Shelter Color Selection of Juvenile Swimming Crabs (Portunus trituberculatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Collection and Maintenance
2.2. Construction of the Animal Behavioral Capture System
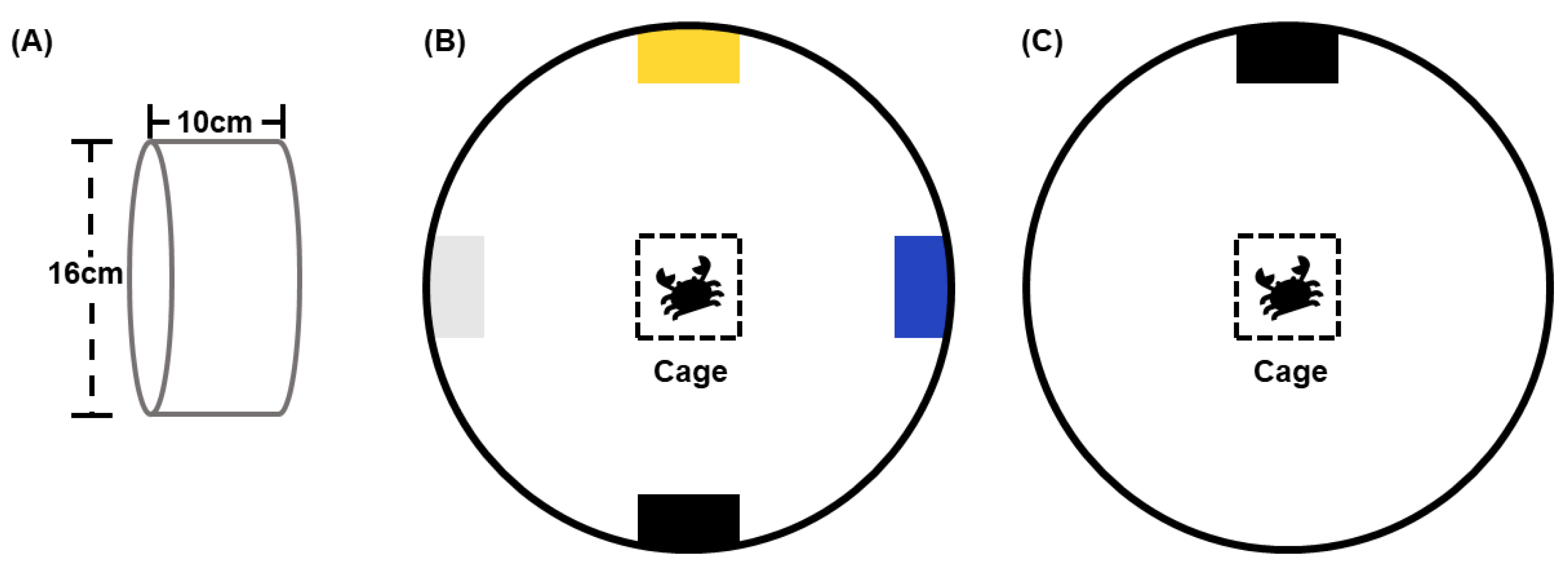
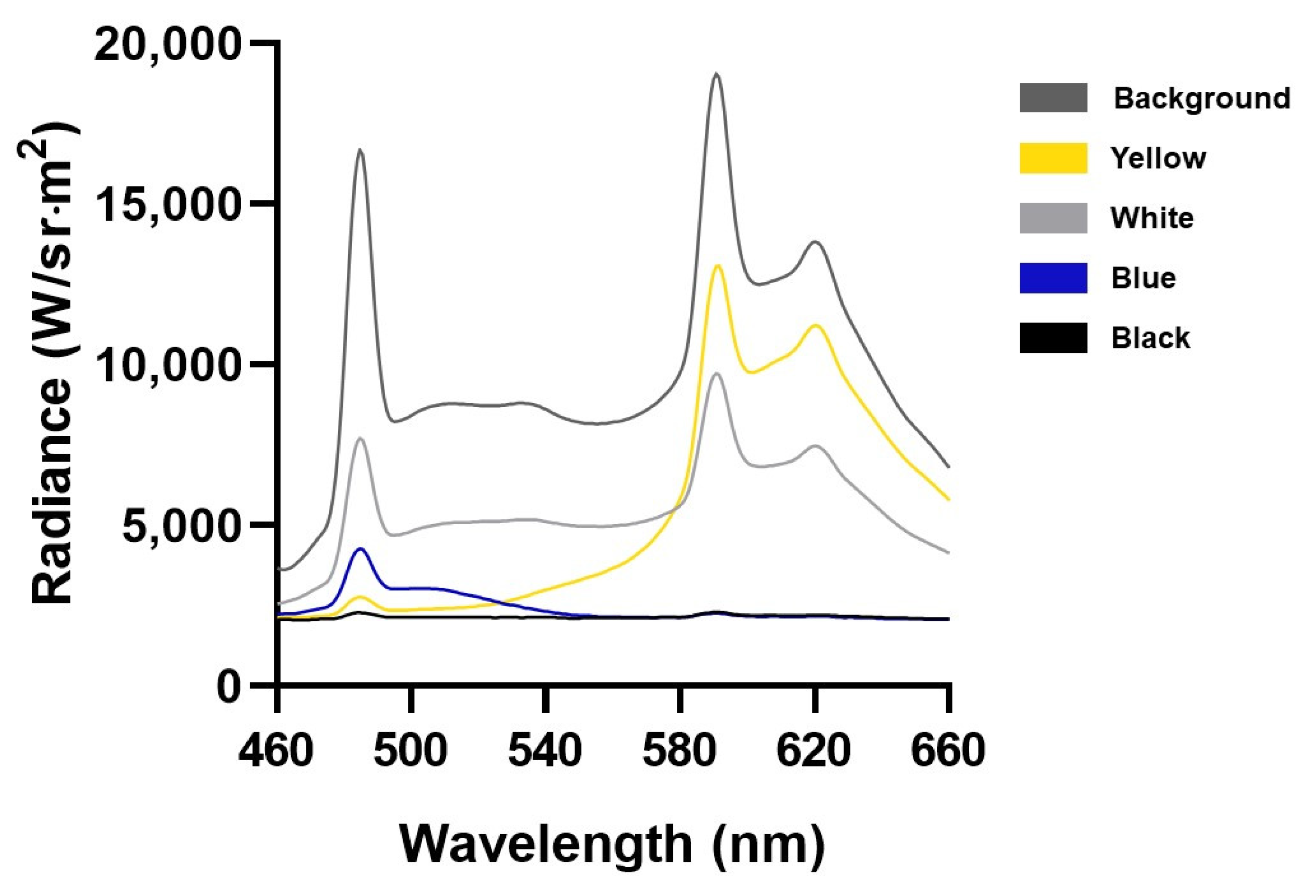
2.3. Construction of Shelters
2.4. Experimental Design
2.4.1. Experiment One—Preference Test of Swimming Crabs for Different Colored Shelters
2.4.2. Experiment Two—Effect of Shelter Color on the Occupation of Shelters by Swimming Crab
2.5. Calculated Parameters
2.5.1. Occupation of the Shelter
2.5.2. The Probability of Fighting upon Encounter
2.5.3. The Probability of Abandoning the Shelter
2.6. Statistical Analysis
3. Results
3.1. Experiment One—Preference Test of Swimming Crabs for Different Colored Shelters
3.1.1. Z-Scores for the Counts of Occupation
3.1.2. Z-Scores for the Duration of Occupation
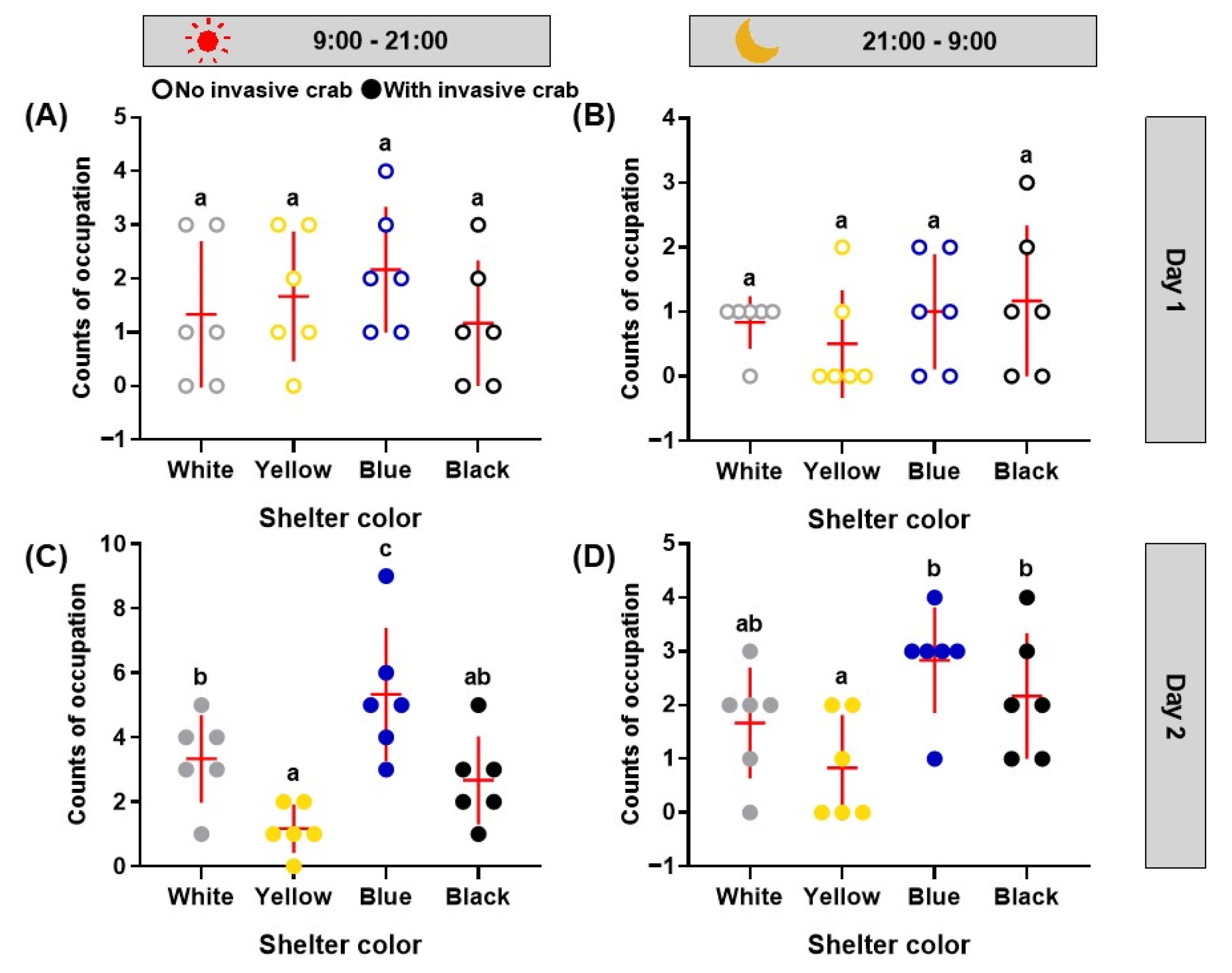
3.2. Experiment Two—Effect of Shelter Color on the Occupation of Shelters by Swimming Crabs
3.2.1. Counts of Occupation
3.2.2. Duration of Occupation
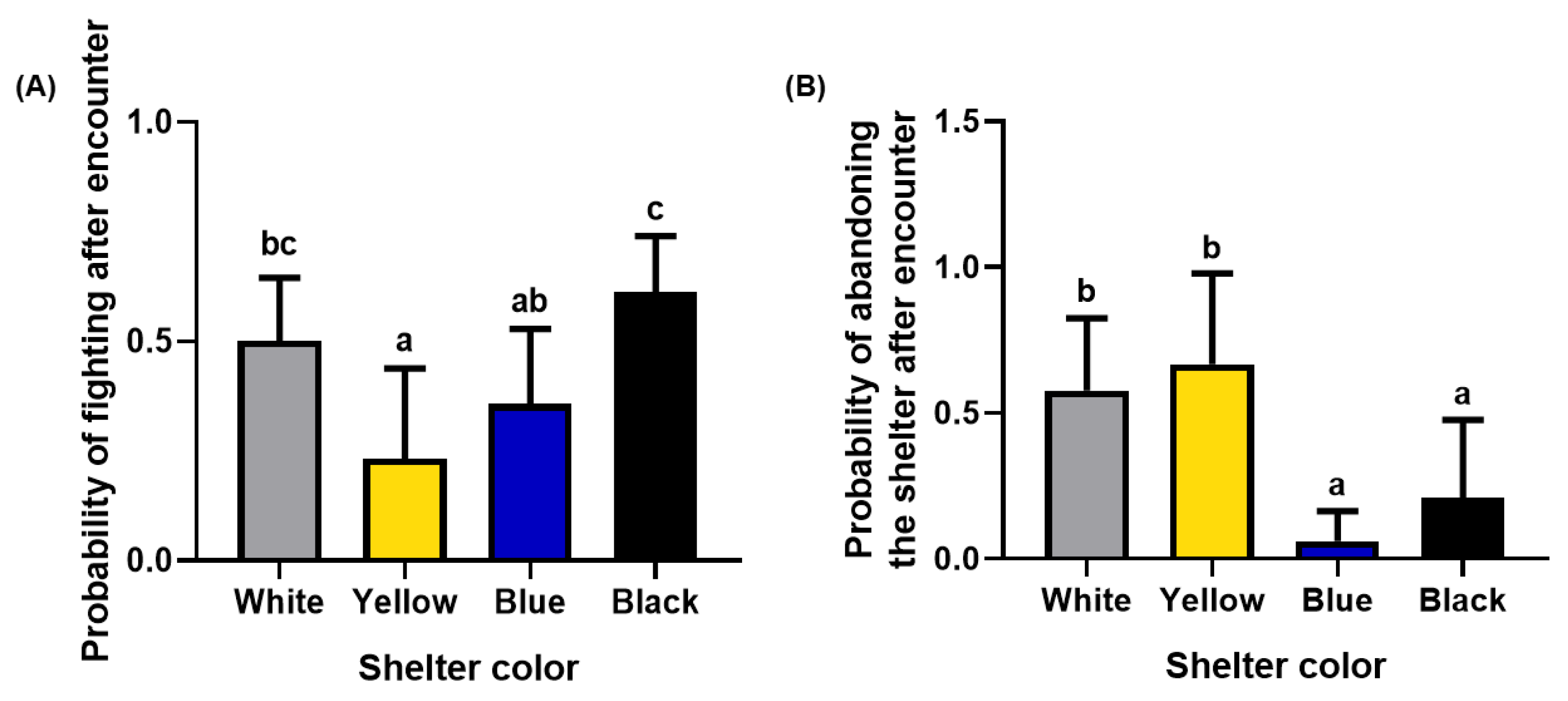
3.2.3. The Probability of Fighting and the Probability of Abandoning the Shelter
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Savolainen, R.; Ruohonen, K.; Tulonen, J. Effects of bottom substrate and presence of shelter in experimental tanks on growth and survival of signal crayfish, Pacifastacus leniusculus (Dana) juveniles. Aquac. Res. 2003, 34, 289–297. [Google Scholar] [CrossRef]
