Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study
Abstract
1. Introduction
2. Materials and Methods
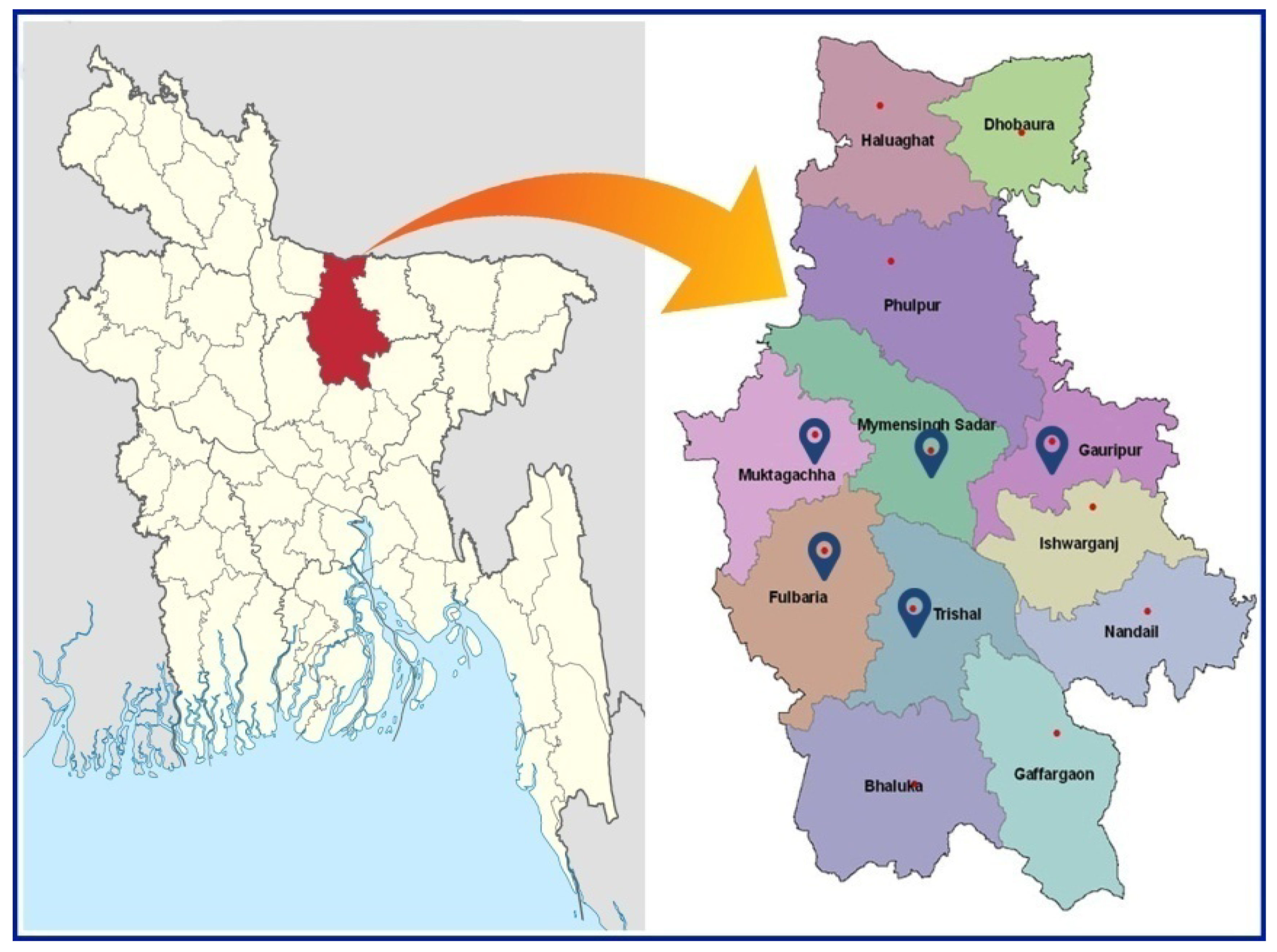
2.1. Site and Hatchery Selection
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. General Characteristics of Hatchery Owners
3.2. Hatchery Owners’ Perceptions of CC and Its Effects
3.3. Relationship between Hatchery Owners’ Perceptions and Key Weather Parameters
3.4. Characteristics of CC Impacts on Fish Hatcheries
3.4.1. Component 1: Poor Reproductive Performance of Fish
3.4.2. Component 2: Poor Survival in Relation to Disease and Poor Water Quality
3.4.3. Component 3: Poor Growth of Broodstock
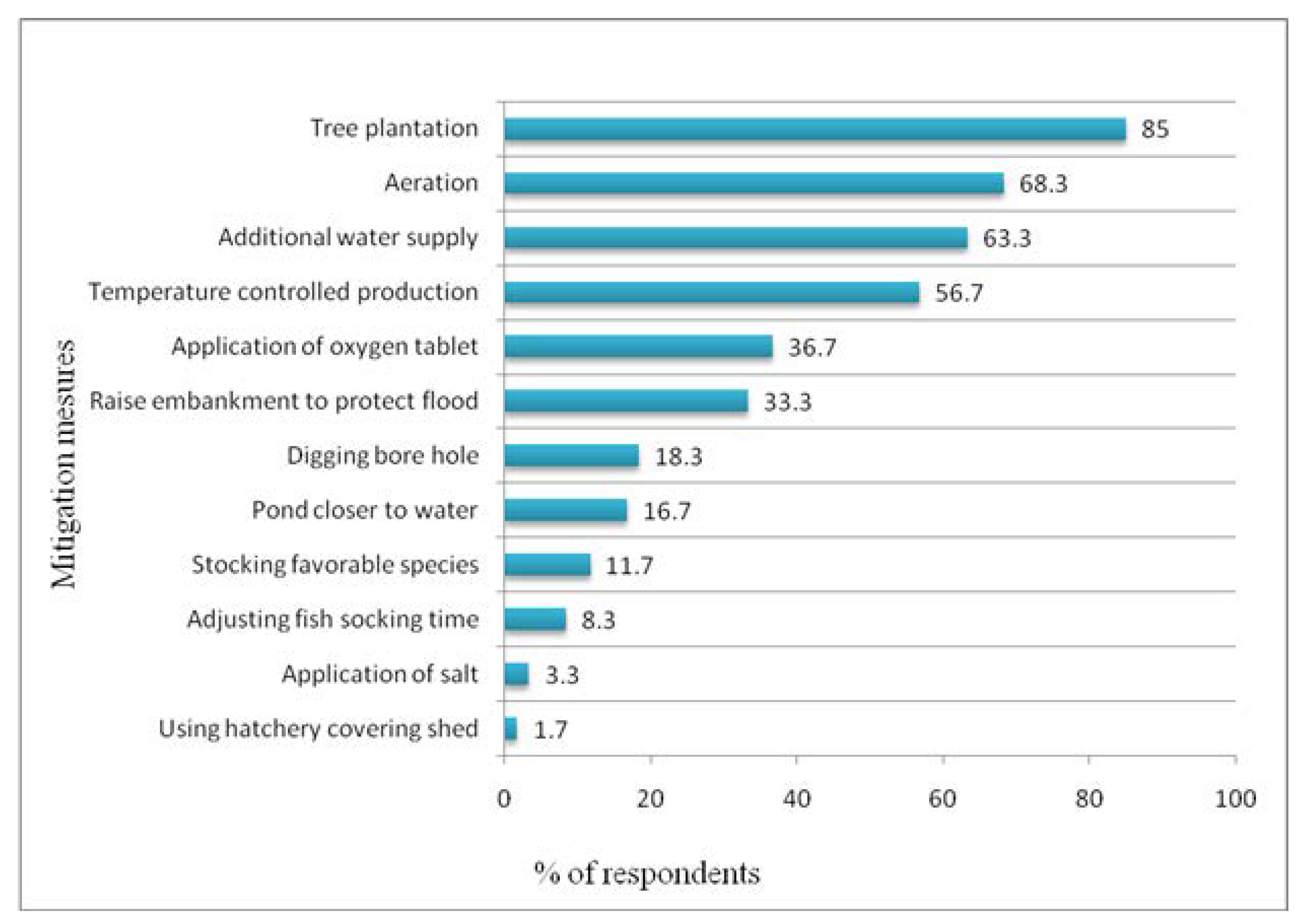
3.5. Mitigation Measures as Perceived by Hatchery Owners
4. Discussion
4.1. General Characteristics of Hatchery Owners
4.2. Hatchery Owners’ Perceptions on CC and Its Effects
4.3. Relationship between Hatchery Owners’ Perceptions and Key Weather Parameters
4.4. Characteristics of CC Impacts on Fish Hatcheries
4.4.1. Poor Reproductive Performance of Fish
4.4.2. Poor Survival in Relation to Disease and Poor Water Quality
4.4.3. Poor Growth of Broodstock
