Abstract
To assess the lipid requirements of O. macrolepis broodstock, five iso-nitrogenous diets (39 g kg−1) with five lipid levels, 50 (5 L), 70 (7 L), 90 (9 L),110 (11 L), and 130 (13 L; g kg−1), were made. A total of 105 three-year-old individuals (50.11 ± 2.86 g per fish) were divided into five groups (triplicate per group) and were fed with the diets, respectively, for eight weeks. Then, the fish were sampled, and items were determined. The results showed that growth rate and feed efficiency ratio were not significantly affected by diets (p > 0.05). A clear dose–response effect of dietary lipid was observed on somatic indexes of gonad indexes of the O. macrolepis brookstock, with the highest values corresponding to fish fed 9 and 11 g kg−1 lipids, in contrast, gonad indexes were reduced as dietary lipid moved away from this level. The other somatic indexes, such as viscerosomatic index, perivisceral fat index, etc., were not significantly affected by diets (p > 0.05). The content of crude lipid and crude protein in carcass, hepatopancreas, and gonad were not significantly affected by dietary lipid levels (p > 0.05). The gonad fatty acids of 16:0 and 22:6n-3 decreased and 18:2n-6 increased with the increasing lipid level, being significantly altered by diets (p < 0.05). The histological features of the gonad showed no significant difference among the five diets (p > 0.05). The relative expression of sex steroid-synthesizing proteins (fshr, 3β-hsd, 17β-hsd, aro., and star.) in the gonad of fish was most significantly highly expressed in the 9 L and 11 L groups (p < 0.05). The results suggested that a proper dietary lipid level of 90–110 g kg−1 could maintain gonad development of O. macrolepis broodstock without affecting growth performance.
1. Introduction
Given the increasing importance of domestication in aquaculture, there is a need for an increased focus on the role of nutrition in broodstock and gonad maturation, as well as in improving larval quality and performance. Izquierdo et al. [1] pointed out that broodstock nutrition of fin fish nutrition was one of the most poorly understood and researched areas almost two decades ago, and significant advances have been achieved in this area [2] in these years; however, there are still important gaps of knowledge related to fish broodstock nutrition. Considering the broad range of species and families important to aquaculture, and the relative scarcity of research into broodstock nutrition, it is difficult to generalize the nutritional requirements of broodfish. Some sporadic research reported the effect of vitamins [3,4,5,6] and protein and carbohydrate levels [7] on the reproductive performance of some broodstock fish. Lipids play an important role as sources of metabolic energy for somatic growth, and they are also the source of essential fatty acids required for the formation of cell membranes, as well as for gonad development and maturation [8,9,10,11]. In this context, the lipid and fatty acid composition of broodstock diets have been identified as major dietary factors that determine successful reproduction and survival of the offspring [1]. In this sense, several studies have shown that higher dietary lipid levels promoted gonad development, resulting in higher fecundity values, larger oocyte diameters, and a greater number of mature oocytes [12,13,14,15,16]. In addition, dietary lipid levels have also been correlated to fish health, immunity, and condition [17], thus revealing the pleiotropic effects of this group of macronutrients on the organism.
The Onychostoma macrolepis (Bleeker, 1871), also named Gymnostomus macrolepis [18], is a freshwater benthopelagic cave fish species belonging to the Cyprinidae family that is distributed in the North of China, including the Jialing, Huai, Wei, Hai, and Yellow rivers [19]. This fish is very delicious and nutritious, being rich in protein and the important highly unsaturated fatty acids EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid), and is very much in demand, resulting in the high price of 200 RMB per kg in the local aquatic market [20]. Although the IUCN classify it as a species of least concern [21], this species has been listed as a National Second-class Protected Animal by the Chinese authorities (List of Aquatic Wild Animal Protection in China). The main reasons for such classification are anthropogenic threats [22] and overfishing and habitat (caves) degradation [23]. During these last years, the efforts for the conservation of this cave fish have increased, and several studies have been published regarding its protection [18], reproductive biology [24,25], and dietary protein requirements [26]. However, up to date, no available data exists on the dietary lipid requirements for this species; information that is critical for the successful artificial propagation of this species.
The objective of the present study was to investigate the optimal dietary lipid levels for the cavefish O. macrolepis broodstock. This assessment was conducted considering several parameters related to the somatic growth, gonad development, and health conditions of broodfish.
2. Materials and Methods
All experimental procedures were conducted in accordance with the Guidelines for Experimental Animals by the Animal Care and Use Committee of Northwest A & F University, China.
2.1. Experimental Diets, Fish and Feeding Procedures
Five isonitrogenous experimental diets (390 g kg−1 protein) with increasing lipid levels (50, 70, 90, 110, and 130 g kg−1) were formulated for O. macrolepis broodfish. Different dietary lipid levels were obtained by increasing soybean oil as the only source of dietary lipids (Table 1). The ingredients and the proximate composition of the experimental diets are presented in Table 1. The diets were named 5 L, 7 L, 9 L, 11 L, and 13 L, according to their percentage of lipid content (5, 7, 9, 11, and 13%, respectively). Regarding diet preparation, all ingredients were weighed and mixed for 15 min, after which, distilled water and oil were added and mixed. Once the desired consistency of the dough was reached, the mixture was then mechanically extruded to obtain suitable-sized pellets (1 mm). The pellets were dried in a convection oven at 25 °C for 24 h, stored in re-sealable plastic bags, and stored at −20 °C until their use.

Table 1.
Ingredients (g kg−1, dry weight) and proximate composition (air dry basis, %) of experimental diets differ in their total crude lipids.
A total of 105 healthy three-year-old O. macrolepis (body weight, BW = 50.1 ± 2.9 g, mean ± standard deviation) were obtained from Tian Yuan Shen Tai Aquaculture Farming (Ankang, Zheng Ping, Shaanxi, China) and transferred to the Ankang Fisheries Experimental and Demonstration Station of Northwest A&F University (Ankang, Shaanxi, China). Then, fish were randomly distributed regardless of their sex (no external sexual differentiation) into 15 rearing tanks of 130 L of volume (three replicates per experimental condition) and maintained in a recirculation aquaculture system (RAS) that guaranteed water quality utilizing ultraviolet, biological, and mechanical filtration. Tanks were equipped with aeration 24 h a day to maintain dissolved oxygen levels at 7.7 ± 0.2 mg L−1 (mean ± standard deviation). The water temperature was 20.0 ± 1.5 °C, and the pH was 7.8 ± 0.5. The photoperiod was 12 h light and 12 h darkness. During the experimental period (8 weeks), fish were fed by hand to apparent satiation levels, three times a day (8:30, 12:30, and 16:30 h).
