Abstract
Fishing of the brown shrimp Penaeus aztecus is an important socioeconomic activity, generating income and different jobs for the fishing sector; however, this species is exposed to contaminants such as toxic metals. The objective of this research was to determine the concentrations of Cd, Pb, Cr, and Ni in brown shrimp P. aztecus from the northwestern Gulf of Mexico, and to analyse morphometric measurements in this species. The determination of toxic metals in shrimp was carried out according to the method proposed by the USEPA. Sexual identification was carried out by examining the first pleopods of P. aztecus specimens; the stages of maturity and proportion of sexes were also determined, finding specimens at maturity stages I and II. Specimens collected in the Veracruz area presented higher morphometric values, with an average TL of 136.15 mm. It was identified in the same area that the batches were formed by a higher proportion of males at 72.2%. Ni was the metal with the highest concentration in the Veracruz area at 15.5 µg g−1, while Pb had a maximum concentration of 8.3 µg g−1. The concentrations obtained for the toxic metals in the shrimp exceeded the values of the international permissible limits established for Pb and Cd.
1. Introduction
Toxic metals are considered as natural trace pollutants in the aquatic environment due to natural and anthropogenic sources such as industrial waste and agricultural and mining activities. Due to the impact of the aforementioned anthropogenic activities, concentrations of trace elements such as Cd, Pb, and Ni are reported to be increasing [1,2].
In aquatic ecosystems, heavy metal contamination is considered a potential and significant risk to invertebrate organisms, fish, and public health [1,3]. When toxic metals such as Pb and Cd are present in high concentrations, they can become highly hazardous to all living things, as chronic exposure to these elements can cause illness and, in some cases, death [4,5,6].
Toxic metals are distributed in different environmental media in the form of particles or complex compounds, which can be detected in water and dissolved in suspended solids, in sediments, and in biota [1,2]. Therefore, the diversity of environmental matrices and chemical forms in which toxic metals occur contributes to their wide distribution in aquatic organisms.
Fish, molluscs, and crustaceans are considered bioindicator organisms and are commonly used for contaminant analysis, due to their level in the aquatic food chain [4,6,7]. Crustaceans as bioindicators of contamination stand out for their wide distribution. They also have other characteristics that allow them to survive in the presence of contaminants, such as their high ecological plasticity and the ability to alter their physiological and biochemical functions [7,8].
Shrimp are a socio-economically important fishery resource in Mexico according to CONAPESCA [9]. Their total value of annual national fishery production varies from 35 to 40%, with shrimp catches in the Gulf of Mexico accounting for approximately 30% of the total. In the Gulf of Mexico from Tamaulipas to the Mexican Caribbean, six species of shrimp are used in fisheries, three of which stand out for their catch volume, namely the brown shrimp P. aztecus, the pink shrimp P. duorarum, and the white shrimp Litopenaeus setiferus [10]. P. aztecus is the predominant species in the catch of juvenile organisms in coastal lagoons and as adults in the high seas, accounting for 91% of the total production in the northern Gulf of Mexico according to the Official Journal [11].
In the coastal region of the Gulf of Mexico, there are important productive activities such as port complexes, commercial fishing, industry, and extensive agriculture; these activities are considered to be the main sources of toxic metal contamination in the coastal environment [7,12,13]. In general, research conducted in Mexico on toxic metals in crustaceans has focused on penaeid species such as the white shrimp L. vannamei, obtained from coastal lagoons in northwestern (NW) Mexico and cultivated in aquaculture farms [14,15,16,17,18].
The amount of research conducted on toxic metals in macroinvertebrate organisms in coastal ecosystems in the Gulf of Mexico in recent decades is considered insufficient compared to that carried out in other regions of Mexico [13]. Evidence of heavy metal inputs and dispersal to freshwater, coastal, and marine ecosystems in sediments and organisms such as oysters, shrimps, and crabs in the Gulf of Mexico has been reported [13,19,20,21]. The objective of this research was to determine the concentrations of Cd, Pb, Cr, and Ni in brown shrimp Penaeus aztecus from the Gulf of Mexico, and to analyse morphometric measurements in this species.
2. Materials and Methods
2.1. Study Area
The study area was the northeastern Gulf of Mexico, which covers the coasts of the states of Tamaulipas and Veracruz and corresponds to the main brown shrimp catching areas. Samples came from one research cruise in Tamaulipas and two in Veracruz carried out by the National Institute of Fisheries and Aquaculture (INAPESCA) during the closed season in July and August 2020. The sampling in Tamaulipas was designed to cover the entire coastline of that state; for those of Veracruz, the sampling was divided into two zones: the northern zone covering the coasts in front of the Tamiahua lagoon and the southern zone, which is located in front of the Tamiahua lagoon, from Alvarado to the mouth of the Coatzacoalcos river (Figure 1).
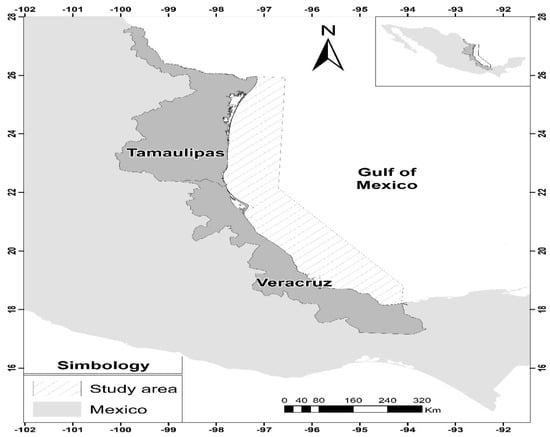
Figure 1.
Sampling sites in the study area for brown shrimp Penaeus aztecus in the Gulf of Mexico.
The sampling design and specifications of these cruises can be found in Wakida-Kusunoki et al. [22]. Sampling in the study area was carried out by the quadrant method; samples were collected in 25 quadrants for the area of the state of Tamaulipas, while for the state of Veracruz, there were 19. The trawling time for each quadrant was two hours, and they were carried out at depths of 8 to 42 m. Sampling was carried out during the P. aztecus closed period, from 31 July to 12 August 2020, for the area of Tamaulipas and Veracruz, because this is the period when the maximum reproductive peaks, sampling, and mobilisation of these organisms occur.
2.2. Ethics and Use of Animals
The handling of specimens in this research was carried out in accordance with the Code of Practice for the Housing and Care of Animals used in Scientific Procedures. This document was published on 14 April 2016 and under Section 1 of the Animals (Scientific Procedures) Act. In the case of aquatic animals, such as shrimp, the stunning of shrimps was done by heat shock, by immersing the animals in salt water with a temperature below 4 °C. This practice is endorsed by the Royal Society for the Prevention of Cruelty to Animals (RSPCA) as a humane method of stunning tropical shrimps. Furthermore, taking into account the above guidelines, this research and the planning and execution thereof followed the commonly accepted ‘3Rs’ guidelines.