4.5. Mitigation Measures as Perceived by the Hatchery Owners
5. Conclusions
6. New Insights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henriksson, P.J.G.; Zhang, W.; Nahid, S.A.A.; Newton, R.; Phan, L.T.; Dao, H.M.; Zhang, Z.; Jaithiang, J.; Andong, R.; Chaimanuskul, K.; et al. Final LCA Case Study Report—Results of LCA Studies of Asian Aquaculture Systems for Tilapia, Catfish, Shrimp, and Freshwater Prawn; Leiden University: Leiden, The Netherlands, 2014; Volume D 3.5, 165p. [Google Scholar]
- DoF. National Fish Week 2022 Compendium; Department of Fisheries, Ministry of Fisheries and Livestock: Dhaka, Bangladesh, 2022; 160p. (In Bengali)
- Alam, M.M.; Haque, M.M.; Aziz, S.B.; Mondol, M.M.R. Development of pangasius–carp polyculture in Bangladesh: Understanding farm characteristics by, and association between, socio-economic and biological variables. Aquaculture 2019, 505, 431–440. [Google Scholar] [CrossRef]
- DoF. Yearbook of Fisheries Statistics 2019–2020; Fisheries Resources Survey System (FRSS), Department of Fisheries, Ministry of Fisheries and Livestock: Dhaka, Bangladesh, 2020; Volume 37, 141p.
