Abstract
This study was designed to elucidate the effects of arsenic (As) on the morpho-behavior, growth development and molecular mechanisms of a commercially important fish, rohu carp, Labeo rohita, in Bangladesh. Fish fry with an average weight of 387.5 ± 169.25 mg and an average length of 3.35 ± 0.37 cm were collected from a local hatchery in Mymensingh, Bangladesh and acclimatized for a week in the Department of Fisheries, University of Dhaka before starting the exposure with arsenic. Fishes were exposed for a period of 14 days with three treatments of NaAsO2, namely treatment 1(T1)—2.5 mg/L; treatment 2 (T2)—15 mg/L; and treatment 3 (T3)—30 mg/L, along with a control (C)—0.0 mg/L, with three replicates. These concentrations were determined based on the LC50 value for 96 h measured for this experiment. This study revealed remarkable morphological abnormalities and deformities in arsenic-exposed rohu carp. In fish exposed to 30 mg/L, caudal fin erosion was a frequent deformity. There was no significant difference in RNA:DNA ratio among the treatments. The overall weight of fish was decreased as the concentration of arsenic was increased. The T3 fish had a statistically significant negative weight gain (−0.05 ± 0.07 g), but the other treatments (T1 and T2) and control fish had no significant weight gain. Different types of histopathological changes were observed in the gills and intestines of arsenic-treated fish. Necrosis and severe damages were found in the secondary lamellae of gills at the highest arsenic concentration (30 mg/L). Epithelial lifting, irregular shape and damages in the gill raker were also observed in the primary lamellae of the gills for the same treatment. In this study, the expression of heat shock protein (HSP 60) and metallothionein (MT) genes was assessed by qPCR, and these genes were upregulated in different treatments compared to controls. The findings of the present study suggest that arsenic pollution significantly changes the morphology, behavior, growth, development, histopathology and molecular mechanisms of this economically important fish, rohu carp, in Bangladesh.
1. Introduction
Environmental contamination, particularly water pollution, has become a major global issue [1]. Water pollution is not only seriously affecting the growth, survival and reproduction of aquatic animals but is also impacting human life through bioaccumulation [1]. Fish development, particularly early development, is very sensitive to water pollution. Heavy metal contamination (e.g., arsenic, cadmium, lead, mercury, etc.) in aquatic environments has a significant impact on fish developmental and physiological processes, such as organ development, breeding and spawning, resulting in a decrease in offspring quantity and quality [2]. The intake of waterborne heavy metals by a fish results in abnormalities and disruptions in the function and structure of many tissues and organs [3].
Among heavy metals, arsenic is one of the most prominent toxicants in the aquatic environment, polluting water severely [4,5]. It is a metalloid toxicant widely found in rivers, canals, ponds, groundwater, lakes and seawater due to the uncontrolled influx of industrial wastes and pesticides in the aquatic environment [6]. The World Health Organization (WHO) has classified arsenic as one of the most dangerous chemicals to public health [7]. Arsenic levels have been documented up to 800 and 2500 ppm in many countries, including Chile and Bangladesh [8]. High-level arsenic exposure is directly associated with many diseases, such as skin and lung cancer as well as cardiovascular disease and liver disorder [9,10,11,12]. Previous research has demonstrated the negative effects of arsenic on fish growth, mortality, development, RNA:DNA ratio, histopathology and genetic expression [13,14,15,16,17,18]. This toxicant can cause biochemical and physiological changes in fish, promoting a decrease in its growth and development to a greater extent [14,19]. Fish exposed to high concentrations of arsenic experience changes in body physiology, including effects on growth, mortality, ion exchange, immune system, reproduction, enzyme activity, histology and gene regulation [20,21,22].
Tissue histopathological investigation is a helpful approach for determining the impacts of environmental contaminants on the specific vital organs of fish in laboratory conditions [23,24]. However, the magnitude of the dose and duration of exposure to pollutants determines the degree of pathological changes in the different organs of fish. Histopathological studies are also helpful to determine the relationships between different biological processes and pollutant exposure [25]. Some researchers have explored the toxic histological effects of heavy metals (mercury, cadmium and iron) on numerous organs in fish, such as the gills, liver, kidneys and intestine [25,26,27]. Heavy metal exposure in fish, especially lethal and sub-lethal arsenic exposure, stimulates different histopathological injuries in various organs in the fish and impacts different physiological processes, such as the nervous, gastrointestinal, respiratory as well as cardiovascular systems [6]. Gills play an important role in fish by carrying out three important functions, viz. gas exchange, ion regulation and the emission of metabolic wastes. Fish gills are considerably infected by water pollutants as a result of continual contact with water, and respiratory problems are one of the major and early indications of pollution exposure [6]. Furthermore, lamellae fusion, epithelial cell hyperplasia, necrosis, cystic formations within secondary lamellae epithelium and secondary lamellae loss were found in gill tissue from heavy-metal-exposed Tilapia mossambica, Channa punctatus, Cyprinus carpio, Anguilla anguilla and Mastacembelus armatus [16,23,28,29].