2.3. Sample Collection
A sample size of approximately 200 g was used in each quadrant in the two study areas for onboard sampling during the research cruises; the samples were composed of 12 to 13 individuals for the Veracruz area and 18 to 19 for the Tamaulipas area. Organisms were selected from each lot with a similar size range, and were then labelled according to the area of capture, set number, and date.
The samples were then frozen without any treatment at a temperature below −4 °C and transferred to the Laboratory of Aquatic Resources Research (LIRA) of the Technological Institute of Boca del Rio (ITBOCA) for analysis.
2.4. Sample Handling in the Laboratory
The batches corresponding to three cruises in the study area were classified in a recording format including five variables for each organism in the batches analysed: total length (TL), head length (HL), weight, sex, and gonadal maturity.
The shrimp samples were handled in the shortest possible time and in temperature conditions below −18 °C to avoid thawing and guarantee the optimal state of conservation of the samples; at the end of their handling, they were stored deep-frozen at a temperature of approximately −20 °C. Samples of whole shrimp batches were refrozen after completion of morphometric analyses. Subsequently, these frozen samples were subjected to a freeze-drying dehydration process with a Thermo Savant ModulyOD-115, for 72 h at −49 °C (Thermo Fisher Scientific Inc., Waltham, MA, USA), and a vacuum pressure of 36 × 10−3 mbar. At the end of this process, the samples were stored in sealed bags and finally ground in a blender with 1800 W of power until a fine particle size was obtained.
2.5. Morphometric Analysis
Morphometric characteristics used in various investigations with penaeid shrimp (total length (TL), head length (HL), and weight) were recorded [23]. Stainless steel vernier calipers were used to measure TL, which was measured from the tip of the rostrum to the tip of the telson of each organism in the batches analysed. Head length was measured from the suborbital ridge of the shrimp to the posterior mid-dorsal edge of the carapace. An OHAUS Explorer Pro analytical balance (Merck KGaA©, Darmstadt, Germany) was used to record the weight of the analysed specimens. The above variables were recorded for the two zones that made up the study area.
2.6. Sexual Identification and Analysis of Gonadal Maturity
Sexual identification was carried out by examining the first pleopods of P. aztecus specimens and determining the presence of the petasma, whose appearance is an indicator of the identification of a male and its absence in the case of females [24,25].
The degree of gonadal maturity was determined using the morphochromatic scale, with which the stages of maturity described by Fenucci [26] and the Official Journal [25] were established. For males, two stages were considered: the immature stage when the petasma has a separate spur and the mature stage when it is attached [24]. In the case of females, five stages of maturity were considered, related to the differentiation of the ovaries through the dorsal exoskeleton, where they are classified by the thickness of the spot [25,26]. The stages used in the case of females were as follows: I—immature, II—in development, III—mature female, IV—the spawning phase, and finally, V—a spawning female.
2.7. Toxic Metal Analysis
The preparation of the material used in the determination of metals was carried out according to the specifications of the official Mexican standard NOM-117-SSA1-1994 [27] and Aguilar-Ucán et al. [28]. The laboratory material was washed with neutral phosphate-free Extran® soap (Merck KGaA©, Darmstadt, Germany) for 24 h and then rinsed under running water. Subsequently, the material was placed in a solution of distilled water with 20% nitric acid HNO3 (J.T.Baker®, Thermo Fisher Scientific Inc., Waltham, MA, USA), for 24 h; at the end of this period, the material was rinsed again with deionized water (Milli-Q) for 24 h. Finally, it was dried and stored until use.
Acid digestion was performed for the determination of metals in shrimp, according to the specifications of the method proposed by the USEPA [29]. Samples were analysed in duplicate for each quadrant in the two study areas. This process started by placing 30 mL of hydrochloric acid HCl J.T.Baker®, (Thermo Fisher Scientific Inc. Waltham, MA, USA) in a beaker and then the samples were slowly added, being previously freeze-dried and ground to avoid effervescence of the calcium carbonate present in the cephalothorax and muscle tissue of the shrimp. Subsequently, 10 mL of nitric acid HNO3 was added to the previous mixture, then a magnetic stirrer was added and, finally, it was covered with a watch glass and placed on a grill at 180 °C for 30 min to obtain an evaporation of up to 3/4 parts of the digested solution.
At the end of the digestion, 0.45 µm Millipore® nitrocellulose filters were used, which were previously moistened with a mixture of 10% acidified water with nitric acid HNO3 and deionized water (Milli-Q). The filtrate finally obtained was placed in a flask and made up to a volume of 50 mL with acidified water. The final extracts were stored in amber polyethylene vials and kept refrigerated at 4 °C until quantification of metals.
Digested samples for the determination of metals in shrimp were analysed on Varian® SpectrAA Model SPECTRAA-2200FS atomic absorption equipment. A calibration curve was prepared for each of the metals, using certified High-Purity Standards (High-Purity Standards, Charleston, SC) to quantify the metals. This curve presented an adequate fit, obtaining a correlation coefficient higher than 0.99.
Weight, total and cephalic length, and metal concentrations in Penaeus aztecus shrimp were analysed by study area with TIBCO Statistica 14.0.0.15 software (TIBCO Software Inc., Palo Alto, CA, USA). The aforementioned variables were transformed by natural logarithms to meet the normality assumptions of ANOVA. A multiple comparison of means using the Kruslak–wallis test was used to determine statistical differences (p < 0.05) between weight, total and head length, and heavy metal concentrations in shrimp. A multivariate analysis of variance (MANOVA) was performed with SPSS Statistics 27.0 software (IBM®, Armonk, NY, USA); a Box’s M test and Pillai’s trace were used to determine the significance values < 0.05, and the behaviour of the covariances. Principal component analysis (PCA) was carried out using the Free Software Past3 version 3.20 (Oyvind Hammer®, Oslo, Norway) to determine the associations of heavy metals in shrimp and the morphometric variables analysed. Maps of heavy metal concentrations in the study areas were made with Arc Gis software (V. 10.3) (ESRI®, Redlands, CA, USA).
3. Results
3.1. Research on Metals in Shrimp in the Gulf of Mexico
There have been few studies on metal analysis in P. aztecus shrimp, and those that were found were carried out with a difference of years in the areas analysed (Table 1). Among the research carried out in the Mexican Pacific those of the species L. vannamei, where found to be the most common because of their aquaculture importance. On the other hand, in the Gulf of Mexico, the most analysed shrimp species was L. setiferus, followed by P. aztecus, while P. duorarum has only been analysed in a single study.

Table 1.
Concentration of toxic metals (µg g−1) in penaeid shrimp species from the Gulf of Mexico coast.