2.2. Sampling Procedures
At the end of the feeding trial, all fish were sedated with tricaine methane sulfonate of water solution (MS-222; 0.01 g L−1, Sigma-Aldrich, Burlington, MA, USA), and their BW and standard length (SL) were determined to the nearest 0.1 g and 1 mm, respectively. Once measured, blood samples were taken from the caudal peduncle vein with heparinized syringes from three fish per tank (n = 9 per diet). The remaining fish from each tank were dissected for sex determination and to obtain the weight of selected tissues, such as the hepatopancreas, spleen, kidney, intestine, ovary, and testis. The gonads of ovary and testis of three fish per group were fixed, respectively, in 4% buffered formalin for 48 h for subsequent histological observation. The hepatopancreas and gonads were stored at −80 °C, respectively, for further biochemical analyses on fatty acid profile and gene expression studies.
2.3. Growth Performance, Feed Utilization, and Somatic Indexes
The effect of experimental diets differing in their lipid levels on the key performance indicators related to growth and feed performances in O. macrolepis, as well as on somatic parameters, were calculated using the following formulae:
Specific growth rate (SGR, %) = (Ln final BW − Ln initial BW) × 100/days;
Feed intake (FI, g fish−1) = feed ingested (g)/number of fish;
Feed efficiency (FE) = BW gain (g)/feed intake (g);
Survival rate (SR, %) = (final number of fish/initial number of fish) × 100;
Viscerosomatic index (VSI, %) = weight of viscera (g)/BW (g) × 100;
Perivisceral fat index (PVFI, %) = weight of intraperitoneal fat (g)/BW (g) × 100;
Gonadosomatic index of female (GSIf, %) = weight of ovary (g)/BW (g) × 100.
Gonadosomatic index of male (GSIm, %) = weight of testis (g)/BW (g) × 100.
Hepatosomatic index (HSI, %) = weight of hepatopancreas (g)/BW (g) × 100;
Splenic somatic index (SSI, %) = weight of spleen (g)/BW (g) × 100;
Renal somatic index (RSI, %) = kidney weight (g)/BW (g) × 100;
Ratio of intestinal length to body length (RILBL, %) = length of intestine (cm)/SL (cm) × 100;
2.4. Proximate and Fatty Acid Composition of Diets and Fish Samples
Proximate composition of experimental diets, fish carcass, and tissues were analyzed according to the Association of Official Analytical Chemists (AOAC 2004) [27]. In particular, crude protein levels were determined by Kjeldahl’s method, crude lipids were determined by the method of ethyl ether extraction, and moisture and ash contents were determined by sample drying in the oven at 105 °C for 24 h and by burning in a muffle furnace at 550 °C for four h, respectively.
For fatty acid analysis, lipids were extracted by chloroform/methanol (2:1 v/v) initiated by Folch et al. (1957) [28]; then, the lipid fraction was saponified and methylated using 12% boron trifluoride in methanol. Briefly, methyl esters were separated using a Gas chromatograph Trace-2000 (ThermoQuest Corporation, Austin, TX, USA) with the following procedure: 60 °C for 20 s, 25 °C min−1; 160 °C for 28 min, 25 °C min−1; 190 °C for 17 min, 25 °C min−1; 220 °C for 9 min. This gas chromatograph was equipped with a 50-m CP-Sil 88 (Chromopack, Agilent) fused silica capillary column (i.d., 0.32 mm). Fatty acids were identified by retention time using standard mixtures of methyl esters (47015-U, Nu-Chek-Prep, Elysian, MN, USA) and quantified using Total Chrom software (version 6.2, Perkin Elmer) as described in Lie and Lambertsen (1991) [29]. The fatty acid composition of five diets was shown in Table 2.

Table 2.
Fatty acid composition (%) of experimental diets differs in their total crude lipids.
2.5. Histological Procedures
Histological procedures for gonad samples from different experimental groups were conducted according to the standard histological techniques [30]. In brief, samples were dehydrated in graded series of ethanol and embedded in paraffin. All tissue blocks were sectioned at the thickness of 5 μm and stained with Haematoxylin and Eosin (H & E), where gonads of ovary and testis were transected. All histological procedures were done at the Pathology Laboratory of the Yangling Demonstration Zone Hospital (Shaanxi, China). The morphology of the gonads were observed under a microscope (Motic BA310, Motic China Group Co., LTD manufacturer, Xiamen, China).
2.6. Gene Expression by Quantitative Real-Time PCR
The impact of dietary lipid level on protein metabolism and sex steroid synthesizing in selected tissues was assessed using the following selected genes: (i) protein metabolism: insulin-like growth factor 1 (igf1), glutamate dehydrogenase (gldh); (ii) sex steroid-synthesizing: follicle stimulating hormone receptor (fshr), 3 beta hydroxysteroid dehydrogenase (3β-hsd), 17β-hydroxysteroid dehydrogenase (17β-hsd), aromatase (aro.), steroidogenic acute regulatory protein (star.).
Genes were determined by quantitative real-time PCR. Firstly, total RNA was extracted from the samples using RNAiso Plus (TaKaRa, Dalian, China) according to the manufacturer’s instructions. Then, RNA was purified using the RNAWizTM protocol (Ambion, Austin, TX, USA) to remove genomic DNA by DNase treatment (DNA-freeTM, Ambion), and its quality and quantity were determined by spectrophotometry (NanoDrop 1000, Thermo Scientific Inc., Wilmington, NC, USA) and agarose gel electrophoresis (2%). Then, RNA (1 μg) was reverse transcribed in a 20 μL reaction volume using random primers (Fermentas Life Science, Hanover, NH, USA); reverse transcription was carried out at 50 °C for 15 min and at 85 °C for 2 min, following the instructions of the kit manufacturer.
Gene expression was measured by quantitative real-time PCR with Sybr-Green (Roche, Switzerland), and reactions were performed using a CFX 96 Real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The total volume of the PCR reaction was 20 μL, where 1 μL of the cDNA product, 0.6 μL of each primer (10 μM), 10 μL of 2 × SYBR® Premix Ex TaqTMII (TaKaRa, Dalian, China), and 7.8μL of double sterile distilled water. The RT-qPCR program started at 95 °C for 3 min, followed by 38 cycles (95 °C for 15 s, 60 °C for 30 s, and 65 °C for 5 s). The PCR primer sequences for each gene were synthesized by Sangon Biotech (Shanghai, China) Co., Ltd. and are shown in Table 3. Relative gene quantification was conducted according to the 2−ΔΔCT method [31], using β-actin as a housekeeping gene.

Table 3.
List of primers used for quantitative PCR analysis.