Some researchers have described the molecular mechanisms in heavy-metal-induced fish. A researcher studied the effect of metal toxicity and the expression of the metallothionein gene in fish and found significant induction of mRNA levels in the liver and gill tissue of copper-, zinc- and cadmium-exposed tilapia [30]. The MT gene is not only responsible for the detoxification of heavy metals but is also involved in the homeostasis of some metals such as copper and zinc [31]. Therefore, the impact of heavy metal exposure can be assessed by the level of MT gene expression. Induction of mRNA synthesis is linked with increased aptitude for binding metals such as lead and safeguarding against the toxicity of metals.
Heat shock proteins (HSPs) are immune-responsive conserved cellular proteins involved in various types of proteome maintenance, such as protein transport, macromolecular complex congregation and apoptosis, as well as the immuno-modulation system [32,33,34]. They have been demonstrated to have a crucial role in the health of aquatic animals, particularly in connection to the host response to environmental contaminants and the production of inflammation [31]. The level of expression of HSPs increases when rebalancing protein homeostasis, to avoid the protein aggregation and misfolding that occurs because of stress. Moreover, the induction of HSP expression has been considered a common response to unfavorable situations; therefore, it works against diseases and is a possible target for new treatments [35].
The literature review revealed that there is a scarcity of scientific information on the overall effects of arsenic toxicity on commercially important and popular fish species rohu carp in Bangladesh. Therefore, the present study was conducted to identify the morphological, behavioral, growth and developmental changes due to arsenic stress on commercially important fish species rohu carp in Bangladesh. This study also revealed the effects of arsenic stress on histopathology, RNA:DNA ratio and different gene expression levels in rohu carp in Bangladesh.
2. Materials and Methods
2.1. Transportation
High-quality and healthy fish fry (Figure 1) of rohu carp (weight: 387.5 ± 169.25 mg, length: 3.35 ± 0.37 cm) were collected from Adarsha Hatchery and Fisheries Ltd., Trisal, Mymensingh, Bangladesh. Then, 5 L capacity oxygenated bags were used to transport fry from the hatchery to the Department of fisheries, University of Dhaka.
Figure 1.
Rohu carp fry collection site in Trishal Upazila of Bangladesh.
2.2. Acclimatization
Fish were acclimatized in a 35 L capacity tank (12 inch × 15 inch × 24 inch) for a period of one week. Acclimatization was performed according to a previous study, with a few modifications [36]. During acclimatization, fish were fed twice daily with floating carp feed (SMS Feeds Limited, Gazipur, Bangladesh) and fifty percent of water in the culture tank was exchanged daily to keep the water free of fish excreta and other wastes. The nutrient composition of SMS Feed was moisture 10%, protein 40%, lipid 7%, carbohydrate 21% fiber 4%, ash 16%, calcium 1.8% and phosphorus 0.7%. Continuous oxygenation was also performed in the tanks. During acclimatization, the physicochemical properties of the water were recorded. After acclimatization, only healthy fish were selected for experiments.
2.3. Stock Solution Preparation and LC50 Determination for Sodium Arsenite
Research-grade sodium arsenite (NaAsO2) (Mumbai, India) was collected from a local distributor. LC50 was determined according to the experiment conducted in an earlier study [16]. Before starting the acute test, a stock solution of sodium arsenite was prepared by adding 10 g of sodium arsenite to 1000 mL of distilled water. Then, an experiment was performed with different concentrations of metals (NaAsO2:0, 15, 30, 35, 40, 45, 50, 55, 70 and 150 mg/L) to identify the LC50 of 96 h. The stocking density of fish was 10 individual fish per 5 L of water in aquaria (12 inch × 8 inch × 8.5 inch) and the weight and length of fish were 171 ± 48.5 mg and 2.5 ± 0.2 cm, respectively. Fish mortality for each concentration was documented at logarithmic time intervals, namely at 6, 12, 24, 48, 72 and 96 h of fish exposure. During the experiment, no feed was given to fish; only aeration was provided in each tank. The physicochemical parameters of water were recorded using a Horiba U-50 series multi-parameter water quality meter (Tokyo, Japan), HACH pH meter and API fresh water master test kit (Ames, IA, USA).