3.2. Sexual Identification and Gonadal Maturity Analysis of P. aztecus
It was identified that the organisms collected in the Veracruz area were larger than those collected in the Tamaulipas area. The specimens collected in Veracruz showed higher morphometric values; the average values were a TL of 136.15 mm, an HL of 31.24 mm, and a weight of 19.57 g. In the state of Tamaulipas, the average values were a TL of 125.05 mm, an HL of 26.68 mm, and a weight of 14.29 g. These differences between zones were reflected in significant statistical differences (p < 0.05) in weight (W) (Figure 2a), total length (TL) (Figure 2b), and head length (HL) (Figure 2c) in P. aztecus from Veracruz and Tamaulipas zones.
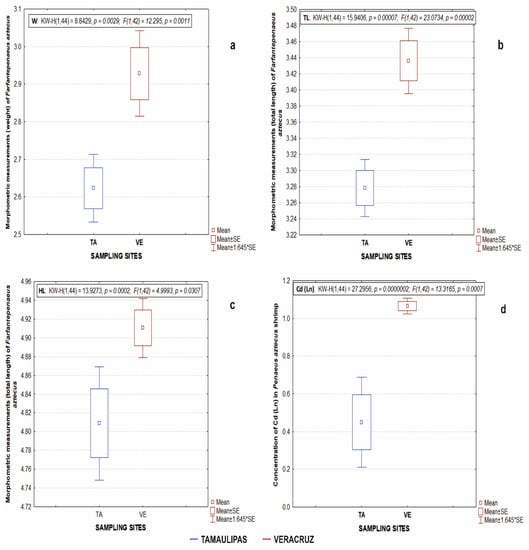
Figure 2.
Morphometric measurements of Penaeus aztecus, weight (a), total length (b), head length (c) and concentration of Cd (d) in the study areas of Veracruz (VE) and Tamaulipas (TA), Mexico.
In relation to sex ratio differentiation, variations were also identified between study areas. Males and females had a similar proportion in the Veracruz area but with differences in the percentage of organisms in each reproductive stage. It was identified that the batches were made up of 72.2% males, which were mainly in reproductive stage II, corresponding to 91.3% of the males. Meanwhile, 27.8% of the females were mainly in reproductive stage I, corresponding to 73.1% of the females analysed.
The Tamaulipas area had the greatest variation in sex proportion; in this area alone, the proportion was 58.4% males, which were mainly in reproductive stage II, with 98.5% for males and 41.6% for females. Females, on the other hand, were found mainly in reproductive stage I at 81.7%, identifying that the degree of maturity detected in both areas was only stages I and II. This indicated that the organisms collected were in immature and developing stages. It would be expected to find greater variability in stages later than the date of this research.
3.3. Toxic Metals in Brown Shrimp P. aztecus
Pb, Cd, Ni, and Cr were detected in the 44 quadrants analysed in the two study areas (Figure 3, Figure 4, Figure 5 and Figure 6). The toxic metals Ni and Pb showed higher concentrations than Cd and Cr. The maximum average concentrations of the toxic metals analysed varied between the two study areas. A statistically significant difference (p < 0.05) was obtained only for Cd in the Veracruz and Tamaulipas areas (Figure 2d).
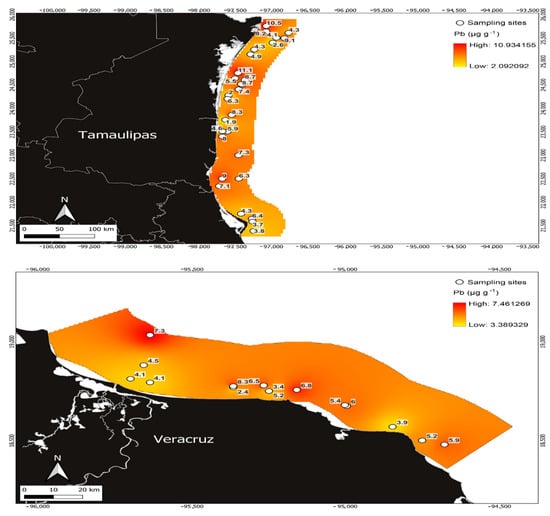
Figure 3.
Pb concentrations in Penaeus aztecus shrimp (whole body) in the areas of Tamaulipas (upper) and Veracruz (below), Mexico.
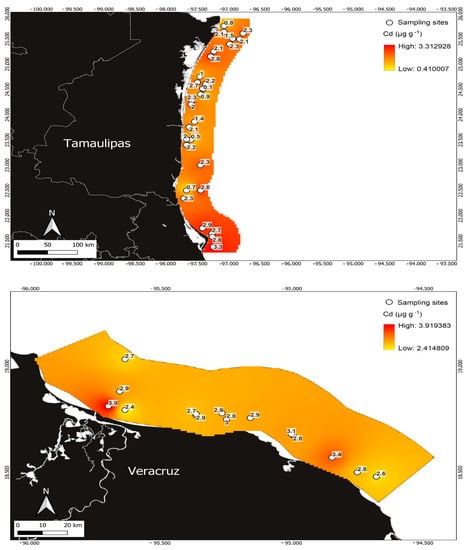
Figure 4.
Cd concentrations in Penaeus aztecus shrimp (whole body) in the areas of Tamaulipas (upper) and Veracruz (below), Mexico.
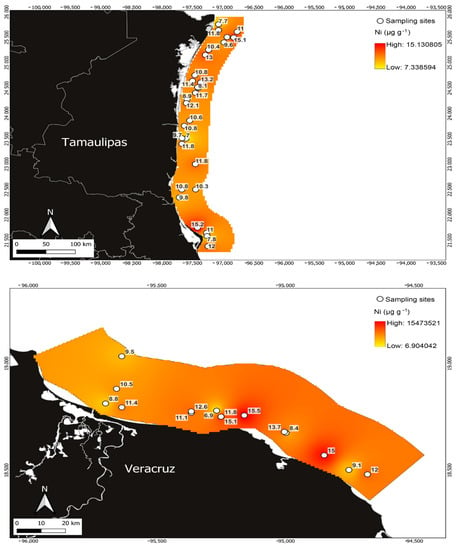
Figure 5.
Ni concentrations in Penaeus aztecus shrimp (whole body) in the areas of Tamaulipas (upper) and Veracruz (below), Mexico.
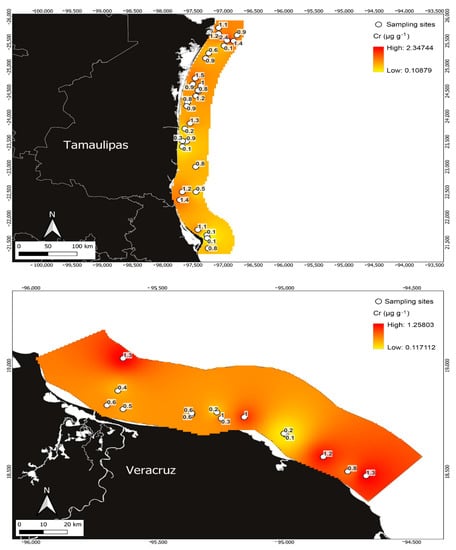
Figure 6.