2.7. Statistical Analyses
Data are shown as means ± standard error. Data expressed as percentages were arcsine-square-root transformed before ANOVA analysis (data previously checked for normality and homoscedasticity). When significant differences were found (p < 0.05), the one-way ANOVA was followed by Duncan’s post hoc test. All analyses were performed using SPSS 16 for Windows Software (SPSS, Chicago, IL, USA).
3. Results
3.1. Growth Performance and Somatic Indexes
At the end of the study, final body weight (BWf), specific growth rate (SGR), feed intake (FI), and feed efficiency ratio (FER) values in O. macrolepis brood fish were not significantly affected by dietary lipid levels (p > 0.05; Table 4).

Table 4.
The effect of dietary crude lipid levels on growth and feed efficiency parameters of Onychostoma macrolepis broodfish.
Regarding the impact of dietary lipid levels of somatic parameters on O. macrolepis females, GSIf, PVFI, and RSI were significantly affected by the diet (p < 0.05; Table 5). In particular, the highest GSIf values were found in broodfish from the 9 L (4.00 ± 0.83%) and 11 L (3.72 ± 0.45%) dietary groups, whereas the lowest values were those of females fed the 13 L diet (2.56 ± 0.12%). The rest of the groups (5 L and 7 L diets) showed intermediate values between maximal and minimal GSIf values. The highest and lowest levels of PVFI were found in females fed the 13 L (2.66 ± 0.44%) and 5 L (1.34 ± 0.09%) diets, respectively. The highest and lowest values in RSI were found in females fed the 13 L (2.67 ± 0.04%) and 5 L (1.34 ± 0.09%) diets, respectively. No statistically significant differences in the VSI, HSI, SSI, and RILBL were found between females fed different experimental diets (p > 0.05; Table 5).

Table 5.
The effect of different dietary lipid levels on somatic indexes in Onychostoma macrolepis broodfish (n = 3; %).
Considering O. macrolepis males, different dietary lipid levels only affected GSIm and SSI values (p < 0.05; Table 5). In particular, the highest and lowest GSIm values were found in fish fed 9 L (1.33 ± 1.66%) and 7 L (0.67 ± 0.19%) diets, respectively (p < 0.05; Table 5), whereas the rest of the dietary groups showed intermediate values. Regarding the SSI, the highest values were found in males fed 9 L (0.25 ± 0.08%) and 11 L (0.23 ± 0.06%) diets, whereas the lowest SSI values were recorded in males fed 5 L (0.18 ± 0.03%) and 7 L (0.17 ± 0.05%) diets (p < 0.05). The rest of the somatic indexes evaluated in males (HSI, VSI, PVFI, RSI, and RILBL) were not significantly different among dietary groups (p > 0.05; Table 5).
3.2. Fatty Acid Profile of the Gonad Tissues
The fatty acid composition of the gonad in O. macrolepis fed diets with different crude lipid levels was relatively stable in terms of total levels of saturated, monounsaturated, n-6, and n-3 polyunsaturated fatty acids (Table 6; p > 0.05). Statistically, significant differences were only found at the level of palmitic acid (16:0), linoleic acid (18:2n-6), and docosahexaenoic acid (22:6n-3) (p < 0.05). In particular, the highest and lowest levels of 16:0 were found in the testes of fish fed the 5 L and 7 L diets, respectively, whereas the rest of the dietary groups showed intermediate levels. Regarding 18:2n-6 levels, the highest values were found in the gonads of fish fed the 13 L diet, whereas the lowest values were found in fish fed the 5 L diet, while the rest of the dietary groups showed intermediate values. The content in 22:6n-3 was highest in the gonad of fish from the 5 L group, whereas the lowest values were found in fish from the 13 L, and the rest of the dietary groups showing intermediate values.

Table 6.
Fatty acid composition (%) of gonad from Onychostoma macrolepis fed diets differing in their crude lipid content.
3.3. Histological Organization of Target Tissues
The cell morphology and structural integrity of cell membranes of the testis and ovary in fish of five dietary groups were exhibited as clear and intact, and no abnormal cells were found (Figure 1A). The ovaries are composed of oocytes at the different developmental stages, including some more giant mature cells, and appeared granulated due to the high concentration of yolk globules, as well as some smaller under mature cells. The mean egg diameter showed no significant difference in different diets, and oocyte atresia was not noted in the ovaries of females among these experimental groups (Figure 1B, data not shown fully).
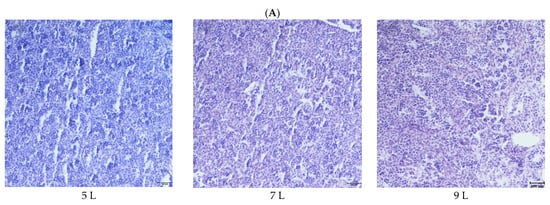
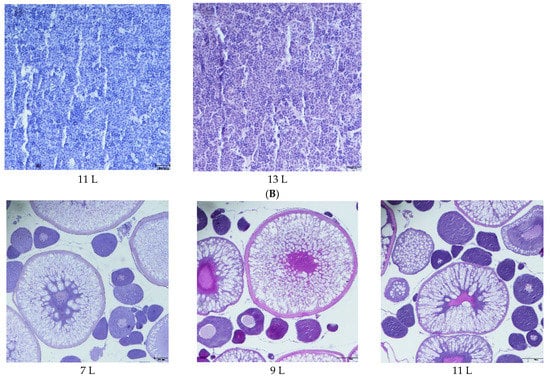
Figure 1.
Histological section of the testis and ovary of the male and female O. macrolepis broodstock, fed graded lipid levels during 8 weeks of feeding trial. (Testis, 100× magnification; ovary, 40× magnification; bar = 100 µm). Here only show histological section of the ovary in 7 L, 9 L and 11 L groups. (A). Gonad of male (testis); (B). Gonad of female (overy).
3.4. Gene Expression
The relative expression of genes involved in growth and protein metabolism in the hepatopancreas of O. macrolepis fed diets with different lipid levels are illustrated in Figure 2A. In particular, relative gene expression values for igf and gldh were similar among other experimental groups (Figure 2A).
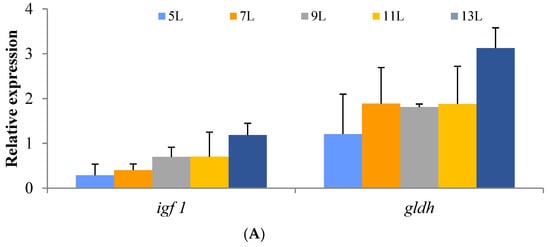
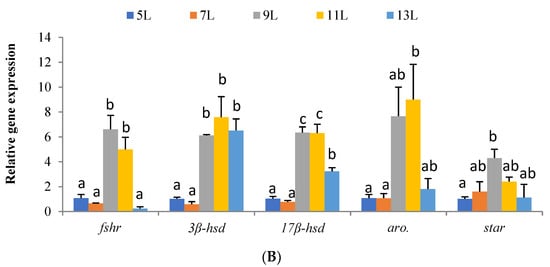
Figure 2.
