 0.06 a | 16.46 ± 0.13 ab | 3.9 ± 0.56 a | |
| 4 °C | 0 h | −1.02 ± 0.08 c | 5.02 ± 0.07 b | −0.85 ± 0.01 a | 15.72 ± 0.21 c | 3.18 ± 0.08 a |
| 6 h | −0.01 ± 0.08 a | 4.87 ± 0.09 b | −0.98 ± 0.08 b | 16.42 ± 0.07 b | 3.77 ± 0.71 a | |
| 24 h | −0.39 ± 0.04 b | 5.00 ± 0.08 b | −0.93 ± 0.06 ab | 16.10 ± 0.09 b | 3.88 ± 0.54 a | |
| 48 h | −1.06 ± 0.01 d | 5.33 ± 0.11 a | −0.96 ± 0.04 b | 16.89 ± 0.20 a | −0.68 ± 0.11 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhu, H.; Yi, X.; Nie, Z.; Zheng, Y.; Yang, X.; Xu, P.; Yu, Y.; Xu, G. Study of the Quality and Nutritional Value of Alosa sapidissima in the Postmortem Process. Fishes 2022, 7, 302. https://doi.org/10.3390/fishes7060302
Li L, Zhu H, Yi X, Nie Z, Zheng Y, Yang X, Xu P, Yu Y, Xu G. Study of the Quality and Nutritional Value of Alosa sapidissima in the Postmortem Process. Fishes. 2022; 7(6):302. https://doi.org/10.3390/fishes7060302
Chicago/Turabian StyleLi, Le, Haojun Zhu, Xiangyu Yi, Zhijuan Nie, Yao Zheng, Xiwei Yang, Pao Xu, Yaqing Yu, and Gangchun Xu. 2022. "Study of the Quality and Nutritional Value of Alosa sapidissima in the Postmortem Process" Fishes 7, no. 6: 302. https://doi.org/10.3390/fishes7060302
APA StyleLi, L., Zhu, H., Yi, X., Nie, Z., Zheng, Y., Yang, X., Xu, P., Yu, Y., & Xu, G. (2022). Study of the Quality and Nutritional Value of Alosa sapidissima in the Postmortem Process. Fishes, 7(6), 302. https://doi.org/10.3390/fishes7060302