- Benhaïm, D.; Leblanc, C.A.; Lucas, G. Impact of a new artificial shelter on Arctic charr (Salvelinus alpinus, L.) behaviour and culture performance during the endogenous feeding period. Aquaculture 2009, 295, 38–43. [Google Scholar] [CrossRef]
- Liu, C.J.; Xu, W.J.; Jiang, Y.M.; He, J.; Zhou, Z.Q.; Shen, L.F. Application of anti-crippling device in “swimming crab and Japanese shrimp” breeding. China Fish. 2019, 7, 72–74. (In Chinese) [Google Scholar]
- Oniam, V.; Arkronrat, W.; Mohamed, N.B. Effect of feeding frequency and various shelter of blue swimming crab larvae, Portunus pelagicus (Linnaeus, 1758). Songklanakarin J. Sci. Technol. 2015, 37, 129–134. [Google Scholar]
- Kazuya, T.; Erika, Y.; Naoyuki, F.; Naoyuki, F.; Toshiki, N. The effects of shelter quality and prior residence on marmorkrebs (marbled crayfish). J. Exp. Biol. 2019, 222, jeb197301. [Google Scholar]
- Ravi, R.; Manisseri, M.K. Efficiency of shelters in reducing cannibalism among juveniles of the marine blue swimmer crab, Portunus pelagicus. Isr. J. Aquacult. Bamid. 2012, 64, 1–6. [Google Scholar] [CrossRef]
- Fatihah, S.N.; Julin, H.T.; Cheng, A.C. Survival, growth, and molting frequency of mud crab Scylla tranquebarica juveniles at different shelter conditions. Aquac. Aquar. Conserv. Legis. 2017, 10, 1581–1589. [Google Scholar]