2.4. Experimental Design for Exposure to Sodium Arsenite
Based on the LC50 value for 96 h of sodium arsenite, a total of three experimental groups were selected: treatment 1(T1)—2.5 mg/L; treatment 2 (T2)—15 mg/L; and treatment 3 (T3)—30 mg/L. A negative control group (0.0 mg/L) was maintained simultaneously. In every group, we randomly selected 10 healthy fish, which were placed in 5 L water in glass aquaria. Each group of treatments was maintained in triplicate and fish were fed daily, two times, at the rate of 3% of their body weight. On every alternate day, 50% of water in each aquaria was siphoned to remove wastes, feed and feces of fish. Subsequently, the same volume of water was added containing the assigned amount of sodium arsenite. The exposure experiment lasted for 14 days and the physicochemical properties of the water, such as temperature, DO, pH, salinity, conductivity, etc., were recorded carefully to maintain the optimum quality of water for fish culture.
2.5. Growth and Mortality Study
The growth of fish was studied by calculating the length, weight gain and condition factor of arsenic-exposed fish. At first, the initial length and weight of fish were recorded, and the final length and weight were recorded at the end of the study, after 12 days of exposure. Before measurement, fish were anaesthetized using 2-phenoxy ethanol anesthetic at the rate of 4 µL per 50 mL of culture water. Weight gain (a), length gain (b), condition factor (c) and mortality rate (d) of fish were calculated using the following formulas [36]:
- (a)
- Weight gain = Mean final fish weight − Mean initial fish weight
- (b)
- Length gain (cm) = Mean final length (cm) − Mean initial length (cm)
- (c)
- (d)
- Mortality rate (%) = × 100
2.6. Morphological Observation
The morphological changes or any abnormalities in exposed fish were also observed and documented throughout the culture period. Photographs of abnormalities and morphological changes in fish were captured using a Samsung Galaxy camera.
2.7. Clinical Signs/Behavioral Changes
According to OECD guidelines, clinical signs of fish are categorized into several groups, including distribution, equilibrium, buoyancy, behaviors and appearance. Based on these clinical groups, the horizontal and vertical distribution, schooling or shoaling behavior, buoyancy control, erratic swimming, ventilation, gulping, etc., of exposed fish were observed at every six-hour interval.
2.8. Histopathology
The gills and intestines of 14-day arsenic-exposed fish were isolated to assess histopathological changes. After collection, tissue was preserved and processed according to the methods described in an earlier study, with minor modification [16]. At first, live fish were sacrificed and fresh gills and intestines were collected in 10 percent formalin immediately for subsequent processing. After fixation, tissue was gradually dehydrated in a serially rising concentration (50 to 90%) of absolute alcohol for a period of one hour. Then, the tissue was also dehydrated for 30 min in acetone. The dehydrated tissue was then properly washed in xylol-1 for 3 h and xylol-2 for 1 h. A paraffin bath was applied in a temperature range of 60 to 70 °C for the impregnation of tissue for 2 h. During embedding, metallic molds were used in melted paraffin. After embedding, the blocks of tissue were kept in the ice chamber of a refrigerator for some time before section cutting. Finally, 3 to 5 µm thick tissue sections were prepared using a microtome machine (Microm HM 325, Thermo Scientific, Waltham, MA, USA). After tissue sectioning, hematoxylin and eosin (H&E) staining was performed by using the Varistain 24-4 automatic slide stainer machine (Thermo Scientific). Finally, histopathological changes in gill and intestine specimens were checked and examined under a microscope with a photographic attachment (Nikon, AFX-DX, Tokyo, Japan)
2.9. Determination of RNA:DNA Ratio
The determination of the RNA:DNA ratio was performed according to [36] Rabbane et al. (2020), with minor modification. For the extraction of DNA and RNA from the target tissue (gill and muscle), the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) was used. In brief, around 10 to 14 mg of fresh tissue was collected from fish and ground with a hand tissue homogenizer. Then, 650 μL of chilled Nuclei Lysis Solution was added to samples and they were transferred into 1.5 μL volume tubes; then, the samples were homogenized for 10 s. The samples were then incubated in a water bath for 20 min at (65 ± 1.5 °C) temperature. After ending incubation, 200 μL of Protein Precipitation Solution was added to the samples and they were kept on ice for 5 min. Then, the samples were centrifuged for 4 min at 14,000 rpm in a refrigerated centrifuge machine and the supernatant of each sample was collected very carefully in a fresh tube containing 600 μL of room-temperature molecular-grade isopropanol. The tube was then inverted gently to mix the sample with isopropanol. Again, the sample was centrifuged for 1 min at 14,000 rpm, supernatant was removed, and 600 μL of 70% ethanol was added and mixed gently. The sample was then centrifuged at 14,000 rpm for 1 min.