Relative expression values of genes from the hepatopancreas involved in growth and protein metabolism (igf1, gldh; (A)) and the gonad involved in sex steroid-synthesizing proteins (fshr, 3β-hsd, 17β-hsd, aro. and star.; (B)) in Onychostoma macrolepis broodfish fed diets differing in their crude lipid levels. Different letters indicate significant differences.
Gene expression of markers involved in gonad development is shown in Figure 2B. In particular, we found that dietary lipid levels modulated the expression of fshr, 3β-hsd, 17β-hsd, aro, and star in the gonad of O. macrolepis (p < 0.05). Maximal relative expression values of fshr were found in fish fed 9 L and 11 L diets, whereas the rest of the dietary groups showed similar expression levels. Regarding 3β-hsd, the highest expression values were found in fish fed the highest dietary lipid levels (9 L, 11 L, and 13 L diets), whereas the lowest gene expression levels were found between fish fed 5 L and 7 L diets. The expression levels of 17β-hsd were maximal in testes of fish fed 9 L and 11 L diets, whereas the lowest ones were in testes from the 5 L and 7 L groups. In contrast, testes from animals fed the 13 L diet showed intermediate expression levels.
4. Discussion
4.1. Effect of Lipid Level on Growth of O. macrolepis Broodstock
Many previous results usually showed that higher lipid levels induced higher growth of broodstock fish, such as Snakehead murrel [14] and European sea bass [32], because dietary protein can be readily used as a source of energy [33], and the catabolism of dietary lipid over protein can help spare the use of this costly nutrient, demonstrating the protein-sparing effect of lipid. In contrast, high dietary lipid levels did not inevitably induce higher growth of fish, which had also been observed in many fish, such as large yellow croaker [34], hybrid snakehead fingerling [35], Senegalese sole juveniles [36], giant croaker [37], and hybrid yellow catfish [38], which was in agreement with the present study that high dietary lipid levels did not induce higher growth of O. macrolepis broodstock fish (Table 4).
To further explore the growth of O. macrolepis broodstock fish, two genes growth-related properties were determined. The ifg1, being a growth hormone/insulin-like growth factor (IGF), regulates growth and cellular metabolism [39] and plays essential role in the growth and development of many fish, e.g., Micropterus salmoides [39], Oncorhynchus kisutch [40], and Lateolabrax japonicus [41], and ifg1 gene expression was generally affected by nutritional status in fish [42,43]. The gldh, glutamate dehydrogenase (GDH), reversibly catalyzes glutamate deamination with the production of ammonia and plays a key role in nitrogen metabolism and the production of plasma ammonia [44,45]. In the present study, an increased trend of ifg1, gldh was observed in O. macrolepis broodstock fish fed diets with lipid levels from 5% to 13%, which was in agreement with the growth of fish. The present non-positive response of growth of O. macrolepis broodstock fish to the high dietary lipid intake is probably due to the slow growth characteristics of O. macrolepis, being a small-sized cave fish and a sub-cold-water fish [46]. In fact, the mean weight of 2–3-year-old adult female and male O. macrolepis is less than 38.79 ± 0.80 g and 17.56 ±0.33 g, respectively, in the natural environment [26], still being tiny size fish, which indicates the growth rate inevitably is relatively low. This low and slow growth feeding with high dietary lipid levels had also been reported in white seabream [47] and hybrid snakehead fish [35].
4.2. Effect of Lipid Level on Gonad Development of O. macrolepis Broodstock
Optimizing lipid in a diet during feed formulation promotes the growth of fish. A conflict between somatic and gonadal growth, especially under restricted food conditions, has often been discussed [48], while in the present study, the O. macrolepis broodstock was fed under the condition of sufficient nutrition supply, then the somatic and gonadal growth were sufficiently supported by diet nutrients.
Gonadosomatic indexes (GSI, typically expressed as 100 × gonad mass/total body mass) show the proportion of body tissue devoted to gamete production, and can be an appropriate proxy for gonadal development and gonad expenditure [49]. The mass increase in GSI is attributable to the growth of nutritive phagocytes that accumulate nutrients of protein and lipids derived from ingested food [50]. GSI, being an important indicator to evaluate fish gonadal development, provides a quantitative basis for assessing fish gonadal development potential and was generally affected by many factors, such as photothermal [51] influence, and nutrients, such as vitamin A, C, and E [3,4,5,6], protein and carbohydrate levels [7], and lipid levels [14,15,16]. In the present study, the gonad indexes of female (GSIf) in O. macrolepis broodstock increased with increasing dietary lipid levels from 5% to 11%, and they reached the highest in group 9 L–11 L, being 3.72–4%, while with further increasing of lipid to 13%, it decreased (p < 0.05; Table 5). This result was a little different from previous findings in snakehead murrel Channa striatus, where GSIf of snakehead murrel increased with dietary lipid level (100–180 g kg−1; 10–18%) [13]. This difference probably was caused by the fact that O. macrolepis is an omnivorous and phytophagous fish; the lipid level of 13% (130 g kg−1) probably is too high and excessive for its ovary growth and development; while snakehead murrel is a carnivorous fish [52], and it requires higher dietary lipid (e.g., 180 g kg−1) for gonad development.
Between male and female fish, previous studies paid less attention to gonadosomatic indexes of males (GSIm) compared to that of females [14,16], and in male fish, researchers paid more interest to sperm motility and sperm freeze–thaw fertilizing ability [53]. Different polyunsaturated fatty acids affecting sperm fertilizing ability were reported in fish. Eicosapentaenoic (EPA) and arachidonic acid (ARA) levels exerted a marked effect on fertilization in species such as rainbow trout [54,55] and European seabass [56] by affecting their sperm motility, while no effect of dietary fatty acids n-3 and n-6 polyunsaturated fatty acids on sperm freeze–thaw fertilizing ability was found by Labbe et al. [55]. Although the present result did not provide data on sperm motility or sperm fertilizing ability, the GSIm showed less correlation with dietary lipid levels (R2 = 0.1898) compared with GSIf (R2 = 0.5965), probably indicating that gonad development of male O. macrolepis broodstock is less affected by dietary lipid levels, and the morphology and membrane integrity of sperm cells were not found to be different among dietary lipid groups (Figure 1), which probably provided more evidence for these findings.