- He, J.; Gao, Y.; Xu, W.; Yu, F.; Su, Z.; Xuan, F. Effects of different shelters on the molting, growth and culture performance of Portunus trituberculatus. Aquaculture 2017, 481, 133–139. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, B.; Yu, L.; Liu, D.; Wang, F.; Lu, Y. Selection of shelter shape by swimming crab (Portunus trituberculatus). Aquac. Rep. 2021, 21, 100908. [Google Scholar] [CrossRef]
- Luchiari, A.C.; Pirhonen, J. Effects of ambient colour on colour preference and growth of juvenile rainbow trout Oncorhynchus mykiss (Walbaum). J. Fish Biol. 2008, 72, 1504–1514. [Google Scholar] [CrossRef]
- Batzina, A.; Sotirakoglou, K.; Karakatsouli, N. The preference of 0+ and 2+ gilthead seabream Sparus aurata for coloured substrates or no-substrate. Appl. Anim. Behav. Sci. 2014, 151, 110–116. [Google Scholar] [CrossRef]
- Li, X.; Chi, L.; Tian, H.; Meng, L.; Zheng, J.; Gao, X.; Liu, Y. Colour preferences of juvenile turbot (Scophthalmus maximus). Physiol. Behav. 2016, 156, 64–70. [Google Scholar] [CrossRef] [PubMed]
- McLean, E. Background color and cultured invertebrates—A review. Aquaculture 2021, 537, 736523. [Google Scholar] [CrossRef]
- Kawamura, G.; Yong, A.S.K.; Roy, D.C.; Lim, L.-S. Shelter colour preference in the purple mud crab Scylla tranquebarica (Fabricius). Appl. Anim. Behav. Sci. 2020, 225, 104966. [Google Scholar] [CrossRef]
- Maia, C.M.; Volpato, G.L. Environmental light color affects the stress response of Nile tilapia. Zoology 2013, 116, 64–66. [Google Scholar] [CrossRef]
- Maia, C.M.; Alves, N.P.C.; Tatemoto, P. Juvenile Nile tilapia fish avoid red shelters. J. Appl. Anim. Welfare Sci. 2021, 24, 98–106. [Google Scholar] [CrossRef]