The ethanol was removed from the sample, and the pellet was left to dry for roughly 15 min. Finally, the pellet in the bottom of the tube was rehydrated by adding 40 μL of DNA Rehydration Solution for 1 h. Then, the sample was kept at −20 °C temperature for quantification with a spectrophotometer (Nanodrop one, Thermo Scientific) with 260/280 and 260/230 nm absorbance levels. Before determination, the NanoDrop was calibrated using DNA Rehydration Solution for DNA measurement and nuclease-free water for RNA measurement. Following calibration, 1 μL of each sample was used to determine RNA and DNA concentrations
2.10. Gene Expression Study
For the gene expression study, 14-day exposed gills and muscles of fish were collected and preserved for RNA extraction and further analysis. Several processes, including total RNA extraction, RNA quantification, cDNA synthesis, primer selection and qPCR analysis, were rigorously carried out for the gene expression study.
Total RNA of fish gill and muscle tissue was extracted using the SV Total RNA Isolation System (Promega, Madison, WI, USA). Approximately 20 mg of tissue was used to extract total RNA. After extraction, the quality and quantity of RNA was assessed using gel electrophoresis and a NanoDrop Spectrophotometer, respectively. The GoScriptTM Reverse Transcription System (Promega, Madison, WI, USA) was used for cDNA synthesis. Several procedures were adopted. For cDNA synthesis, we altered up to 5 μg of total RNA or up to 500 ng of poly (A) RNA into first-strand cDNA. For the gene expression study, the qTOWER3 real-time PCR thermal cycler (Analytic Jena, Jena, Germany) was used and fluorescent dye (Syber green) as well as GoTaq® qPCR Master Mix (Promega, Madison, WI, USA) were applied. In the gene expression study, three primers were used: housekeeping gene primer (β-actin), heat shock protein (HSP 60) and metallothionein (MT) gene. Table 1 presents the sequence of the target primers. The qPCR cycling program was 95 °C for 2 min, followed by 44 cycles at 95 °C for 15 s and 52 °C for 15 s. Dissociation curves and threshold cycles (Ct) were automatically calculated by qPCRsoft 3.2. The delta Ct method was applied to calculate the level of expression.

Table 1.
List of gene with their primer sequences [37] for the assessment of gene expression.
2.11. Data Analysis
The analysis of water quality and growth parameters such as weight gain, length gain and condition factor were carried out using IBM SPSS Statistics 20 software. One-way ANOVA and Tukey’s HSD post-hoc multiple comparison test was carried out to identify statistically significant differences among the treatments. Probit analysis was performed to determine the 96-h lethal concentration (LC) value of NaAsO2 using the same IBM SPSS Statistics 20 software. The Ct value obtained from qPCRsoft 3.2 was analyzed by the delta delta Ct method to determine the expression level of HSP 60 and the MT gene.
3. Results
3.1. Physicochemical Parameters of Culture Water
Different physicochemical parameters of the culture water were recorded to ensure optimum water quality for fish culture and treatment. Table 2 presents the properties of the water during acclimatization, LC50 determination and final exposure treatment. Almost all parameters indicated suitable conditions for fish culture. Considerable physicochemical parameter differences were not noticed during acclimatization, LC50 determination and the final exposure treatment period.

Table 2.
Physicochemical properties (Mean ± Std. error) of water during acclimatization, LC50 determination and final exposure period.
3.2. Cumulative Mortality and Determination of LC50
The cumulative mortality in percentage for rohu carp during the 96 h experiment is shown in Figure 2. Mortality was increased gradually with the increasing concentration of NaAsO2. No mortality was observed in control fish but up to 30 to 40% mortality was recorded at the 15 to 40 mg/L concentration of NaAsO2. Moreover, 80 to 90 percent mortality was calculated at 50 to 70 mg/L exposure of NaAsO2. Finally, 100% mortality occurred at the 150 mg/L concentration of NaAsO2. From the data of 96 h mortality, the lethal concentration (LC) value was calculated using probit analysis through SPSS software. The calculated LC50 and LC90 values for the 96 h study were 32.98 and 90.02 mg/L, respectively (Figure 3). The 95% confidence limit values for LC50 and LC90 were 23.28 to 41.33 mg/L and 65.05 to 205.49 mg/L, respectively.
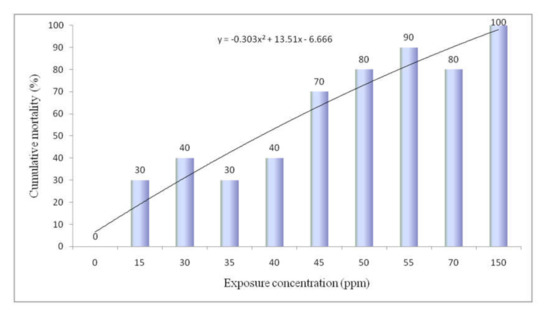
Figure 2.
Cumulative mortality of rohu carp exposed to sodium arsenite for a period of 96 h.