The previous results in O. macrolepis broodstock showed that the oocytes were increasingly more prominent and more mature with the increase of dietary protein levels [26]. The present research is the first to study the effect of dietary lipid levels on gonad development of O. macrolepis broodstock. The current results showed that no significant differences were noted in the histological features of ovary sectioning in O. macrolepis broodstock among five dietary groups; a similar result was found in orange mud crabs’ ovaries [57]. While in Chinese sturgeon, higher dietary lipid levels improved ovarian development, with a higher percent of mature oocytes and larger giant oocyte (diameter), especially for the 24-month feeding period [58]. The difference between the result of O. macrolepis and Chinese sturgeon probably is caused by the different fish species, feeding periods, and specific lipid levels used.
Previously the use of hormone injections for spermiation and reproduction of O. macrolepis broodstock had been reported [59], while the research about the histology of O. macrolepis broodstock testes during maturation generally is scarcely concerned about consulting the same kinds of literature, or about the effect of dietary lipid levels on the histology of O. macrolepis broodstock testis, which is firstly reported in the present study. No significant differences in the histological features of testis sectioning were observed in O. macrolepis broodstock among the five dietary groups.
Sex-steroid hormones mainly include testosterone and estradiol. Testosterone can promote spermatogenesis, promote the development and differentiation of the reproductive duct, and maintain reproductive function. Estradiol can promote the growth and maturation of follicular cells and directly affect the secretory function of the ovary. They both can stimulate gonad maturation and function, and their level can reflect the maturity degree of the gonad, being a vital index to accurately judge the reproductive state of broodfish [51]. Conversion of cholesterol to sex-steroid hormones in gonad cells is a complex progress mainly regulated by the steroid metabolic pathway [60]. In this pathway, the genes of fshr, hsd, aromatase (aro.), and steroidogenic acute regulatory protein (star) were taken as the markers for steroidogenesis in gonad development and maturation [61,62,63,64,65]. Star is a hormone-induced mitochondria-targeted protein that has been shown to initiate cholesterol transfer into mitochondria, which is considered a rate-limiting step in hormone-induced steroid formation [64].
By feeding experiment, the present investigation studied the effect of lipid levels in feed on fish steroid hormone synthesis, including the critical process of steroid hormone synthesis (i.e., response to gonadotropin (fshr), cholesterol transport (star.), and enzymatic reaction process (3β-hsd, 17β-hsd, aro.), respectively, by molecular biological methods, to reveal the relevant mechanism and process of LL regulating steroid hormone synthesis. These fundamental processes are the core processes of steroid hormone synthesis, but there are few studies on their regulation by feed nutrients [66]. The present results showed that most of these genes were highly expressed in groups 9 L and 11 L (Figure 2), indicating that proper dietary lipid level is suitable for steroidogenesis and gonad maturation for O. macrolepis broodfish.
With the deepening of study, the effect of dietary fatty acids are more substantially concentrated than lipid levels on the reproductive performance of fish [1,67,68,69,70,71], especially when one of the polyunsaturated fatty acids, arachidonic acid (ARA), is found to be the precursor of eicosanoids and has been proven to influence sex steroidogenesis [65,72]. Although Zhang et al. (2017) [66] found that ARA inhibited the estradiol and testosterone production in immature turbot Scophthalmus maximus broodstock, Norambuena et al. (2013) [73] found that ARA in feeds could increase the content of 11 keto testosterone and testosterone in the blood of fish, and the latter finding was supported by cell experiments in vitro in goldfish (Carassius auratus) [74,75,76]. Although the content of the sex hormone in the blood of O. macrolepis broodfish is unknown in the present study, the ARA content of the gonads was not significantly affected by dietary lipid levels (p > 0.05; Table 5). More proof is required to certify the relationship between the sex hormone in the blood of fish and the dietary lipid level, while the relative expression values of genes involved in sex steroid-synthesizing proteins speaks for itself.
5. Conclusions
In summary, the present data revealed that these five dietary lipid levels did not affect the growth of O. macrolepis broodstock, while the dietary lipid levels of 90–110 g kg−1 promoted higher gonad development with considerable higher gonadosomatic indexes and relative expression of steroid hormone synthesis related genes, suggesting that a proper dietary lipid level of 90–110 g Kg−1 could maintain the growth performance and gonad development of O. macrolepis broodstock. The same growth of broodstock in five lipid levels probably is related to the fact that it is a relatively small-sized and naturally slow-growing cave fish. The GIS, especially the GSIf, firstly increased with the increasing dietary lipid level and then decreased with the further increasing dietary lipid level, probably indicating that dietary lipid was mainly used in the growth of the gonad. This is an interesting finding, and further research is required to explore this phenomenon.
Author Contributions
Conceptualization, H.J.; methodology, Y.L.; validation, P.F.; formal analysis, J.Z.; writing—original draft preparation, J.Z.; writing—review and editing, all authors; visualization, E.G.; supervision, J.Z.; project administration, J.Z.; funding acquisition, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key R & D Program of China (2019YFD0900200), Special R & D Program Project of Chinese Academy of Se-enriched Industry (2019ZKG-1), Key R & D plan of Shaanxi Province (2018ZDXM-NY-045) and Shaanxi Special plan project of technological innovation guidance (2022QFY12-03).
Institutional Review Board Statement
In This study was conducted following the Guiding Principles for Biomedical Research Involving Animals (EU2010/63), the guidelines of the Spanish laws (law 32/2007 and RD 53/2013) and authorized by the Ethical Committee of IRTA (Spain) and by the Ethical committee of NWAFU (China) for the use of laboratory animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings are available upon direct request to the corresponding author.