- Gaffney, L.P.; Franks, B.; Weary, D.M.; von Keyserlingk, M.A. Coho salmon (Oncorhynchus kisutch) prefer and are less aggressive in darker environments. PLoS ONE 2016, 11, e0151325. [Google Scholar] [CrossRef]
- Forward, R.B.; Cronin, T.W.; Douglass, J.K. The visual pigments of crabs. J. Comp. Physiol. 1988, 162, 463–478. [Google Scholar] [CrossRef]
- Zheng, W.; Luo, H. Phototropic response of Portunus trituberculatus to color light. Mar. Sci. 1979, 3, 15–18. (In Chinese) [Google Scholar]
- Takahashi, M.; Suzuki, N.; Koga, T. Burrow defense behaviors in a sand-bubbler crab, Scopimera globosa, in relation to body size and prior residence. J. Ethol. 2001, 19, 93–96. [Google Scholar] [CrossRef]
- Wong, M.C.; Peterson, C.H.; Kay, J. Prey size selection and bottom type influence multiple predator effects in a crab–bivalve system. Mar. Ecol. Prog. Ser. 2010, 409, 143–156. [Google Scholar] [CrossRef]
- Liu, D.; Wang, F.; Yang, C.; Hu, N.; Sun, Y. Starvation and a conspecific competitor influence multiple predator effects in a swimming crab (Portunus trituberculatus)—Manila clam (Ruditapes philippinarum) foraging system. J. Exp. Mar. Biol. Ecol. 2017, 495, 35–42. [Google Scholar] [CrossRef]
- Shi, C.; Wang, J.; Peng, K.; Mu, C.; Ye, Y.; Wang, C. The effect of tank colour on background preference, survival and development of larval swimming crab Portunus trituberculatus. Aquaculture 2019, 504, 454–461. [Google Scholar] [CrossRef]