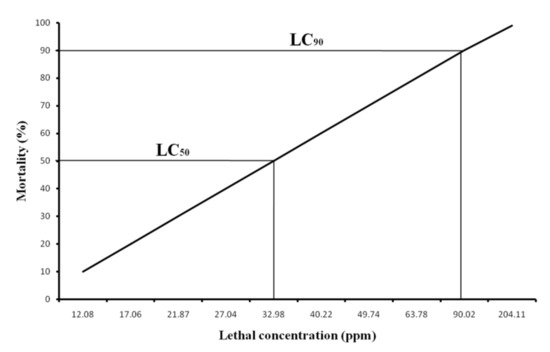
Figure 3.
The calculated LC50 and LC90 values for 96 h study period.
3.3. Morphological Anomalies and Deformities
Remarkable morphological anomalies and deformities in arsenic-exposed rohu carp were observed in this study. Caudal fin erosion of some fish was a prominent deformity in 30 mg/L exposed fish (Figure 4b,c). In particular, the dorsal lobe of the caudal fin was strongly affected by arsenic. Some fish exhibited an irregular body shape with skin discoloration (Figure 4b,c). Other anomalies included outward protrusion of the eyes, a large head, a narrow posterior end and a split fin (Figure 4d–f).

Figure 4.
Morphological anomalies and deformities of arsenic-exposed rohu carp. Protrusion eye (A), caudal fin erosion (B,C), irregular body shape (D,F), skin discoloration (E).
3.4. Clinical Signs/Behavioral Changes
In this study, no significant clinical signs were recorded in control and T1 (3.00 mg/L) fish. However, different clinical and behavioral changes were detected in arsenic-exposed fish in the experiment. The clinical signs were increased with the increasing concentration of heavy metals, especially at 30 mg/L for NaAsO2-treated fish. The reaction of heavy metals started after only a few minutes of exposure. Initially, hyperactivity and jumping out from the water were observed in T2- and T3-stressed fish. Gulping, hyperventilation, abnormal swimming and appearance were not recorded in control fish throughout the study period. Hyperventilation, erratic swimming and gulping were increased with increasing arsenic concentration, particularly in T2 and T3 treatment fish. The fish did not show any significant schooling behavior variation among the treatments during the experimental period. Before death, the fish showed a loss of balance, heavy mucus secretion and were distributed at the near-surface of the water.
3.5. RNA and DNA Ratio
In the study, the RNA and DNA ratio of muscles and gills was calculated. Figure 5 presents a bar diagram of the calculated RNA and DNA ratio of muscles and gills. In muscle tissue, the highest ratio was recorded in control fish (0.96) and the lowest in T1 (0.72), whereas in gill tissue, the maximum ratio was found in control and T1 fish (0.78) and the lowest in T2 (0.35). An increasing or decreasing pattern was not observed in the RNA:DNA ratio for both muscle and gill tissue.
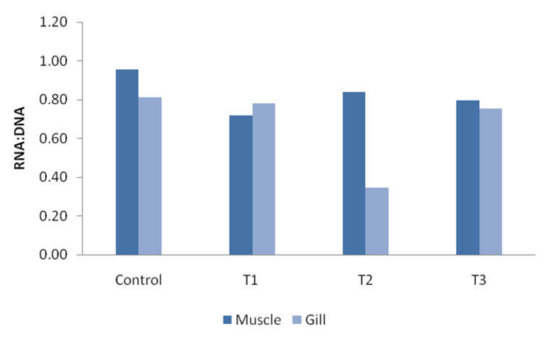
Figure 5.
RNA and DNA ratio of 14-day exposed muscle and gills. Control: 0.0 mg/L, T1: 2.5 mg/L, T2: 15 mg/L and T3: 30 mg/L.
3.6. Growth Parameter Study
The growth of fish was studied by calculating the weight gain, length gain and condition factor. The overall weight of fish was decreased with increasing arsenic concentration. Statistically significant negative weight gain (−0.05 ± 0.07 g) was observed in T3 fish, whereas there was no significant weight gain difference among other treatments (T1 and T2) and control fish (Table 3). A similar result was obtained for length gain. Significantly, the lowest and most negative length gain (−0.35 ± 0.12 cm) was calculated for T3 fish when compared with control and other treatments. However, no significant length gain difference was observed among control, T1 and T2 fish. The condition factor of fish was estimated at the beginning (initial) and end (final) of the experiment by calculating the length and weight of fish. The highest initial condition factor (0.98 ± 0.07) was recorded in control fish and the lowest (0.82 ± 0.03) in T3 fish. The lowest final condition factor (0.86 ± 0.04) was observed in T1 fish and the highest (0.95 ± 0.06) in T2 fish. However, there was no statistically significant difference observed among treatments and control fish regarding the initial and final condition factor (Table 3).

Table 3.