- Dutta, J.; Sen, T.; Mitra, A.; Zaman, S.; Mitra, A. Brief commentary on the impact of global climate change on fisheries and aquaculture with special reference to India. Bangladesh J. Zool. 2020, 48, 457–463. [Google Scholar] [CrossRef]
- WorldFish Centre. Global Vulnerability of Fisheries Systems to Climate Change; ISSUES, Brief/1701; WorldFish Centre: Penang, Malaysia, 2007. [Google Scholar]
- Haque, M.M.; Alam, M.R.; Alam, M.M.; Basak, B.; Sumi, K.R.; Belton, B.; Murshed-E-Jahan, K. Integrated floating cage aquageoponics system (IFCAS): An innovation in fish and vegetable production for shaded ponds in Bangladesh. Aquac. Rep. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- FAO. Climate Change Implications for Fisheries and Aquaculture. Summary of the findings of the IPCC Fifth Assessment Report. In FAO Fisheries and Aquaculture Circular: Vol. FIAP/C1122; Food and Agricultural Organization: Rome, Italy, 2016; 65p. [Google Scholar]
- Parvez, M.S.; Rahman, M.A.; Hasan, M.J.; Rasel, M.S.E.; Shaikh, M.M.; Molla, M.H.R.; Chowdhury, S.H.; Billah, M.M. Role of Hatchery on Fish Seed Production in Patuakhali District of Bangladesh: An Overview. Int. J. Chem. Environ. Biol. Sci. 2018, 6, 1–7. [Google Scholar]
- Hemal, S.; Uddin, M.S.; Uddin, M.S.; Majumdar, B.C.; Rasul, M.G.; Alam, M.T. Present status and problems of fish seed marketing in Sylhet district, Bangladesh. Res. Agric. Livest. Fish. 2017, 4, 45–54. [Google Scholar] [CrossRef]
- Debnath, S.; Hossen, S.; Sharker, M.R.; Ghosh, A.; Ferdous, A.; Zannat, L.K.; Ali, M.M. Fish Seed Producing Hatcheries in Southern Bangladesh: An Overview. Middle-East J. Sci. Res. 2020, 28, 199–206. [Google Scholar]
- Haque, M.M. Fisheries and Aquaculture in Seasonal Bangladesh: Implications of Climate Change. In Impacts of Climate Change on Livelihoods, Agriculture and Aquaculture & Fisheries Sector of Bangladesh; Wahab, M.A., Salam, M.A., Eds.; Department of Aquaculture, Bangladesh Agricultural University: Mymensingh, Bangladesh, 2009; pp. 47–53. [Google Scholar]
- Kausar, R.; Salim, M. Effect of Water Temperature on the Growth Performance and Feed Conversion Ratio of Labeo rohita. Pak. Vet. J. 2006, 26, 105–108. [Google Scholar]
- Khater, E.S.G.; Ali, S.A.; Mohamed, W.E. Effect of Water Temperature on Masculinization and Growth of Nile Tilapia Fish. J. Aquac. Res. Dev. 2017, 8, 8–12. [Google Scholar] [CrossRef]
- Faruk, M.A.R.; Mausumi, M.I.; Anka, I.Z.; Hasan, M.M. Effects of temperature on the egg production and growth of monosex Nile Tilapia Oreochromis niloticus fry. Bangladesh Res. Publ. J. 2012, 7, 367–377. [Google Scholar]
- Usman, I.; Auta, J.; Abdullahi, S.A. Effect of monthly variation in water temperature on artificial breeding of common carp (Cyprinus carpio L.) in Zaria, Nigeria. Int. J. Fish. Aquat. Stud. 2015, 3, 353–356. [Google Scholar]
- Remen, M.; Nederlof, M.A.J.; Folkedal, O.; Thorsheim, G.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J.; Oppedal, F.; Olsen, R.E. Effect of temperature on the metabolism, behaviour and oxygen requirements of Sparus aurata. Aquac. Environ. Interact. 2015, 7, 115–123. [Google Scholar] [CrossRef]
- O’Gorman, E.J.; Ólafsson, Ó.P.; Demars, B.O.L.; Friberg, N.; Guðbergsson, G.; Hannesdóttir, E.R.; Jackson, M.C.; Johansson, L.S.; McLaughlin, Ó.B.; Ólafsson, J.S.; et al. Temperature effects on fish production across a natural thermal gradient. Glob. Chang. Biol. 2016, 22, 3206–3220. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.K.; De, M.; Mazlan, A.G.; Zaidi, C.C.; Rahim, S.M.; Simon, K.D. Impact of global climate change on fish growth, digestion and physiological status: Developing a hypothesis for cause and effect relationships. J. Water Clim. Chang. 2015, 6, 200–226. [Google Scholar] [CrossRef]
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Munganga, B.P.; Phiri, K.J.; Nsekanabo, J.D.M. Climate Change Effects on Aquaculture Production: Sustainability Implications, Mitigation, and Adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Adhikari, S.; Chaudhury, A.K.; Gangadhar, B.; Ramesh, R.; Mandal, R.N.; Sarosh, I.; Saha, G.S.; De, H.K.; Sivaraman, I.; Mahapatra, A.S.; et al. Adaptation and Mitigation Strategies of Climate Change Impact in Freshwater Aquaculture in some states of India. J. FisheriesSciences.com 2018, 12, 16–21. [Google Scholar]
- Hossan, M.S.; Ulka, S.B.; Motin, M.A.; Tarafder, M.A.K.; Sukhan, Z.P.; Rashid, H. Egg and fry production performance of female tilapia related to fluctuating temperature and size variation. In Proceedings of the 4th the International Conference on Environmental Aspects of Bangladesh, Fukuoka, Japan, 24–25 August 2013; pp. 105–108. [Google Scholar]
- Okunsebor, S.; Ofojekwu, P.; Kakwi, D.; Audu, B. Effect of Temperature on Fertilization, Hatching and Survival Rates of Heterobranchus bidorsalis Eggs and Hatchlings. Br. J. Appl. Sci. Technol. 2015, 7, 372–376. [Google Scholar] [CrossRef]