Cr concentrations in Penaeus aztecus shrimp (whole body) in the areas of Tamaulipas (upper) and Veracruz (below), Mexico.
The highest concentrations of toxic metals in the samples analysed in this research could be associated with the process used for their analysis, i.e., whole shrimps were used, including the cephalothorax and muscle tissue. Although the conventional form of consumption of these crustaceans normally considers only the muscle, occasionally the cephalothorax is also consumed. Therefore, the whole shrimp has been considered as an indicator of public health risk.
Ni was the metal that presented the highest concentration with respect to the rest of the toxic metals (Figure 5). In the samples from the Veracruz area, the mean concentration was 11.43 ± 2.63 µg g−1 (Table 1), the maximum concentration was 15.501 µg g−1, and the minimum was 6.89 µg g−1. Meanwhile, in the Tamaulipas area, the maximum concentration showed similar behaviour to the Veracruz area, with a mean of 10.79 ± 1.74 µg g−1, where the minimum concentration recorded was 7.04 µg g−1 and the maximum was 15.05 µg g−1. The Veracruz area presented higher Ni concentrations, with the exception of the minimum value, which was higher for Tamaulipas.
Pb showed the second-highest concentration in this research; the maximum value of this element obtained was 11.09 µg g−1, which was reported in the area of Tamaulipas (Figure 3). The mean Pb concentration for the Tamaulipas area was 6.50 ± 2.48 µg g−1 and the minimum was 1.88 µg g−1, which was lower than that obtained for the Veracruz area, with a minimum of 2.36 µg g−1. In the Veracruz area, the maximum Pb concentration was 8.26 µg g−1, with a mean of 5.11 ± 1.51 µg g−1 (Table 1).
Variations were identified in the detection of maximum concentrations of toxic metals between one zone and another; in the case of Cd, the maximum concentration was obtained in Veracruz at 3.92 µg g−1, with a minimum of 2.41 µg g−1 and a mean of 2.91 ± 0.33 µg g−1 (Figure 4). In contrast, in the Tamaulipas zone, the maximum concentration obtained was 2.76 µg g−1, with a mean value of 1.84 ± 0.75 µg g−1 and a minimum concentration of 0.108 µg g−1.
Regarding Cr, the maximum concentration detected in the state of Tamaulipas was 2.44 µg g−1, with a mean of 0.94 ± 0.50 µg g−1 and a minimum of 0.106 µg g−1. (Figure 6). Meanwhile, in Veracruz, the mean value was 0.65 ± 0.407 µg g−1, the minimum concentration was 0.100 µg g−1, and the maximum was 1.25 µg g−1 (Figure 6). Among the metals analysed, Cr was identified as the metal with the lowest concentration in shrimp from the Gulf of Mexico (Table 1).
The concentrations of Cd, Pb, and Ni in this research showed variations and, in some cases, exceeded the concentrations reported for P. aztecus and other commercially important penaeid species in the Gulf of Mexico (Table 1). This highlights that differences exist between the different penaeid shrimp species with regard to the accumulation of different metals in their tissues.
In the multivariate analysis of variance, it was identified that there is an interaction between the morphometric variables of weight, total length, cephalic length and for the metal Cd, which showed a significance value p < 0.05. This indicated that there were significant differences between the group means considering the linear combination of the dependent variables analysed. This coincided with the ANOVA, which showed a significant statistical difference only for Cd in the two study areas. In contrast, Pb, Ni, and Cr had significance values > 0.05, indicating that there is no significant effect of the study area interaction on these response variables. Therefore, the multivariate contrast showed that there is a pattern between the study area on morphometric measurements and at least one toxic metal.
However, in relation to ACP for the morphometric variables and the concentration of heavy metals in F. aztecus, it was identified that component 1 and 2 together explain 80.10% of the variability; the former explains 53.85% of the variability and the latter 26.25%. ACP, it was identified that the values of the variables with the highest weight corresponded to the heavy metals Cr and Pb, while the variables with the lowest weight were the variables weight (W) and total length (TL).
The results of the comparison with ANOVA and MANOVA for the three metals indicated above showed that the concentrations in both areas were similar and that they were only weakly related to the variables analysed. However, it was shown that Cd behaved differently to the rest of the metals.
4. Discussion
4.1. Research on Metals in Shrimp in the Gulf of Mexico
Research on the presence of toxic metals in penaeid shrimps can be considered limited, as most of this work has been carried out in geographical areas centred on the coasts of Mexico [34], such as the case of the Gulf of California, which stands out as one of the most studied coastal ecosystems in Mexico [16]. However, it should be noted that there is a small amount of research on the concentration of Cd and Pb in freshwater crustaceans in Mexico; therefore, the information reported on environmental contaminants is insufficient [13].
4.2. Sexual Identification and Gonadal Maturity Analysis of P. aztecus
Variations in the average values of shrimp species lengths in the two analysed areas may be associated with the difference in depths at which the settings were made in these areas (26.2 for Veracruz and 21.3 for Tamaulipas), because shrimp lengths increase with depth [35,36]. In addition, referring to variations in shrimp collection periods and area, Wakida-Kusunoki et al. [12] indicated that during July and early August, shrimp catches in Tamaulipas correspond to a size less than 120 mm TL.
Differences in the size of P. aztecus, even for the same region, could be related to the environmental characteristics of each locality and possible genetic variation between populations [37]. P. aztecus presented two different generations (cohorts) throughout the year. On the coast of Veracruz, it was identified that one group was characterised by its small size between 100 and 108 mm TL; meanwhile, large organisms with a TL of up to 200 mm appeared during the autumn–winter seasons from October to February [10,36]. F. californiensis also showed significant differences in mean size between study areas, indicating that shorter organisms had a mean TL value of 14.78 cm ± 0.53 cm and HL = 3.78 cm ± 0.12 cm, whereas larger specimens had a TL of 16.10 cm ± 1.42 cm and HL = 4.04 cm ± 0.49 cm [37].
The difference in sex ratio is associated with size differences between females and males, and size in the present study may also be related to sampling periods [10,35,36]. Trejo-López [38] indicated that P. aztecus females have a wider size distribution and a better representation towards large sizes (190 mm TL) compared to males, where the size of large organisms is smaller [38]. In addition, March is the most important sampling month for P. aztecus females stage I, while stages III and IV were detected from October to November, when the most important peak of reproduction of this species occurs [38,39]. Brown shrimp P. aztecus have mature females throughout the year, but there are two main reproductive periods: the first is in late winter and early spring and the other is in autumn. However, winter is the most important as it generates the cohort, on the other hand, May and June is when most of the population is growing after being recruited at sea [35,36].