Growth parameters of arsenic-exposed rohu carp. Data represented as Mean ± Std. Error and analyzed by one-way ANOVA and Tukey’s HSD post-hoc test. Data with different subscript lowercase letters represent significant differences (p < 0.05) among treatments.
3.7. Mortality Rate Study
The mortality of rohu carp was recorded for 14 days of exposure. Mortality was simultaneously increased with increasing arsenic concentration. No fish mortality was observed in the control and T1 groups. The highest fish mortality was recorded in T3 (16.66%) and the lowest was in T2 (3.33%) (Figure 6).
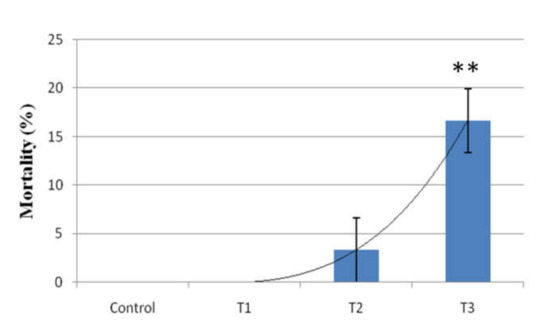
Figure 6.
Mortality rate of arsenic-exposed rohu carp in different treatment groups. Column with (**) sign indicates significant difference (p < 0.01) compared to other groups. Control: 0.0 mg/L, T1: 2.5 mg/L, T2: 15 mg/L and T3: 30 mg/L.
3.8. Histopathology
Different types of histopathological changes were observed in the gills of arsenic-treated rohu carp. Necrosis and severe damages were found in the secondary lamellae in T3-stressed fish (Figure 7A). Epithelial lifting was also observed in the primary lamellae of the gills of T3 fish (Figure 7A). Irregular shape and damages also occurred in the gill rakers of some T3 fish (Figure 7B). Fish treated with 30 mg/L sodium arsenite showed necrosis of primary lamellae, as well as fusion and hyperplasia in the secondary lamellae (Figure 7C). Vasodilation was also detected in the primary gill lamellae in T2-treated fish (Figure 7D). Moreover, edema and epithelial cell proliferation were found in the gill filaments of T2 fish. In addition, blood congestion was noticed in primary gill lamellae and proliferation of the vascular space also appeared toward the end of the primary gill lamellae in T1-treated fish (Figure 7E,F), whereas in control fish, no considerable histopathological changes were observed.
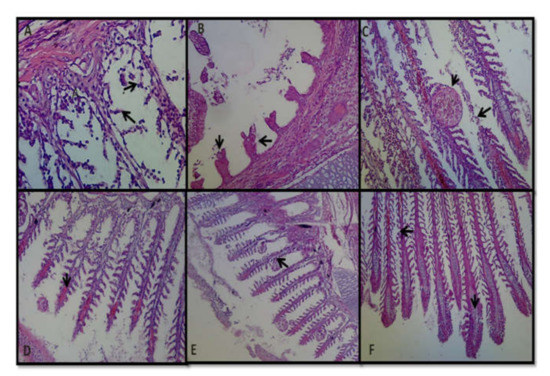
Figure 7.
Hematoxylin and eosin staining slide of arsenic-treated fish gill (400×). Arrowhead (→) sign indicates the change in gill structure due to arsenic treatment. (A): Necrosis and damages of secondary gill lamellae (T3). (B): Irregular shape and damage of gill rakers (T3). (C): Necrosis of primary gill lamellae and fusion and hyperplasia of secondary gill lamellae (T3). (D): Vasodilation in primary gill lamellae (T2). (E): Edema and epithelial proliferation. (F): Lamellar blood congestion and proliferation of vascular space toward the end of primary gill lamellae (T1). Photos were taken using Nikon AF-S DX, Japan.
In this study, histopathological changes in the intestine were also evaluated at the end of the experiment. In rohu carp, the tissue of control fish intestines showed a normal shape, size and structure. Well-defined mucosa, submucosa, goblet cells and serosa were noticed in the control intestine tissue (Figure 8A). Minor hemorrhage and blood congestion in the blood vessels was noticed in the mucosa and submucosal layer of the intestines in T1-treated fish (Figure 8B). Degeneration and damages of the mucosal layer were found in the intestines of some T2-treated fish (Figure 8C). Significant exhaustion of intestinal villi was observed in T3-treated fish (Figure 8D), whereas fusion of the muscularis and submucosal layer was detected in the intestinal tissue of T3-treated fish (Figure 8E). Severe necrosis and disintegration of intestinal villi were also significantly observed in the T3 fish (Figure 8F).