- Valeta, J.S.; Likongwe, J.S.; Kassam, D.; Maluwa, A.O. Temperature-dependent egg development rates, hatchability and fry survival rate of Lake Malawi Tilapia (Chambo), Oreochromis karongae (Pisces: Cichlidae). Int. J. Fish. Aquac. 2013, 5, 55–59. [Google Scholar]
- Hossain, M.I.; Khatun, M.; Kamal, B.M.M.; Habib, K.A.; Tumpa, A.S.; Subba, B.R.; Hossain, M.Y. Effects of Seasonal variation on Growth Performance of Mirror carp (Cyprinus carpio ver. specularis) in Earthen Nursery Ponds. Our Nat. 2015, 12, 8–18. [Google Scholar]
- Politis, S.N.; Mazurais, D.; Servili, A.; Zambonino-Infante, J.L.; Miest, J.J.; Sørensen, S.R.; Tomkiewicz, J.; Butts, I.A.E. Temperature effects on gene expression and morphological development of European eel, Anguilla anguilla larvae. PLoS ONE 2017, 12, e0182726. [Google Scholar] [CrossRef]
- Yoo, G.Y.; Lee, J.Y. The effect of feeding frequency, water temperature, and stocking density on the growth of river puffer Takifugu obscurus reared in a zero-exchange water system. Fish. Aquat. Sci. 2016, 19, 23. [Google Scholar] [CrossRef]
- Parvin, G.A.; Fujita, K.; Matsuyama, A.; Shaw, R.; Sakamoto, M. Climate Change, Flood, Food Security and Human Health: Cross-Cutting Issues in Bangladesh. In Food Security and Risk Reduction in Bangladesh; Habiba, U., Hassan, A.W.R., Abedin, M.A., Shaw, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 187–212. [Google Scholar]
- Chowdhury, M.T.H.; Sukhan, Z.P.; Hannan, M.A. Climate change and its impact on fisheries resource in Bangladesh. In Proceedings of the International Conference on Environmental Aspects of Bangladesh (ICEAB10), Fukuoka, Japan, 4 September 2010; pp. 95–98. [Google Scholar]
- Mallick, A.; Panigrahi, A.K. Effect of temperature variation on disease proliferation of common fishes in perspective of climate change. Int. J. Exp. Res. Rev. 2018, 16, 40–49. [Google Scholar] [CrossRef]
- Islam, M.A.; Islam, M.S.; Wahab, M.A. Impacts of climate change on shrimp farming in the South-West coastal region of Bangladesh. Res. Agric. Livest. Fish. 2016, 3, 227–239. [Google Scholar] [CrossRef]
- Alam, S.M.A.; Sarkar, M.S.I.; Miah, M.M.A.; Rashid, H. Management strategies for Nile Tilapia (Oreochromis niloticus) hatchery in the face of climate change induced rising temperature. Aquac. Stud. 2021, 21, 55–62. [Google Scholar] [CrossRef]
- Hasan, N.A.; Heal, R.D.; Bashar, A.; Bablee, L.A.; Haque, M.M. Impacts of COVID-19 on the finfish aquaculture industry of Bangladesh: A case study. Mar. Policy 2021, 130, 104577. [Google Scholar] [CrossRef]
- Ahmed, N.; Toufique, K. Greening the blue revolution of small-scale freshwater aquaculture in Mymensingh, Bangladesh. Aquac. Res. 2015, 46, 2305–2322. [Google Scholar] [CrossRef]
- Alam, M.M.; Haque, M.M. Presence of antibacterial substances, nitrofuran metabolites and other chemicals in farmed pangasius and tilapia in Bangladesh: Probabilistic health risk assessment. Toxicol. Rep. 2021, 8, 248–257. [Google Scholar] [CrossRef]
- Malinowski, R.M. Factor Analysis in Chemistry, 2nd ed.; Wiley: New York, NY, USA, 1991; 368p. [Google Scholar]
- Statheropoulos, M.; Vassiliadis, N.; Pappa, A. Principal component and canonical correlation analysis for examining air pollution and meteorological data. Atmos. Environ. 1998, 32, 1087–1095. [Google Scholar] [CrossRef]
- Mabe, F.N.; Donkoh, S.A.; Al-Hassan, S. Technology adoption typology and rice yield differentials in Ghana: Principal component analysis approach. Afr. J. Sci. Technol. Innov. Dev. 2019, 11, 555–567. [Google Scholar] [CrossRef]
- Pravakar, P.; Sarker, B.S.; Rahman, M.; Hossain, M.B. Present Status of Fish Farming and Livelihood of Fish Farmers in Shahrasti Upazila of Chandpur District, Bangladesh. Am.-Eurasian J. Agric. Environ. Sci. 2013, 13, 391–397. [Google Scholar]
- Khatun, S.; Adhikary, R.K.; Rahman, M.; Nurul, M.; Sikder, A.; Hossain, M.B. Socioeconomic Status of Pond Fish Farmers of Charbata, Noakhali, Bangladesh. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 2–12. [Google Scholar]
- Mithun, M.; Kowsari, M.; Sheheli, S. Socioeconomic characteristics and constraints of participatory pond fish farmers in Mymensingh district, Bangladesh. Int. J. Agric. Res. Innov. Technol. 2021, 10, 170–176. [Google Scholar] [CrossRef]
- Uddin, M.N.; Kabir, K.H.; Roy, D.; Hasan, M.T.; Sarker, M.A.; Dunn, E.S. Understanding the constraints and its related factors in tilapia (Oreochromis sp.) fish culture at farm level: A case from Bangladesh. Aquaculture 2021, 530, 735927. [Google Scholar] [CrossRef]
- Ali, H.; Azad, M.A.K.; Anisuzzaman, M.; Chowdhury, M.M.R.; Hoque, M.; Shariful, M.I. Livelihood status of the fish farmers in some selected areas of Tarakanda upazila of Mymensingh district. J. Agrofor. Environ. 2010, 3, 85–89. [Google Scholar]
- BBS. Statistical Yearbook of Bangladesh Bureau of Statistics; Statistical Division, Ministry of Planning, Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2002; 660p.