- Nilsson, D.E. Evolutionary links between apposition and superposition optics in crustacean eyes. Nature 1983, 302, 818–821. [Google Scholar] [CrossRef]
- Nilsson, D.E.; Hallberg, E.; Elofsson, R. The ontogenetic development of refracting superposition eyes in crustaceans: Transformation of optical design. Tissue Cell 1986, 18, 509–519. [Google Scholar] [CrossRef]
- Kawamura, G.; Yong, A.S.K.; Wong, J.S.; Daning Tuzan, A.; Lim, L.S. The giant freshwater prawn Macrobrachium rosenbergii alters background colour preference after metamorphosis from larvae to postlarvae: In association with nature of phototaxis. Aquac. Res. 2020, 51, 3711–3717. [Google Scholar] [CrossRef]
- Maciel, C.R.; Valenti, W.C. Effect of tank colour on larval performance of the Amazon River prawn Macrobrachium amazonicum. Aquac. Res. 2014, 45, 1041–1050. [Google Scholar] [CrossRef]
- De los Santos Romero, R.; Garcia Guerrero, M.; Alpuche Osorno, J.; Cortes Jacinto, E. The effect of alpha males and shelter type on growth and survival of the longarm prawn Macrobrachium tenellum (Smith, 1871). Lat. Am. J. Aquat. Res. 2018, 46, 551–557. [Google Scholar] [CrossRef]
- Kawamura, G.; Bagarinao, T.U.; Cheah, H.S.; Saito, H.; Yong, A.S.K.; Lim, L.-S. Behavioural evidence for colour vision determined by conditioning in the purple mud crab Scylla tranquebarica. Fish. Sci. 2020, 86, 299–305. [Google Scholar] [CrossRef]
- Von Frisch, K. Bees: Their Vision, Chemical Senses, and Language, 1st ed.; Cornell University Press: Ithaca, NY, USA, 1971; pp. 1–34. [Google Scholar]