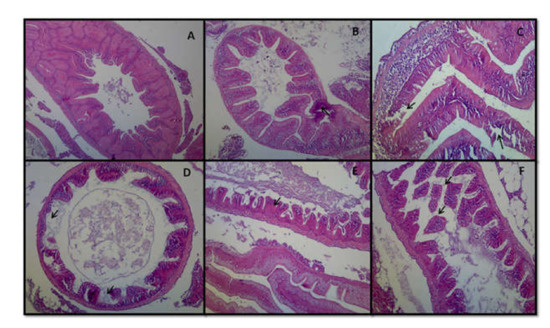
Figure 8.
Representative hematoxylin and eosin stain histopathological image of intestine of rohu carp (400×). (A): Normal intestine of control fish. (B): Hemorrhage in the mucosa and submucosal layer (T1). (C): Degeneration and damages of mucosal layer (T2). (D): Exhaustion of intestinal villi (T3). (E): Fusion of muscularis and submucosal layer (T3). (F): Necrosis and disintegration of intestinal villi (T3). Photos were taken using Nikon AF-S DX, Japan.
3.9. Gene Expression Study
In this experiment, the relative gene expression of mRNA was assessed for some heavy-metal-induced genes such as MT and HSP 60. The impact of arsenic on the relative expression of MT and HSP 60 genes in the muscle and gill tissue of rohu carp is presented in Figure 9. Upregulation of mRNA expression of the MT gene was observed in both arsenic-exposed muscle and gill tissue. The highest MT gene expression was achieved in T1 and T2 for muscle and gill tissue, respectively, and the lowest relative expression was found in T3 and T1 in the muscle and gill tissue when compared with controls (Figure 9a,b). The relative expression of the HSP 60 gene was also upregulated compared with controls, but with the exception of T2 and T3 for arsenic-exposed muscle and gill tissue, respectively, where mRNA expression was downregulated (Figure 9c,d).
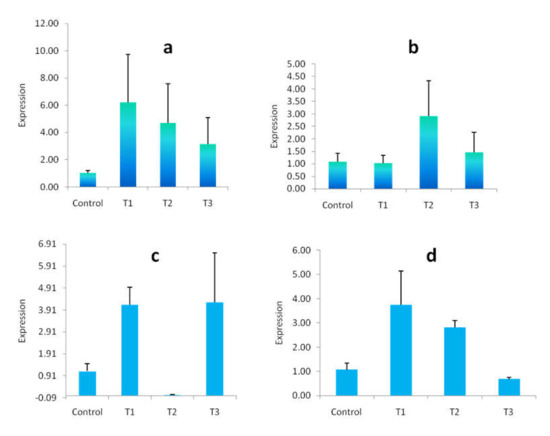
Figure 9.
Effect of arsenic on relative mRNA expression of heavy-metal-induced genes (MT and HSP60). (a): Expression of MT gene in muscle tissue. (b): Expression of MT gene in gill tissue. (c): Expression of HSP 60 gene in muscle tissue. (d): Expression of HSP 60 gene in gill tissue. Expression values are presented as Mean ± SE (presented as bar diagram). Control: 0.0 mg/L, T1: 2.5 mg/L, T2: 15 mg/L and T3: 30 mg/L.
4. Discussion
Arsenic is a toxic element that is abundant in aquatic environments such as rivers, canals, lakes, groundwater and seawater [6]. Heavy metal bioaccumulation and transformation in fish tissue such as the liver, kidneys, muscle, and brain has emerged from the continuous input of industrial wastes, pesticides, mining wastes, pharmaceutical compounds and other contaminants into aquatic habitats [6,38]. The deleterious effect of heavy metals on fish, particularly on growth, survival, internal abnormalities, embryonic development and so on, has been widely investigated during the last few decades [39]. In this experiment, the 96 h LC50 value of NaAsO2 for rohu carp was calculated to be 32.98 mg/L. An earlier scientist reported that the 96 h LC50 value of arsenic trioxide for Catla catla was found to be 20.41 mg/L [40]. The 48 h LC50 was reported to be 8.4 mg/L in Clarias batrachus [41], 84 mg/L in C. batrachus [42] and 76 mg/L in Channa punctatus [43]. The estimated 96 h LC50 value for NaAsO2 was found to be 28.219 mg/L for tilapia and 18.211 mg/L for Climbing Perch [16,44].
The foregoing studies show that the level of toxicity of arsenic varies from one species to another, and even from one strain of the same species to another. A previous research work reported that arsenic toxicity depends on the exposure duration, dose, species, sex, life state and inorganic and organic forms of chemicals [45]. Likewise, the environmental condition is another factor that can affect the toxicity of arsenic [16].