- Ali, M.; Hossain, M.D.; Hasan, A.N.G.M.; Bashar, M.A. Assessment of the livelihood status of the fish farmers in some selected areas of Bagmara Upazilla under Rajshahi district. J. Bangladesh Agric. Univ. 2008, 6, 367–374. [Google Scholar] [CrossRef]
- Zaman, T.; Jewel, M.A.S.; Bhuiyan, A.S. Present Status of Pond Fishery Resources and Livelihood of the Fish Farmers of Mohanpur Upazila in Rajshahi District. Univ. J. Zool. Rajshahi Univ. 2006, 25, 31–35. [Google Scholar] [CrossRef]
- Tasnoova, S.; Iqbal, K.M.; Iwamoto, I.; Haque, M.M. Economic performance of fish-based farming systems in Bangladesh. J. Fish. Aquat. Sci. 2008, 3, 206–212. [Google Scholar] [CrossRef]
- Islam, A.B.M.S.; Farouque, M.G.; Roy, D. Problem Confronted by the Fish Farmers in Practicing Semi-intensive climbing perch (Anabus testubineus) farming. Bangladesh J. Ext. Educ. 2013, 25, 53–61. [Google Scholar]
- Rahman, M.A.; Alam, M.M.; Barman, S.K.; Hossain, M.J.; Tikadar, K.K. Effect of COVID-19 on Fisheries Products Exported from Southwest Bangladesh: A Case Study. J. Bangladesh Agric. Univ. 2022, 20, 217–224. [Google Scholar] [CrossRef]
- Azad, A.K.; Wadood, S.N. The Impact of Climate Change on Fish Production in Bangladesh: An Assessment; Bureau of Economic Research (BER), University of Dhaka: Dhaka, Bangladesh, 2020. [Google Scholar] [CrossRef]
- Faruque, M.H.; Kabir, M.A. Climate change effects on aquaculture: A case study from north western Bangladesh. Int. J. Fish. Aquat. Stud. 2016, 4, 550–556. [Google Scholar]
- Alam, E.; Mallick, B. Climate change perceptions, impacts and adaptation practices of fishers in southeast Bangladesh coast. Int. J. Clim. Chang. Strateg. Manag. 2022, 14, 191–211. [Google Scholar] [CrossRef]
- Halim, M.A.; Mondal, D.K.; Salam, M.A.; Hossain, M.S. Impacts of climate change on pond fish farming in Amtoli, Borguna, Bangladesh. Int. J. Fish. Aquat. Stud. 2017, 5, 38–41. [Google Scholar]
- Rahman, M.L.; Shahjahan, M.; Ahmed, N. Tilapia farming in Bangladesh: Adaptation to climate change. Sustainability 2021, 13, 7657. [Google Scholar] [CrossRef]
- McNamara, J.M.; Barta, Z.; Klaassen, M.; Bauer, S. Cues and the optimal timing of activities under environmental changes. Ecol. Lett. 2011, 14, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increases and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Aziz, M.S.B.; Hasan, N.A.; Mondol, M.M.R.; Alam, M.M.; Haque, M.M. Decline in fish species diversity due to climatic and anthropogenic factors in HakalukiHaor, an ecologically critical wetland in northeast Bangladesh. Heliyon 2021, 7, e05861. [Google Scholar] [CrossRef]
- Dastansara, N.; Vaissi, S.; Mosavi, J.; Sharifi, M. Impacts of temperature on growth, development and survival of larval Bufo (Pseudepidalea) viridis (Amphibia: Anura): Implications of climate change. Zool. Ecol. 2017, 27, 228–234. [Google Scholar] [CrossRef]
- Pereira, B.F. Effect of Temperature on Sperm Motility in Fishes—A Review. Int. J. Sci. Res. Methodol. 2016, 5, 81–89. [Google Scholar]
- Muchlisin, Z.A. A General Overview on Some Aspects of Fish Reproduction. Aceh Int. J. Sci. Technol. 2014, 3, 43–52. [Google Scholar] [CrossRef]
- Karmakar, S.; Purkait, S.; Das, A.; Samanta, R.; Kumar, K. Climate change and inland fisheries: Impact and mitigation strategies. J. Exp. Zool. 2018, 21, 329–335. [Google Scholar]
- Tucker, J.W. Marine Fish Culture; Springer: Boston, MA, USA, 1998; 750p. [Google Scholar] [CrossRef]