- Yuan, W.; Chen, H.; Zhang, H.; Sheng, C. Morphology and ultrastructure of photoreceptors in Portunus trituberculatus. Acta Zool. Sin. 2001, 47, 578–582. (In Chinese) [Google Scholar]
- Kelber, A.; Vorobyev, M.; Osorio, D. Animal colour vision—Behavioural tests and physiological concepts. Biol. Rev. 2003, 78, 81–118. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, L.E.; Tansley, L.K. Some new forms of vsual purple found in sea fishes with a note on the visual cells of origin. Proc. R. Soc. Lond. 1936, 120, 95–113. [Google Scholar]
- Clarke, G.L. On the depth at which fish can see. Ecology 1936, 17, 452–456. [Google Scholar] [CrossRef]
- Johnson, M.L.; Gaten, E.; Shelton, P.M.J. Spectral sensitivities of five marine decapod crustaceans and a review of spectral sensitivity variation in relation to habitat. J. Mar. Biol. Assoc. U. K. 2002, 82, 835–842. [Google Scholar] [CrossRef]
- Wang, C.; Mu, C.; Li, R.; Song, W.; Yi, X.; Han, C. Efficient production technology for monoculture of Portunus trituberculatus. China Fish. 2013, 4, 72–75. (In Chinese) [Google Scholar]
- Shen, L.; Hong, T.; Gu, J.; Yi, X.; Liu, C.; Dai, H.; Lin, X. Research on stereoculture technology of Portunus trituberculatus monoculture in baskets. Mod. Agric. Sci. Technol. 2013, 6, 264. (In Chinese) [Google Scholar]
- Islam, M.L.; Siddiky, M.; Yahya, K. Growth, survival and intactness of green mud crab (Scylla Paramamosain) broodstock under different captive grow out protocols. SAARC J. Agri. 2018, 16, 169–180. [Google Scholar] [CrossRef]