Acknowledgments
The authors would like to acknowledge Wang Gang for his kind donation of fish used in this study and to Liu Hai Xia for the provision of her result of transcriptome in O. macrolepis being helpful to design the primers of these genes tested in the present paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Izquierdo, M.S.; Fernandez-Palacios, H.; Tacon, A.G.J. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Jobling, M. Fish nutrition research: Past, present and future. Aquacult. Int. 2016, 24, 767–786. [Google Scholar] [CrossRef]
- Xiao, D.Y.; Liang, M.Q.; Wang, X.X.; Zheng, K.K.; Zhao, M.; Li, Q.H. Effects of dietary vitamin A supplementation on the reproduction and offspring quality of tongue sole Cynoglossus semilaevis. Prog. Fish. Sci. 2014, 35, 50–59. (In Chinese) [Google Scholar]
- Xiao, D.Y.; Liang, M.Q.; Zheng, K.K.; Zhao, M. Effects of vitamin C on reproductive performance and offspring quality of tongue sole (Cynoglossus semilaevis) Broodstocks. Chin. J. Anim. Nutr. 2014, 26, 2664–2674. (In Chinese) [Google Scholar]
- Xiao, D.Y.; Liang, M.Q.; Wang, X.X.; Zheng, K.K.; Zhao, M. Effects of different levels of dietary vitamin E on the reproductive performance and offspring quality of Tongue sole Cynoglossus semilaevis. Prog. Fish. Sci. 2015, 36, 125–132. (In Chinese) [Google Scholar]
- Zhang, H.T.; Liang, M.Q.; Zheng, K.K.; Wang, X.X. Impact of dietary vitamin C on the reproductive performance of turbot Scophthalmus maximus. Prog. Fish. Sci. 2013, 32, 73–81. (In Chinese) [Google Scholar]
- Cuesta-Gomez, D.M.; Sánchez-Saavedra, M.D.P. Effects of protein and carbohydrate levels on survival, consumption and gonad index in adult sea urchin Strongylocentrotus purpuratus (Stimpson 1857) from Baja California, Mexico. Aquac. Res. 2017, 48, 1596–1607. [Google Scholar] [CrossRef]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and beta-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.R.; Henderson, R.J.; Tocher, D.R. The lipids. In Fish Nutrition; Halver, J.E., Ed.; Academic Press: New York, NY, USA, 1989; pp. 154–158. [Google Scholar]
- Parpoura, A.C.R.; Alexis, M.N. Effects of different of dietary oils in sea bass (Dicentrarchuslabrax) nutrition. Aquacult. Int. 2001, 9, 463–476. [Google Scholar] [CrossRef]
- Carboni, S.; Hughes, A.D.; Atack, T.; Tocher, D.R.; Migaud, H. Influence of broodstock diet on somatic growth, fecundity, gonad carotenoids and larval survival of sea urchin. Aquac. Res. 2015, 46, 969–976. [Google Scholar] [CrossRef]
- Duary, M.; Kohno, H.; Pascual, F. The effect of lipid enriched broodstock diet on spawning, egg and larval quality of hatchery-bred rabbitfish (Siganus guttatus). Philipp. Sci. 1994, 31, 42–57. [Google Scholar]
- Ghaedi, A.; Kabir, M.A.; Hashim, R. Effect of lipid levels on the reproductive performance of Snakehead murrel, Channastriatus. Aquac. Res. 2016, 47, 983–991. [Google Scholar] [CrossRef]
- Yildiz, M.; Ofori-Mensah, S.; Arslan, M.; Ekici, A.; Yamaner, G.; Baltacı, M.A.; Korkmaz, F. Effects of different dietary oils on egg quality and reproductive performance in rainbow trout Oncorhynchus mykiss. Anim. Reprod. Sci. 2020, 221, 106545. [Google Scholar] [CrossRef] [PubMed]
- Nzohabonayo, E.; Kassam, D.; Kangómbe, J. Effect of lipid levels on reproductive performance of Oreochromis karongae. Aquac. Res. 2017, 48, 1998–2003. [Google Scholar] [CrossRef]
- Du, H.; Yao, J.P.; Zhou, H.; Leng, X.Q.; Wu, J.P.; He, S.; Luo, J.; Liang, X.F.; Wei, Q.W.; Tan, Q.S. Optimal dietary lipid level promoted ovary development of Chinese sturgeon (Acipensersinensis) broodstocks. Aquaculture 2018, 495, 288–294. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef]
- Yin, B.Y.; Dai, Y.G. A review of research progress in fish Onychostoma. Fish. Sci. 2014, 33, 63–68. (In Chinese) [Google Scholar]
- Yue, P.Q.; Shan, X.H.; Lin, R.D.; Chu, X.L. Fauna Sinica: Osteichthyes, Cypriniformes (III); Science Press: Beijing, China, 2000; pp. 168–174. (In Chinese) [Google Scholar]
- Chen, S.W.; Chen, Y.Y.; Qu, G.S. Analysis and evaluation of nutritional composition of Onychostoma macrolepis in Qinling-Bashan Mountain area. Biot. Resour. 2019, 41, 112–118. [Google Scholar]
- IUCN. IUCN Red List of Threatened Species, Version 2010.4. 2010. Available online: https://www.iucnredlist.org/ (accessed on 1 February 2019).
- Chen, C. The ecological habits and development, and utilization of Onychostomamacrolepis. Shandong Fish. 2007, 24, 39. (In Chinese) [Google Scholar]
- Zhao, Y.H.; Gozlan, R.E.; Zhang, C.G. Out of sight out of mind: Current knowledge of Chinese cave fishes. J. Fish Biol. 2011, 79, 1545–1562. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, J.P.; Feng, D.P.; Yang, J.; Yuan, Z.J.; He, Z.G.; Zou, S.X. Reproductive biology of Varicorhinusmacrolepis in Shennongjia. Fish. Sci. 2015, 34, 523–526. (In Chinese) [Google Scholar]
- Song, H.; Wang, S.Y.; Peng, K.M.; Yin, X.H. Histological studies on gonadial differentiation and development of Varicorhinusmacrolepis. J. Fish. Sci. China 2006, 13, 723–729. (In Chinese) [Google Scholar]
- Liu, Y.; Zhou, J.S.; Ji, H.; Yu, H.B.; Dong, W.Z.; Wang, T.; Liu, H.X. Effects of dietary protein levels on growth, body composition and gonad of Varicorhinus macrolepis broodstock. Siliao Gongye 2016, 37, 19–26. (In Chinese) [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; AOAC: Arlington, VA, USA, 2004; Volume 2. [Google Scholar]
- Folch, M.; Lees, G.H.; Stanley, S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Lie, Ø.; Lambertsen, G. Fatty acid composition of glycerophospholipids in seven tissues of cod (Gadusmorhua), determined by combined high-performance liquid chromatography and gas chromatography. J. Chromatogr. A 1991, 565, 119–129. [Google Scholar] [CrossRef]