- Stien, L.H.; Bracke, M.B.M.; Folkedal, O.; Nilsson, J.; Oppedal, F.; Torgersen, T.; Kittilsen, S.; Midtlyng, P.J.; Vindas, M.A.; Øverli, Ø. Salmon Welfare Index Model (SWIM 1.0): A semantic model for overall welfare assessment of caged Atlantic salmon: Review of the selected welfare indicators and model presentation. Rev. Aquac. 2013, 5, 33–57. [Google Scholar] [CrossRef]
- Cascarano, M.C.; Stavrakidis-Zachou, O.; Mladineo, I.; Thompson, K.D.; Papandroulakis, N.; Katharios, P. Mediterranean Aquaculture in a Changing Climate: Temperature Effects on Pathogens and Diseases of Three Farmed Fish Species. Pathogens 2021, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, L.; Munson, D.; Trushenski, J. Climate Change and Considerations for Fish Health and Fish Health Professionals. Fisheries 2016, 41, 396–399. [Google Scholar] [CrossRef]
- Harasawa, H.; Matsuoka, Y.; Takahashi, K.; Hijioka, Y.; Shimada, Y.; Munesue, Y.; Lal, M. Potential Impacts of Global Climate Change. In Climate Policy Assessment Asian-Pacific Integrated Modeling; Kainuma, Y.M., Matsuoka, Y., Morita, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 37–54. [Google Scholar]
- Marcogliese, D.J. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Tech. 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Cochrane, K.; De Young, C.; Soto, D.T.; Bahri, D.T. Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper No. 530; FAO: Rome, Italy, 2009. [Google Scholar]
- Burge, C.A.; Eakin, C.M.; Friedman, C.S.; Froelich, B.; Hershberger, P.K.; Hofmann, E.E.; Petes, L.E.; Prager, K.C.; Weil, E.; Willis, B.L.; et al. Climate Change Influences on Marine Infectious Diseases: Implications for Management and Society. Annu. Rev. Mar. Sci. 2014, 6, 249–277. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.; Kutz, S.; Harvell, C.D. Climate change and infectious diseases: From evidence to apredictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Egan, S.; Gardiner, M. Microbial dysbiosis: Rethinking disease in marine ecosystems. Front. Microbiol. 2016, 7, 991. [Google Scholar] [CrossRef]
- Islam, M.A.; Akber, M.A.; Ahmed, M.; Rahman, M.M.; Rahman, M.R. Climate change adaptations of shrimp farmers: A case study from southwest coastal Bangladesh. Clim. Dev. 2019, 11, 459–468. [Google Scholar] [CrossRef]
- Ahmed, N.; Diana, J.S. Coastal to inland: Expansion of prawn farming for adaptation to climate change in Bangladesh. Aquac. Rep. 2015, 2, 67–76. [Google Scholar] [CrossRef]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Moore, S.K.; Hallegraeff, G.; Kudela, R.M.; Clement, A.; Mardones, J.I.; Cochlan, W.P. Pelagic harmful algal blooms and climate change: Lessons from nature’s experiments with extremes. Harmful Algae 2019, 91, 101591. [Google Scholar] [CrossRef]
- Shafland, P.L.; Pestrak, J.M. Lower lethal temperatures for fourteen non-native fishes in Florida. Environ. Biol. Fishes 1982, 7, 149–156. [Google Scholar] [CrossRef]
- Ngoan, L.D. Effects of climate change in aquaculture: Case study in Thua Thien Hue Province, Vietnam. Biomed. Int. J. Sci. Technol. Res. 2018, 10, 7551–7552. [Google Scholar] [CrossRef]
- Björnsson, B.; Tryggvadóttir, S.V. Effects of size on optimal temperature for growth and growth efficiency of immature Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 1996, 142, 33–42. [Google Scholar] [CrossRef]