- Xu, Y.; Shentu, J.; Ding, Z. Effect of stocking space size on feeding behavior and growth characteristics of Portunus trituberculatus in a monobasic basket culture system. Oceanol. Limnol. Sin. 2014, 6, 1346–1352. [Google Scholar]
- Barcellos, L.J.G.; Kreutz, L.C.; Quevedo, R.M.; da Rosa, J.G.S.; Koakoski, G.; Centenaro, L.; Pottker, E. Influence of color background and shelter availability on jundiá (Rhamdia quelen) stress response. Aquaculture 2009, 288, 51–56. [Google Scholar] [CrossRef]
- Amano, M.; Amiya, N.; Mizusawa, K.; Chiba, H. Effects of background color and rearing density on stress-related hormones in the juvenile Japanese eel Anguilla japonica. Fish. Sci. 2021, 87, 521–528. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, B.; Yu, L.; Wang, F. Shelter Color Selection of Juvenile Swimming Crabs (Portunus trituberculatus). Fishes 2022, 7, 296. https://doi.org/10.3390/fishes7050296
Zhang H, Zhu B, Yu L, Wang F. Shelter Color Selection of Juvenile Swimming Crabs (Portunus trituberculatus). Fishes. 2022; 7(5):296. https://doi.org/10.3390/fishes7050296
Chicago/Turabian StyleZhang, Hanzun, Boshan Zhu, Liye Yu, and Fang Wang. 2022. "Shelter Color Selection of Juvenile Swimming Crabs (Portunus trituberculatus)" Fishes 7, no. 5: 296. https://doi.org/10.3390/fishes7050296
APA StyleZhang, H., Zhu, B., Yu, L., & Wang, F. (2022). Shelter Color Selection of Juvenile Swimming Crabs (Portunus trituberculatus). Fishes, 7(5), 296. https://doi.org/10.3390/fishes7050296