- Humason, G.L. Animal Tissu Techniques; Freeman Company: New York, NY, USA, 1961; pp. 25–55. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lanari, D.; Poli, B.M.; Ballestrazzi, R.; Lupi, P.; Dágaro, E.; Mecatti, M. The effects of dietary fat and NFE levels on growing European sea bass (Dicentrarchuslabrax L.). Growth rate, body and fillet composition, carcass traits and nutrient retention efficiency. Aquaculture 1999, 179, 351–364. [Google Scholar] [CrossRef]
- Kleiber, M. The Fire of Life; Robert, E., Ed.; Kleiber Publishing Co.: New York, NY, USA, 1975; pp. 236–252. [Google Scholar]
- Yan, J.; Liao, K.; Wang, T.; Mai, K.; Xu, W.; Ai, Q. Dietary lipid levels influence lipid deposition in the liver of large yellow croaker (Larimichthyscrocea) by regulating lipoprotein receptors, fatty acid uptake and triacylglycerol synthesis and catabolism at the transcriptional level. PLoS ONE 2015, 10, e0129937. [Google Scholar]
- Zhang, Y.F.; Sun, Z.Z.; Wang, A.L.; Ye, C.X.; Zhu, X. Effects of dietary protein and lipid levels on growth, body and plasma biochemical composition and selective gene expression in liver of hybrid snakehead (Channamaculata ♀ × Channaargus ♂) fingerlings. Aquaculture 2017, 468, 1–9. [Google Scholar] [CrossRef]
- Bonacic, K.; Estevez, A.; Bellot, O.; Conde-Sieira, M.; Gisbert, E.; Morais, S. Dietary fatty acid metabolism is affected more by lipid level than source in senegalese sole juveniles: Interactions for optimal dietaryformulation. Lipids 2016, 51, 105–122. [Google Scholar] [CrossRef]
- Han, T.; Li, X.Y.; Wang, J.T.; Hu, S.X.; Jiang, Y.D.; Zhong, X.D. Effect of dietary lipid level on growth, feed utilization and body composition of juvenile giant croaker Nibea japonica. Aquaculture 2014, 434, 145–150. [Google Scholar] [CrossRef]
- Fei, S.Z.; Xia, Y.; Chen, Z.; Liu, C.; Liu, H.K.; Han, D.; Jin, J.Y.; Yang, Y.X.; Zhu, X.M.; Xie, S.Q. A high-fat diet alters lipid accumulation and oxidative stress and reduces the disease resistance of overwintering hybrid yellow catfish (Pelteobagrus fulvidraco♀×P. vachelli♂). Aquacul. Rep. 2022, 23, 101043. [Google Scholar] [CrossRef]
- Li, X.H.; Bai, J.J.; Ye, X.; Hu, Y.C.; Li, S.J.; Yu, L.Y. Polymorphisms in the 5′ flanking region of the insulin-like growth factor I gene are associated with growth traits in largemouth bass Micropterus salmoides. Fish. Sci. 2009, 75, 351–358. [Google Scholar] [CrossRef]
- Duan, C.; Plisetskaya, E.M.; Dickhoff, W.W. Expression of insulin-like growth factor I in normally and abnormally developing coho salmon (Oncorhynchus kisutch). Endocrinology 1995, 136, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Men, K.; Ai, Q.; Mai, K.; Xu, W.; Zhang, Y.; Zhou, H. Effects of dietary corn gluten meal on growth, digestion and protein metabolism in relation to IGF-I gene expression of Japanese seabass, Lateolabrax japonicus. Aquaculture 2014, 428–429, 303–309. [Google Scholar] [CrossRef]
- Duan, C.; Ren, H.; Shan, G. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: Roles in skeletal muscle growth and differentiation. Gen. Comp. Endocr. 2010, 167, 344–351. [Google Scholar] [CrossRef]
- Moriyama, S.; Ayson, F.G.; Kawauchi, H. Growth regulation by insulin-like growth factor-I in fish. Biosci. Biotechnol. Biochem. 2000, 64, 1553–1562. [Google Scholar] [CrossRef]
- Melo, J.F.B.; Lundstedt, L.M.; Metón, I.; Baanante, I.V.; Moraes, G. Effects of dietary levels of protein on nitrogenous metabolism of Rhamdia queen (Teleostei: Pimelodidae). Comp. Biochem. Phys. 2006, 145A, 181–187. [Google Scholar] [CrossRef]
- Metón, I.; Mediavilla, D.; Caseras, A.; Cantó, E.; Fernández, F.; Baanate, I.V. Effect of diet composition and ratio size on key enzymes activity of glycolysis–gluconeogenesis, the pentose phosphate pathway and amino acid metabolism in liver of gilthead sea bream (Sparus aurata). Br. J. Nutr. 1999, 82, 223–232. [Google Scholar] [CrossRef]
- Chui, C. The ecological habits and development of Onychostomamacrolepis. Shandong Fish. 2007, 24, 39. (In Chinese) [Google Scholar]
- Ozorio, R.O.; Valente, L.M.; Pousao-Ferreira, P.; Oliva-Teles, A. Growth performance and body composition of white seabream (Diplodus sargus) juveniles fed diets with different protein and lipid levels. Aquac. Res. 2006, 37, 255–263. [Google Scholar] [CrossRef]
- Scheibling, R.E.; Hatcher, B.G. Strongylocentrotus droebachiensis. In Sea Urchins: Biology and Ecology; Lawrence, J.M., Ed.; Academic Press: London, UK, 2013; pp. 381–412. [Google Scholar]
- Parker, G.A.; Ramm, S.A.; Lehtonen, J.; Henshaw, J.M. The evolution of gonad expenditure and gonadosomatic index (GSI) in male and female broadcast-spawning invertebrates. Biol. Rev. 2018, 93, 693–753. [Google Scholar] [CrossRef] [PubMed]
- Unuma, T.; Walker, C.W. Relationship between gametogenesis and food quality in sea urchin gonads. In Aquaculture Technologies for Invertebrates, Proceedings of the 36th U.S.-Japan Aquaculture Panel Symposium, Durham, New Hampshire, 29–30 October 2009; Stickney, R., Iwamoto, R., Rust, M., Eds.; The NOAA Technical Memorandum NMFS-F/SPO-99; U.S. Department Commerce: Milfford, CT, USA, 2007; pp. 45–53. [Google Scholar]
- Xu, Y.J.; Liu, X.Z.; Wang, Q.Y.; Ma, A.J.; Zhao, M.; Ni, N.; Qu, J.Z. Relationships between serum sex steriods levels and gonadal development and photothermal regulation during the annual maturation of captive Cynoglossus semillaevis günther. Oceanol. Et Limnol. Sin. 2011, 42, 64–74. (In Chinese) [Google Scholar]
- Chen, L.C. (Ed.) Aquaculture in Taiwan; Blackwell Scientific Publication: London, UK, 1990; pp. 57–89. [Google Scholar]
- Labbe, C.; Maisse, G. Influence of rainbow trout thermal acclimation on sperm cryopreservation: Relation to change in the lipid composition of the plasma membrane. Aquaculture 1996, 145, 281–294. [Google Scholar] [CrossRef]