- Fang, J.; Tian, X.; Dong, S. The influence of water temperature and ration on the growth, body composition and energy budget of tongue sole (Cynoglossus semilaevis). Aquaculture 2010, 299, 106–114. [Google Scholar] [CrossRef]
- Amin, M.N.; Carter, C.G.; Barnes, R.S.K.; Adams, L.R. Protein and energy nutrition of brook trout (Salvelinus fontinalis) at optimal and elevated temperatures. Aquac. Nutr. 2016, 22, 527–540. [Google Scholar] [CrossRef]
- Charo, H.K. Selection for Growth of Nile tilapia (Oreochromis niloticus L.) in Low Input Environments. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2006. [Google Scholar]
- Miegel, R.P.; Pain, S.J.; van Wettere, W.H.E.J.; Howarth, G.S.; Stone, D.A.J. Effect of water temperature on gut transit time, digestive enzyme activity and nutrient digestibility in yellowtail kingfish (Seriola lalandi). Aquaculture 2010, 308, 145–151. [Google Scholar] [CrossRef]
- Lemasson, A.J.; Hall-Spencer, J.M.; Fletcher, S.; Provstgaard-Morys, S.; Knights, A.M. Indications of future performance of native and nonnative adult oysters under acidification and warming. Mar. Environ. Res. 2018, 142, 178–189. [Google Scholar] [CrossRef]
| General Characteristics | Number | % | |
|---|---|---|---|
| Age of hatchery owners | Young age (15–30) | 4 | 6.67 |
| Middle age (31–50) | 36 | 60.00 | |
| Old age (51–65) | 20 | 33.33 | |
| Gender | Male | 60 | 100.00 |
| Female | 0 | 0.00 | |
| Education | Illiterate | 1 | 1.67 |
| Adult literacy/informal education | 1 | 1.67 | |
| Class I to V | 11 | 18.33 | |
| Class VI to X | 23 | 38.33 | |
| Class XI to above | 24 | 40.00 | |
| Age of hatchery | 1–12 Years | 35 | 58.33 |
| 13–24 Years | 18 | 30.00 | |
| 25–36 Years | 7 | 11.67 | |
| Size of hatchery | Small | 28 | 46.67 |
| Medium | 13 | 21.67 | |
| Large | 19 | 31.67 | |
| Year of fish production | 1–5 Years | 15 | 25.00 |
| 6–10 Years | 14 | 23.33 | |
| Above 10 Years | 31 | 51.67 | |
| Training experience | Yes | 60 | 100.00 |
| No | 0 | 0.00 | |
| Run other business | Yes | 30 | 50.00 |
| No | 30 | 50.00 | |
| Challenges face in the hatchery production | Yes | 60 | 100.00 |
| No | 0 | 0.00 |
| Perceptions of the Causes Responsible for Climate Change | Responses (%) | ||||
|---|---|---|---|---|---|
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | |
| Rising air temperature | 6.67 | 63.33 | 30.00 | 0.00 | 0.00 |
| Rising water temperature | 6.67 | 68.33 | 25.00 | 0.00 | 0.00 |
| Frequent natural calamities such as flood, cyclones, heavy rainfall, water logging, flash flood, etc. | 1.69 | 45.76 | 52.54 | 0.00 | 0.00 |
| Prolonged drought | 0.00 | 22.03 | 76.27 | 1.69 | 0.00 |
| River erosion | 0.00 | 15.00 | 83.33 | 1.67 | 0.00 |
| Rising sea level | 0.00 | 35.00 | 63.33 | 1.67 | 0.00 |
| Intense thunderstorm | 0.00 | 21.67 | 76.67 | 1.67 | 0.00 |
| Model Summary | ||||
|---|---|---|---|---|
| Model | R | R Square | Adjusted R Square | Std. Error of the Estimate |
| 1 | 0.372 a | 0.139 | 0.122 | 1.495 |
| 2 | 0.493 b | 0.243 | 0.214 | 1.414 |
| 3 | 0.558 c | 0.312 | 0.270 | 1.362 |
| ANOVA a | ||||||
|---|---|---|---|---|---|---|
| Model | Sum of Squares | df | Mean Square | F | Sig. | |
| 1 | Regression | 18.680 | 1 | 18.680 | 8.363 | 0.006 b |
| Residual | 116.153 | 52 | 2.234 | |||
| Total | 134.833 | 53 | ||||
| 2 | Regression | 32.808 | 2 | 16.404 | 8.200 | 0.001 c |
| Residual | 102.026 | 51 | 2.001 | |||
| Total | 134.833 | 53 | ||||