Sex ratio differentiation has been associated with differences in size between females and males, [10,35,36]. Trejo-López [38] indicated that P. aztecus females have a wider size distribution and a better representation towards large sizes (190 mm TL) compared to males, where the size of large organisms is smaller [38]. In addition, March is the most important month of samplig for stage I P. aztecus females, while stages III and IV were detected from October to November, when the most important peak of reproduction of this species occurs [38,39]. Brown shrimp P. aztecus have mature females throughout the year, but there are two main reproductive periods: the first is in late winter and early spring and the other is in autumn. However, winter is the most important as it generates the cohort, and May and June are when most of the population is growing after being recruited at sea [35,36].
The differences in the ratio of the percentage of sexual maturity of females and males and the size of P. aztecus in this research could be related to multiple factors such as the period of collection, the study area, and the genetics, as the reproductive peaks of each species are related to different stages (I and II) of sexual maturity obtained in this research and the periods when the population occurs after sampling.
4.3. Toxic Metals in Brown Shrimp P. aztecus
The concentrations of the metals Pb, Cd, Ni, and Cr in the body of P. aztecus at all sites in the two sampling areas indicate the presence and bioavailability of these elements in the environment, as well as the bioconcentration capacity of the species. This shows that P. aztecus shrimp, being a benthic organism, comes into contact with the sediments where it lives through this route of exposure [33,34], which is considered a recurrent route of exposure to different types of pollutants such as metals.
Variations in metal detection in different regions of the world are associated with the species of aquatic organisms analysed in their environment [3,7] and the sources of contamination and pathways that contribute to the variability of metals [1,2,5,40]. Coinciding with the above, the Tamaulipas study area reported in this research receives inputs of pollutants from the surrounding areas, specifically from the Laguna Madre and the Rio Bravo delta, with the latter reporting the highest concentrations of metals in the study area (Figure 3, Figure 4, Figure 5 and Figure 6).
The differences in the results of this research are related to the capacity of P. aztecus to incorporate toxic metals throughout its body, as well as by different routes such as its diet and through its gills [4,21,33]. Variations in the bioaccumulation of toxic metals in biota may be associated with the particular detoxification capacity of each species to dispose of an element [34,40]. The hepatopancreas has been reported to be the main organ of accumulation and detoxification of toxic metals in crustaceans, demonstrated by histological analysis reflecting the damage received in this organ [4,18,31,32].
Temporality in P. aztecus collection is another important factor regarding the concentration of toxic metals. The analysis of P. aztecus species was carried out during the closed season; this period was established according to regulations to allow the organisms to reproduce and reach the adult stage. Migration of this species from estuarine waters to the coastal zone also occurs during this period, which involves exposure to various pollutants such as toxic metals.
Reproductive periods in various aquatic organisms have also been associated with the accumulation of lipids that act as potential reservoirs for contaminants such as toxic metals [19,20]. It is therefore important to relate the reproductive periods of P. aztecus and the presence of metals, because this species has up to two important reproductive peaks and the most important one is during winter, as it generates the cohort that produces the highest catches in the lagunar and marine zones [36,41]. The potential sublethal effects and variations of toxic metals in the different breeding seasons of these species must also be considered.
The toxic metal values reported in this research are heterogeneous with respect to the concentrations reported in other penaeid species, as is the case for L. setiferus [21,31,32], P. duorarum [30], and L. vanammei [17,18,41], among others (Table 1). It should also be noted that increased concentrations of toxic metals in shrimp analysed in this research represent an important indicator of dietary exposure. P. aztecus is a fishery resource that is used in the production of feed, meal, and other byproducts used in aquacultural and agricultural activities, indicating other alternative pathways of exposure to toxic metals by penaeid shrimp.
The concentrations of Pb and Cd obtained in this research in both areas exceeded the permissible limit of 0.5 µg g−1 established for both metals in crustaceans by NOM-242-SSA1 [42], NOM-029-SSA1 [43], and EC Regulation [44]. Therefore, the permissible limits are an important indicator to assess the risk to public health from the consumption of P. aztecus. In contrast, for metals such as Ni and Cr, the official standard NOM-242-SSA1 [42] has not established a maximum limit (Table 2). However, according to the permissible limit of 80 µg g−1 established for crustaceans by the National Shellfish Health Programme [45], the maximum Ni concentrations obtained in this research did not exceed the aforementioned permissible limit. In the case of Cr, In the case of Cr, the FDA [46] considers the permissible limit for crustaceans to be 12 µg g−1; none of the values obtained in this research exceeded this reference value. This indicates that the Cr concentration reported in this research does not represent a risk to public health. Likewise, the minimum Cr concentration detected in P. aztecus analysed in this study could be associated with the lower bioaccumulation capacity for biota [35,41].

Table 2.
Permissible concentration limits of toxic metals (µg g−1) in crustaceans for human consumption to establish the risk to public health.
The detection of concentrations above the permissible limits of metals such as Pb and Cd, which do not have a biological function, represents a stress factor from exposure to these elements for aquatic organisms such as shrimp. Ramírez-Ayala et al. [13] indicated that the levels of contamination reported in aquatic ecosystems in Mexico are similar to those found in other regions of the world. Nonetheless, the presence of the toxic metals analysed in this research and reported in other shrimp species is an indicator that should be considered to assess the public health risk of this important fishery resource in the Gulf of Mexico.
The processes that regulate the accumulation and detoxification of toxic metals in organisms are very complex and include biotic and abiotic factors. Therefore, the explanation of this phenomenon cannot be explained with the determination of these elements in the tissues of an organism; it is necessary to analyse multiple variables that contribute to the analysis of the factors that influence these mechanisms. Therefore, it is necessary to further investigate the relationship with other morphometric variables to help explain whether the behaviour of toxic metals in P. aztecus is due to the synergistic effect between these elements (Figure 2a–d).
However, few studies have analysed the interaction between the presence of toxic metals and variables complementary to the values determined in the body tissues of aquatic organisms. Nevertheless, some investigations have associated the analysis of different morphometric variables and their relationship with the concentration of toxic metals in some aquatic species such as crustaceans and fish. One of these was carried out by Oladunjoye et al. [47] in Macrobrachium vollenhovenii, who indicated a negative correlation between body weight and Fe concentration in prawns; however, they reported a positive relationship between body weight and Cu and Zn. This is consistent with the associations obtained for P. aztecus in this research between morphometric variables such as weight and total, cephalic length and Cd.
The analyses performed in this research (such as MANOVA and ACP) showed that some of the morphometric variables analyzed have an interaction with specific types of metals, with these the association of a certain specific type of metals was demonstrated. According to the above-mentioned importance of specific morphometric variables such as weight, Oladunjoye et al. [47] reported that in the species M. vollenhovenii, females generally have a relatively higher weight, which is why they accumulate a higher concentration of metals compared to males. Meanwhile, Fajana and Adeboyejo [48] indicated differences in heavy metal accumulation in muscle and morphometric variables in five crustacean and demersal fish species.
It should also be noted that the organisms were collected during one of the reproductive peaks of P. aztecus, characterised by fluctuation in the size of the organisms with their sampling, as well as the accumulation of lipid reserves for the production of gametes. Therefore, biological processes such as migration and reproductive periods can be considered important in the process of incorporation of pollutants such as toxic metals into crustaceans such as P. aztecus.