- Watanabe, T.; Takeuchi, T.; Saito, M.; Nishimura, K. Effect of low protein-high calorie or essential fatty acid deficiency diet on reproduction of rainbow trout. Nippon Suisan Gakk. 1984, 50, 1207–1215. [Google Scholar] [CrossRef]
- Labbe, C.; Loir, M.; Kaushik, S.; Maisse, G. The Influence of Both Rearing and Dietary Lipid Origin on Fatty Acid Composition of Spermatozoan Polar Lipids in Rainbow (Oncorrhynchus mykiss). Effect on Sperm Cryopreservation Tolerance. Fish Nutrition in Practice, Biarritz, France, 1993, 24–27 June 1991; INRA: Paris, France, 1993; Les Colloques; pp. 49–59. [Google Scholar]
- Asturiano, J.F. El Proceso Reproductivo de la Lubina Europea (Dicentrarchus labrax L.). Efectos Delos Ácidos Grasos dela Dieta: Estudios In Vivo e In Vitro. Ph.D. Thesis, Valencia University, Valencia, Spain, 1999; p. 251. [Google Scholar]
- Aaqillah-Amr, M.A.; Hidir, A.; Waiho, K.; Fazhan, H.; Ahmad-Ideris, A.R.; Muhammad-Zulhilmi, R.; Peng, T.H.; Abualreesh, M.H.; Noordiyana, M.N.; Ma, H.; et al. Morphological, histological, and physiological responses of female orange mud crab, scylla olivacea (Herbst, 1796), fed with different dietary lipid levels. Aquacult. Nutr. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Leng, X.Q.; Zhou, H.; Tan, Q.S.; Du, H.; Wu, J.P.; Liang, X.F.; He, S.; Wei, Q.W. Integrated metabolomic and transcriptomic analyses suggest that high dietary lipid levels facilitate ovary development through the enhanced arachidonic acid metabolism, cholesterol biosynthesis and steroid hormone synthesis in Chinese sturgeon (Acipenser sinensis). Br. J. Nutr. 2019, 122, 1230–1241. [Google Scholar] [PubMed]
- Dong, W.Z.; Wang, T.; Ma, L.; Liu, Y.; Wang, L.Q.; Jiang, R.M.; Ji, H. Artificial reproduction of Varicorhinus macrolepis in Qin-Ba mountains. J. Anim. Sci. Vet. Med. 2016, 35, 27–35. (In Chinese) [Google Scholar]
- Flück, C.E.; Pandey, A.V. Impact on CYP19A1 activity by mutations in NADPH cytochrome P450 oxidoreductase. J. Steroid Biochem. 2017, 165, 64–70. [Google Scholar] [CrossRef]
- Stocco, D.M. StAR protein and the regulation of steroid hormone biosynthesis. Annu. Rev. Physiol. 2001, 63, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Wang, X.J.; Jo, Y.; Manna, P.R. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: More complicated than we thought. Mol. Endocrinol. 2005, 19, 2647–2659. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Walsh, L.P.; Reinhart, A.J.; Stocco, D.M. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J. Biol. Chem. 2000, 275, 20204–20209. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Z.H.; Shen, W.J.; Azhar, S. Review Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef]
- Norberg, B.; Kleppe, L.; Andersson, E.; Thorsen, A.; Rosenlund, G.; Hamre, K. Effects of dietary arachidonic acid on the reproductive physiology of female Atlantic cod (Gadusmorhua L.). Gen. Comp. Endocr. 2017, 250, 21–35. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Xu, H.G.; Cao, L.; Wei, Y.L.; Zheng, K.K.; Liang, M.Q. Effects of dietary arachidonic acid on the sex steroid hormone synthesis in turbot broodstock before maturation. J. Fish. China 2017, 41, 588–601. (In Chinese) [Google Scholar]
- Baeza, R.; Peñaranda, D.S.; Vílchez, M.C.; Tveiten, H.; Pérez, L.; Asturiano, J.F. Exploring correlations between sex steroids and fatty acids and their potential roles in the induced maturation of the male European eel. Aquaculture 2015, 435, 328–335. [Google Scholar] [CrossRef]
- Chang, G.; Wu, X.; Cheng, Y.; Zeng, C.; Yu, Z. Reproductive performance, offspring quality, proximate and fatty acid composition of normal and precocious Chinese mitten crab Eriocheir sinensis. Aquaculture 2017, 469, 137–143. [Google Scholar] [CrossRef]
- Luo, L.; Ai, L.; Liang, X.; Hu, H.; Xue, M.; Wu, X. N-3 Long-chain polyunsaturated fatty acids improve the sperm, egg, and offspring quality of Siberian sturgeon (Acipenser baerii). Aquaculture 2017, 473, 266–271. [Google Scholar] [CrossRef]
- Peng, S.; Gao, Q.; Shi, Z.; Zhang, C.; Wang, J.; Yin, F.; Zhang, Y. Effect of dietary n-3 LC-PUFAs on plasma vitellogenin, sex steroids, and ovarian steroidogenesis during vitellogenesis in female silver pomfret (Pampus argenteus) broodstock. Aquaculture 2015, 444, 93–98. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.M.; Xie, D.Z.; Tian, X.; Zhang, Y.R.; Wu, L.M.; Liu, H.F.; Wang, L.; Dong, C.J.; Li, X.J.; et al. Effect of dietary linolenic/linoleic acid ratios on growth performance, ovarian steroidogenesis, plasma sex steroid hormone, and tissue fatty acid accumulation in juvenile common carp, Cyprinus carpio. Aquacult. Rep. 2020, 18, 100452. [Google Scholar] [CrossRef]
- Xu, H.; Cao, L.; Zhang, Y.; Johnson, R.B.; Wei, Y.; Zheng, K.; Liang, M. Dietary arachidonic acid differentially regulates the gonadal steroidogenesis in the marine teleost, tongue sole (Cynoglossus semilaevis), depending on fish gender and maturation stage. Aquaculture 2017, 468, 378–385. [Google Scholar] [CrossRef]
- Norambuena, F.; Estévez, A.; Mañanós, E. Effects of graded levels of arachidonic acid on the reproductive physiology of Senegalese sole (Solea senegalensis): Fatty acid composition, prostaglandins and steroid levels in the blood of broodstock bred in captivity. Gen. Comp. Endocr. 2013, 191, 92–101. [Google Scholar] [CrossRef]
- Wade, M.G.; Van der Kraak, G.; Gerrits, M.F. Releaseand steroidogenic actions of polyunsaturated fatty acidsin the goldfish testis. Biol. Reprod. 1994, 51, 131–139. [Google Scholar] [CrossRef]
- Mercure, F.; Van Der Kraak, G. Inhibition of gonadotropin-stimulated ovarian steroid production by polyunsaturated fatty acids in teleost fish. Lipids 1995, 30, 547–554. [Google Scholar] [CrossRef]
- Mercure, F.; Van Der Kraak, G. Mechanisms of action of free arachidonic acid on ovarian steroid production in the goldfish. Gen. Comp. Endocr. 1996, 102, 130–140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).