| 3 | Regression | 42.024 | 3 | 14.008 | 7.547 | 0.000 d |
| Residual | 92.810 | 50 | 1.856 | |||
| Total | 134.833 | 53 | ||||
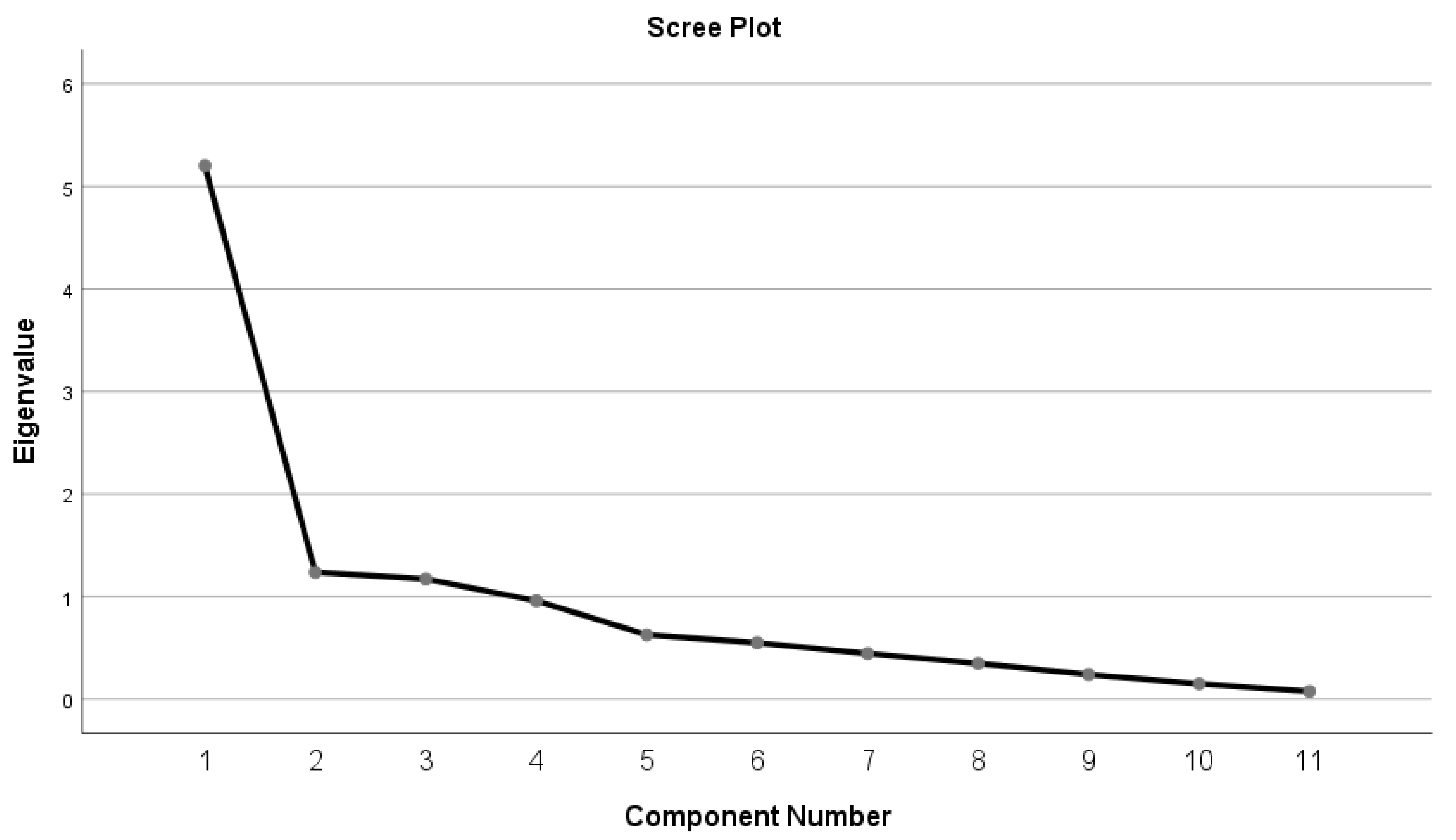
| Principal Component | Eigenvalue | Difference | Proportion (%) | Cumulative (%) |
|---|---|---|---|---|
| 1 | 6.009 | 4.771 | 50.08 | 50.08 |
| 2 | 1.238 | 0.060 | 10.32 | 60.39 |
| 3 | 1.178 | 0.217 | 9.82 | 70.21 |
| 4 | 0.961 | 0.333 | 8.01 | 78.22 |
| 5 | 0.628 | 0.079 | 5.23 | 83.45 |
| 6 | 0.548 | 0.103 | 4.57 | 88.02 |
| 7 | 0.445 | 0.057 | 3.71 | 91.73 |
| 8 | 0.389 | 0.129 | 3.24 | 94.97 |
| 9 | 0.260 | 0.102 | 2.17 | 97.14 |
| 10 | 0.158 | 0.047 | 1.32 | 98.45 |
| 11 | 0.111 | 0.036 | 0.93 | 99.38 |
| 12 | 0.075 | - | 0.62 | 100.00 |
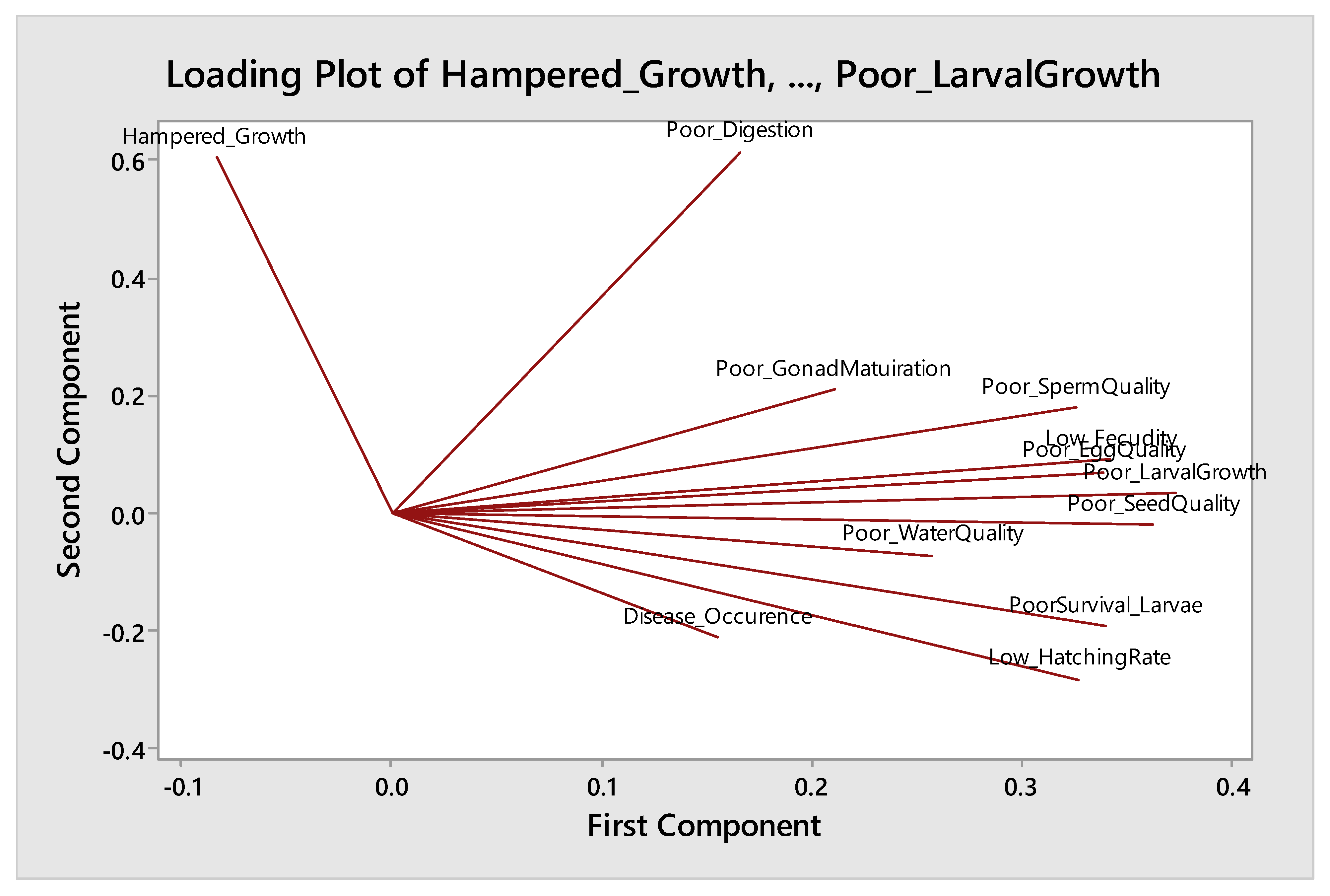
| Rotated Component Matrix a | |||
|---|---|---|---|
| Component | |||
| PC 1 | PC 2 | PC 3 | |
| Poor larval growth | 0.874 | ||
| Poor gonadal maturation | 0.861 | ||
| Low hatching rate | 0.859 | ||
| Poor egg quality | 0.849 | ||
| Poor seed quality | 0.823 | ||
| Low fecundity | 0.784 | ||
| Poor sperm quality | 0.736 | ||
| Disease occurrence | 0.810 | ||
| Poor survival of larvae | 0.750 | ||
| Poor water quality | 0.530 | ||
| Poor digestion | 0.701 | ||
| Hampered growth rate of broodstock | 0.661 | ||
| Mitigation and Adaptation Strategies | Method | Timeframe | Impact |
|---|---|---|---|
| Increasing the inside air temperature | Use of polythene sheets to cover the hatchery | During the seed production cycle, when the temperature falls below the optimum | Positive |
| Maintaining favorable nursery and broodfish pond water temperature | Supply clean additional water to increase the volume of water in the hatchery tank and broodfish pond | If the seed and broodfish pond temperature exceeds tolerable limits. | Positive |
| Maintaining water oxygen levels | Use of a simple aerator system and oxygen tablets | If oxygen levels drop during hatchery seed production and pond broodfish management. | Positive |
| Preventing fish from escaping during seasonal floods | Repairing and raising pond dikes | In the dry season | Unknown |
| Creating a cool production environment | Suitable tree planting in pond dikes and the hatchery compound | In the wet season | Environmentally friendly hatchery compound |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddique, M.A.B.; Ahammad, A.K.S.; Mahalder, B.; Alam, M.M.; Hasan, N.A.; Bashar, A.; Biswas, J.C.; Haque, M.M. Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study. Fishes 2022, 7, 270. https://doi.org/10.3390/fishes7050270
Siddique MAB, Ahammad AKS, Mahalder B, Alam MM, Hasan NA, Bashar A, Biswas JC, Haque MM. Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study. Fishes. 2022; 7(5):270. https://doi.org/10.3390/fishes7050270
Chicago/Turabian StyleSiddique, Mohammad Abu Baker, A. K. Shakur Ahammad, Balaram Mahalder, Md. Mehedi Alam, Neaz A. Hasan, Abul Bashar, Jatish Chandra Biswas, and Mohammad Mahfujul Haque. 2022. "Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study" Fishes 7, no. 5: 270. https://doi.org/10.3390/fishes7050270
APA StyleSiddique, M. A. B., Ahammad, A. K. S., Mahalder, B., Alam, M. M., Hasan, N. A., Bashar, A., Biswas, J. C., & Haque, M. M. (2022). Perceptions of the Impact of Climate Change on Performance of Fish Hatcheries in Bangladesh: An Empirical Study. Fishes, 7(5), 270. https://doi.org/10.3390/fishes7050270