5. Conclusions
The coasts of the Gulf of Mexico are impacted by different sources of pollution due to the types of activities that take place there. The concentrations of toxic metals obtained in this study are related to different sources of metals in the coastal zone.
In this research, differences were identified in the values of morphometric variables and in the degree of sexual maturity in the areas of Tamaulipas and Veracruz; these variations could be related to the conditions of food supply and the state of maturity of the individuals during the sampling season. These variables influence physiological processes such as growth, accumulation mechanisms, and detoxification of toxic metals.
In the geographical coastal area of the Gulf of Mexico, several species of shrimp are caught for human consumption. It is necessary to follow up through continuous monitoring to analyse the behaviour of these elements, which will support management and governance of our seas and the possible risks to public health.
Author Contributions
Conceptualisation, M.d.R.C.-C., D.R.-V. and A.T.W.-K.; methodology, G.N.-R. and E.B.-G.; validation, M.d.R.C.-C. and A.T.W.-K.; research, M.d.R.C.-C., D.R.-V. and E.B.-G.; resources, M.d.R.C.-C. and D.R.-V.; writing—original draft, M.d.R.C.-C., G.N.-R. and A.T.W.-K.; writing—review and editing, M.d.R.C.-C., G.N.-R. and E.B.-G.; supervision, G.N.-R., D.R.-V. and A.T.W.-K.; project administration, M.d.R.C.-C. and A.T.W.-K. All authors have read and agreed to the published version of the manuscript.
Funding
Tecnológico Nacional de México/Instituto Tecnológico de Boca del Río (TecnM/ITBOCA), and CONACYT (Consejo Nacional de Ciencia y Tecnología).
Institutional Review Board Statement
The handling of specimens in this research was conducted in accordance with the Code of Practice for the Housing and Care of Animals used in scientific procedures. This document was issued on 14 April 2016 and under Section 1 of the Animals (Scientific Procedures) Act. Project identification code: 10300.21-P; approval date: January to current date, as granted by the Institutional Postgraduate and Research Committee, Instituto Tecnológico de Boca del Río (ITBOCA).
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge the Tecnológico Nacional de México/Instituto Tecnológico de Boca del Río (TecnM/ITBOCA) for facilitating the development of this project. The authors thank Guillermo Acosta Barbosa, Mario Gámez Díaz, and Ariel López Salazar (INAPESCA staff) regarding the coordination of shrimp research in the Gulf of Mexico and the Caribbean Sea for the shrimp samples obtained on the research cruises, with fishing exploitation permits no. DGOPA PF-01–052/20, DGOPA PF-01–053/20, and DGOPA PF-01–053/20/. The authors also thank Cuauhtemoc Ruiz Pineda for his support in the elaboration of the maps used in this work.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Plavan, G.I.; Jitar, O.; Teodosiu, C.; Nicoara, M.; Micu, D.; Strungaru, S.-A. Toxic metals in tissues of fishes from the Black Sea and associated human health risk exposure. Environ. Sci. Pollut. Res. 2017, 24, 7776–7787. [Google Scholar] [CrossRef] [PubMed]
- Strungaru, S.-A.; Nicoara, M.; Teodosiu, C.; Baltag, E.; Ciobanu, C.; Plavan, G.I. Patterns of toxic metals bioaccumulation in a cross-border freshwater reservoir. Chemosphere 2018, 207, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Teodosiu, C.; Jitar, O.; Nicoara, M.; Plavan, G. Study of heavy metal pollution and bioaccumulation in the black sea living environment. Environ. Eng. Manag. J. 2013, 12, 271–276. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Aguilar, M.; Osuna, I.; Abad, S.; Izaguirre, G.; Voltolina, D. Metals and shrimp farming in Mexico. Hidrobiológica 2011, 21, 217–228. (In Spanish) [Google Scholar]
- Strungaru, S.-A.; Nicoara, M.; Jitar, O.; Plavan, G. Influence of urban activity in modifying water parameters, concentration and uptake of heavy metals in Typha latifolia L. into a river that crosses an industrial city. J. Environ. Health Sci. Eng. 2015, 13, 5. [Google Scholar] [CrossRef]
- Jitar, O.; Teodosiu, C.; Oros, A.; Plavan, G.; Nicoara, M. Bioaccumulation of heavy metals in marine organisms from the Romanian sector of the Black Sea. New Biotechnol. 2015, 32, 369–378. [Google Scholar] [CrossRef]
- Mendoza-Díaz, F. Determination of Heavy Metals, Cd, Cr, Cu and Pb in Farfantepenaues Aztecus (Ives, 1891) Collected in the Tampamachoco Lagoon, Veracruz. Master’s Thesis, Management of Marine and Coastal Ecosystems, Universidad Veracruzana, Tuxpan, Mexico, 2010; p. 84. (In Spanish). [Google Scholar]
- Frías-Espericueta, M.G.; Osuna-López, J.I.; Izaguirre-Fierro, G.; Aguilar-Juárez, M.; Voltolina, D. Cadmium and lead in organisms of commercial importance in the coastal zone of Sinaloa, Mexico: 20 years of studies. CICIMAR Oceánides 2010, 25, 121–134. (In Spanish) [Google Scholar] [CrossRef]
- CONAPESCA (Comisión Nacional de Acuacultura y Pesca). Mexican Shrimp Production in the 2019–2020 Capture Season. CONAPESCA. Mexico. 2020. Available online: https://gob.mx/conapesca/articulos/produjo-mexico-47-mil-664-toneladas-de-camaron-en-la-temporada-de-captura-2019-2020-agricultura?idiom=es (accessed on 25 June 2022). (In Spanish).
- Wakida-Kusunoki, A.T.; Solana-Sansores, R.; Sandoval, M.E.; Núñez-Márquez, G.; Uribe-Martínez, J.A.; González-Cruz, A.; Medellín-Ávila, M. Shrimp from the Gulf of Mexico and the Caribbean Sea. In Sustainability and Responsible Fishing in Mexico; Arreguín-Sánchez, F., Beléndez-Moreno, L., Méndez-Gómez-Humarán, I., Solana-Sansores, R., Rangel-Davalos, C., Eds.; Instituto Nacional de la Pesca: Ciudad de México, Mexico, 2006; pp. 427–476. (In Spanish) [Google Scholar]
- Official Gazette (Diario Oficial). AGREEMENT Establishing Closed Seasons and Areas for the Capture of All Shrimp Species in Marine Waters and Estuarine Lagoon Systems under Federal Jurisdiction in the Gulf of Mexico and the Caribbean Sea for 2022. Published in the Official Gazette in 3 May 2022. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5650823&fecha=03/05/2022#gsc.tab=0 (accessed on 18 May 2022). (In Spanish).
- Frías-Espericueta, M.; Voltolina, D.; Osuna-López, I.; Izaguirre-Fierro, G. Toxicity of metal mixtures to the Pacific white shrimp Litopenaeus vannamei postlarvae. Mar. Environ. Res. 2009, 68, 223–226. [Google Scholar] [CrossRef]
- Ramírez-Ayala, E.; Arguello-Pérez, M.A.; Tintos-Gómez, A.; Pérez-Rodríguez, R.Y.; Díaz-Gómez, J.A.; Borja-Gómez, I.; Sepúlveda-Quiroz, C.A.; Patiño-Barragán, M.; Lezama-Cervantes, C.; Salomé-Baylón, J. Review of the biomonitoring of persistent, bioaccumulative, and toxic substances in aquatic ecosystems of Mexico: 2001-2016. Lat. Am. J. Aquat. Res. 2020, 48, 705–738. [Google Scholar] [CrossRef]
- Páez, F.; Ruiz, A. Trace metals in the Mexican shrimp Penaeus vannamei from estuarine and marine environments. Environ. Pollut. 1995, 87, 243–247. [Google Scholar] [CrossRef]
- Páez, F.; Tron, L. Concentration and distribution of heavy metals in tissues of wild and farmed shrimp Penaeus vannamei from the northwest coast of Mexico. Environ. Int. 1995, 22, 443–450. [Google Scholar] [CrossRef]
- Ruelas-Inzunza, J. Distribution and Concentration of Trace Metals in Tissues of Three Penaeid Shrimp Species from Altata-Ensenada del Pabellón Lagoon (S.E. Gulf of California). Bull. Environ. Contam. Toxicol. 2004, 72, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Frías-Espericueta, M.G.; Osuna-López, J.I.; Estrada-Toledo, F.J.; López-López, G.; Izaguirre-Fierro, G. Heavy metals in the edible muscle of shrimp from coastal lagoons located in Norhwest Mexico. Bull. Environ. Contam. Toxicol. 2005, 74, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Frías-Espericueta, M.G.; Osuna-López, I.; Voltolina, D.; Beltrán-Velarde, M.A.; Izaguirre-Fierro, G.; López-López, G.; Muy-Rangel, M.D.; Rubio-Carrasco, W. The contents of Cd, Cu, Pb and Zn of the white shrimp Litopenaeus vannamei (Boone, 1931) of six coastal lagoons of Sinaloa, NW Mexico. Rev. de Biol. Mar. y Oceanogr. 2009, 44, 197–201. [Google Scholar] [CrossRef]
- Lango-Reynoso, F.; Castaneda-Chavez, M.D.R.; Landeros-Sanchez, C.; Galaviz-Villa, I.; Rodríguez, G.N.; Soto-Estrada, A. Cd, Cu, Hg and Pb, and Organochlorines Pesticides in Commercially Important Benthic Organisms Coastal Lagoons SW Gulf of Mexico. Agric. Sci. 2013, 1, 63–79. [Google Scholar] [CrossRef]
- Castañeda-Chavez, M.D.R.; Rodríguez, G.N.; Lango-Reynoso, F.; Galaviz-Villa, I.; Landeros-Sánchez, C. Heavy Metals in Oysters, Shrimps and Crabs from Lagoon Systems in the Southern Gulf of México. J. Agric. Sci. 2014, 6, 108–117. [Google Scholar] [CrossRef][Green Version]
- Vázquez-Sauceda, M.L.; Sanchez-Martinez, J.G.; Rabago-Castro, J.L.; Benavides-Gonzalez, F.; Blanco-Martinez, Z.; Garrido-Olvera, L.; Perez-Castañeda, R. Heavy metals in áter, sediment and shrimp (Litopenaeus setiferus) from a tropical estuarine ecosystem of the Gulf of Mexico. Fresenius Environ. Bull. 2019, 28, 7924–7932. [Google Scholar]
- Wakida-Kusunoki, A.T.; Solana-Sansores, R.; González-Cruz, A. Estimated abundance of brown shrimp (Farfantepenaeus aztecus) off the coast of Tamaulipas, 2002. Oceánides 2005, 20, 17–28. (In Spanish) [Google Scholar] [CrossRef]
- Díaz, R.; Márquez, M.E.; Espinosa, G.; Berovides, V. Morphometric and electrophoretic study of three species of commercial penaeid shrimp in culture. Rev. de Investig. Mar. 1995, 16, 83–88. (In Spanish) [Google Scholar]
- Fischer, W.; Krupp, F.; Schneider, W.; Sommer, C.; Carpenter, K.E.; Niem, V.H. FAO Guide for the Identification of Species for Fishing Purposes. Eastern Central Pacific; FAO: Rome, Italy, 1995; Volume I. Plants and Invertebrates, pp. 1–646. (In Spanish) [Google Scholar]
- Official Gazette. Agreement that Announces the Fishing Management Plan for Brown Shrimp (Farfantepenaeus aztecus) and White Shrimp (Litopenaeus setiferus) on the Coasts of Tamaulipas and Veracruz. Published in the Official Gazette on 12 March 2014. Available online: https://inapesca.gob.mx/portal/documentos/Planes-de-Manejo-Pesquero/Golfo/Plan-de-Manejo-Pesquero-de-Camaron-Cafe-y-Blanco.pdf (accessed on 2 July 2022). (In Spanish).
- Fenucci, J.L. Manual for Breeding Penaeid Shrimp. Field Document; Food and Agriculture Organization (FAO): Rome, Italy, 1988; pp. 1–88. (In Spanish) [Google Scholar]
- Official Gazette. OFFICIAL MEXICAN STANDARD NOM-117-SSA1-1994. Goods and Services. Test Method for the Determination of Cadmium, Arsenic, Lead, Tin, Copper, Iron, Zinc and Mercury in Food, Drinking Water and Purified Water by Atomic Absorption Spectrometry. Official Gazette of the Federation 16 August 1995. Available online: http://transparencia.cofepris.gob.mx/index.php/es/?option=com_content&view=article&id=36&Itemid=101 (accessed on 25 June 2022). (In Spanish).
- Aguilar-Ucán, C.A.; Montalvo-Romero, C.; Cerón-Bretón, J.G.; Anguebes-Fransesch, F. Heavy metal levels in marine species: Oyster (Crassostrea virginica), Crab (Callinectes sapidus) and Shrimp (Litopenaeus setiferus), from Ciudad del Carmen, Campeche, Mexico. Lat. Am. J. Nat. Resour. 2014, 10, 9–17. (In Spanish) [Google Scholar]
- USEPA. Method 7010: Graphite Furnace Atomic Absorption Spectrophotometry. USA. 2007. Available online: https://epa.gov/hw-sw846/sw-846-test-method-7010-graphite-furnace-atomic-absorption-spectrophotometry (accessed on 25 January 2022).
- Vázquez, F.G.; Sharma, V.K.; Mendoza, Q.A.; Hernandez, R. Metals in Fish and Shrimp of the Campeche Sound, Gulf of Mexico. Bull. Environ. Contam. Toxicol. 2001, 67, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Martínez, V.; Aguirre-Macedo, M.L.; Rodriguez, R.E.D.R.; Gold-Bouchot, G.; Osten, J.R.-V.; Miranda-Rosas, G. The pink shrimp Farfantepenaeus duorarum, its symbionts and helminths as bioindicators of chemical pollution in Campeche Sound, Mexico. J. Helminthol. 2006, 80, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Palomarez-García, J.M.; Castañeda-Chávez, M.R.; Lango-Reynoso, F.; Landeros-Sánchez, C. Heavy metal levels in brown shrimp Farfantepenaeus aztecus from the Tamiahua lagoon, Veracruz, Mexico. Rev. Investig. Mar. 2009, 30, 63–69. (In Spanish) [Google Scholar]
- Vázquez-Sauceda, M.L.; Pérez-Arriaga, E.; Benavides-González, F.; Blanco-Martínez, Z.; Hernández-Contreras, S. Heavy Metals (Pb, Cd and Hg) in the Shrimp Farfantepenaeus Aztecus from Laguna Madre, Tamaulipas and the Risk to Public Health. Tecno Intelecto Magazine/Tecnológico Nacional de México. 2017, p. 19. Available online: https://www.itvictoria.edu.mx/investigacion/tecnointelecto/TecnoINTELECTO-Vol%2014-2-2017-FINAL-24ABR-2018.pdf#page=21 (accessed on 7 May 2022).
- Baruch-Garduza, E.; Castañeda-Chávez, M.R.; Wakida-Kusunoki, A.T.; Reynier-Valdés, D.; Navarrete-Rodríguez, G. Heavy metals in macroinvertebrates (Penaeus) in the Mexican coasts of the Gulf of Mexico: Status, sources, and regulations. Lat. Am. J. Aquat. Res. 2022, 50, 331–342. [Google Scholar] [CrossRef]
- Wakida-Kusunoki, A.T.; García-Solorio, L.; Vázquez Benavides, N.G. Abundance of commercial penaeid shrimp juveniles in the northern zone of Laguna Madre, Mexico. Hidrobiológica 2008, 18, 85–88. (In Spanish) [Google Scholar]
- Wakida-Kusunoki, A.T.; González Cruz, A.; Medellín-Ávila, M.; Arreguín-Sánchez, F. Estimated emigration of the brown shrimp Farfantepenaeus aztecus through the mouth of Mezquital, Tamaulipas, Mexico. Hidrobiológica 2010, 20, 256–265. (In Spanish) [Google Scholar]
- Barbosa-Saldaña, M.L.; Díaz-Jaimes, P.; Uribe-Alcocer, M. Morphological variation of the brown shrimp (Farfantepenaeus californiensis) in the Mexican Pacific. Mex. J. Biodivers. 2012, 83, 42–50. (In Spanish) [Google Scholar]
- Trejo-López, B. Temporal Variation of the Brown Shrimp Farfantepenaeus aztecus off the Coast of Veracruz during 1999–2000. Master’s Thesis, Ecology and Fisheries, Institute of Marine Sciences and Fisheries, Universidad Veracruzana, Xalapa, Mexico, 2015; p. 39. Available online: https://uv.mx/veracruz/mep/files/2012/10/Tesis_Beatriz-Trejo-Lopez.pdf (accessed on 5 July 2022). (In Spanish).
- Ventura-Flores, A.; Giménez-Hurtado, E.; Delgado-Miranda, G.; Rosquete-Miranda, C.M. Maturation of female pink shrimp Farfantepenaeus notialis (Pérez-Farfante, 1967) in the Gulf of Guacanayabo, Cuba during the periods 1992–1994 and 2010–2013. Rev. Electrónica de Vet. 2017, 18, 1–13. (In Spanish) [Google Scholar]
- Sultana, S.; Hossain, M.B.; Choudhury, T.R.; Yu, J.; Rana, S.; Abu Noman, M.; Hosen, M.M.; Paray, B.A.; Arai, T. Ecological and Human Health Risk Assessment of Heavy Metals in Cultured Shrimp and Aquaculture Sludge. Toxics 2022, 10, 175. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Osuna-López, J.I.; Delgado-Álvarez, C.G.; Muy-Rangel, M.D.; López-López, G.; Izaguirre-Fierro, G.; Jaimes-Bustamante, F.; Zazueta-Padilla, H.M.; Aguilar-Juárez, M.; Rubio-Carrasco, W.; et al. Changes in metal contents in shrimp cultured in NW Mexico (2000–2010). Environ. Monit. Assess. 2015, 187, 1–7. [Google Scholar] [CrossRef]
- Official Gazette. NOM-242-SSA1-2009. Productos y Servicios. Productos de la Pesca Frescos, Refrigerados, Congelados y Procesados. Especificaciones Sanitarias y Métodos de Prueba. Publicado Diario Oficial de la Federación 10 de Febrero de 2011. Reviewed: 15 June 2022. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5177531&fecha=10/02/2011 (accessed on 17 May 2022).
- Official Gazette. Proyecto de Norma Oficial Mexicana NOM-029-SSA1-1993, Bienes y Servicios. Productos de La pesca. Crustáceos Fresco-Refrigerados y Congelados. Especificaciones Sanitarias. Publicado en 15 April 1994. Available online: https://dof.gob.mx/nota_detalle.php?codigo=4687630&fecha=15/04/1994#gsc.tab=0 (accessed on 13 April 2022).
- Reglamento (CE) No. 1881/2006; Contenido Máximo de Determinados Contaminantes en los Productos Alimenticios. Reglamento Comunidad Europea (CE): Brussels, Belgium, 2008; Volume 8, pp. 1–18.
- ISSC. National Shellfish Sanitation Program. Guide for the Control of Molluscan Shellfish; Interstate Shellfish; ISSC: Columbia, SC, USA, 2007; p. 549. [Google Scholar]
- USFDA. Guidance Document for Lead in Shellfish; Center for Food Safety and Applied Nutrition US Food and Drug Administration: Silver Spring, MD, USA, 1993.
- Oladunjoye, R.Y.; Fafioye, O.O.; Asiru, R.A.; Solola, T.D.; Isiaka, O.F. Morphometric and Metals Relationships in African River Prawn, Macrobranchium Vollenhovenii (Herklot, 1852) of Asejire Lake, Nigeria. In Proceedings of the International Conference on Developmental Sciences and Technologies, Federal University of Agriculture, Abeokuta, Nigeria; 2019; pp. 124–135. Available online: https://unaab.edu.ng/wp-content/uploads/2020/07/16.-Oladunjoye2.pdf (accessed on 19 June 2022).
- Fajana, O.P.; Adeboyejo, A. Morphometric and Heavy Metals Accumulation in the Muscles of Five Demersal Seafoods Sampled in Nigerian Coastal Waters. Adv. J. Grad. Res. 2022, 12, 20–35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).