Different morphological abnormalities and deformities were reported in arsenic-exposed fish in the present investigation, such as fin erosion, outward protrusion of the eyes, large head, narrow posterior end and split fin, etc. An earlier work reported that arsenic-exposed zebrafish larvae showed a significant increase in eye diameter at 14 days postfertilization [7]. A previous study reported that heavy metals may negatively impact different metabolic process in the embryonic development system of fish, resulting in functional and morphological abnormalities, growth retardation and even death in the most vulnerable fish [2]. Caudal fin malformation and degeneration, fin blistering, abnormal body posture, etc., were observed in cadmium-exposed zebrafish by some researchers [46,47].
Significant clinical and behavioral changes were observed in arsenic-exposed fish in the experiment, particularly at 30 mg/L in NaAsO2-treated fish. Gulping, inconsistent swimming and hyperventilation were increased with increasing levels of arsenic. Behavior, as a sole factor, relates the ecology and physiology of an individual to its environment. A small amount of pollutants can alter the physiology and behavior of fish [6]. Some previous works reported that exposure to sodium arsenate resulted in a loss of balance, irregular swimming, jumping out from the water, erratic swimming and hyperventilation [44,48]. The abnormal behavior of arsenic-exposed fish was caused by the neurotoxic impacts of pollutants and the frustration to the sensory organs [6]. Jumping out and erratic swimming are two indicators of the avoidance of arsenic exposure in fish. Excessive mucus secretion may be due to the direct contact of arsenic with the fish skin.
The analysis of the RNA:DNA ratio has been widely used as an indicator for the measurement of the condition and growth of larval and adult fish, macroinvertebrates, phytoplankton and zooplankton [36,49,50,51,52,53,54,55]. This method is based on the conception that the quantity of DNA is almost constant in the individual cell but the concentration of RNA is increased based on the synthesis of proteins [56,57]. In addition, some studies documented that the RNA:DNA ratio has a relationship with the chemical exposure of fish [15]. The RNA:DNA ratio has been shown to decrease when fish are exposed to different organic and inorganic chemicals, such as mercury, cadmium, methyl parathion, benzophenone, etc. However, no significant reduction in RNA:DNA ratio was determined in this experiment. Nonetheless, the value of the ratio was higher in control fish for both muscle and gill tissue compared to all treatments. According to a previous study, the RNA:DNA ratio has not yet been confirmed as a biomarker for environmental toxicants [58]. The RNA:DNA ratio was significantly inhibited when common carp juveniles were exposed to tributyltin (TBT) for a period of 60 days [58]. Some other studies also documented that the RNA:DNA ratio was significantly reduced when fish were exposed to organic contaminants and metals for a long period [59]. However, some other researchers did not find any statistical significant relationship between environmental pollutants (DDT, Al3+, Cu2+) and RNA:DNA ratio [60,61]. The measurement of the RNA:DNA ratio could be a useful indicator for monitoring aquatic pollutant or toxicants in the early life stages of fish, when RNA synthesis is high [62]. Moreover, the estimation of this index is necessary to investigate the effects of pollutants in the life stages of fish relative to their molecular mechanisms.
The effect of arsenic on fish growth parameters and mortality was clearly identified in the present investigation. Both the length and weight of fish were reduced with the increasing exposure time and concentration of the pollutant. A similar result was reported by some other researchers when fish were treated with different pollutants [13,17]. At 4 weeks, arsenic-exposed starry flounder Platichthys stellatus showed a decreased rate of daily weight gain, daily length gain, condition factor and feed efficiency [13]. Significant reductions in the growth of Labeo rohita, Catla catla and Cirrhina mrigala were determined when fish were exposed to a sublethal concentration of manganese for a period of 30 days [17]. In general, fish weight and heavy metal concentration show the opposite relationship, whereas mortality shows a positive relationship with heavy metal concentration [63]. Mercury, chromium and zinc are more effective in delaying the growth of fish than other metals [64]. The condition factor is another index that estimates the general wellbeing of fish. A higher value of the condition factor indicates a better environmental situation, whereas a lower value shows a poor environmental condition [23]. Nevertheless, very few researchers have used the condition factor as a biomarker of environmental pollution. No condition factor variation occurred when researchers studied the effect of heavy metals in Gobio gobio, Rutilus rutilus and Perca fluviatilis inhabiting the Scheldt River, Belgium [65]. In the study, no significant condition factor variation was measured among the treatments and control. A previous work also explained that the condition factor is not only affected by pollutants but also some other factors are also responsible, such as food availability, temperature, etc. [23]. Therefore, heavy metal toxicity may affect the growth, mortality, physiological functions and reproduction of fish [66]. Similarly, in this experiment, fish mortality was greatly affected by arsenic exposure because mortality was increased with the increasing arsenic concentration and we found the highest mortality in T3 (16.66%), followed by T2 (3.33%).
Tissue histopathology is an important tool for determining the effects of environmental pollutants. In this study, different histopathological changes were observed in the gill and intestine tissue of 14-day arsenic-exposed rohu carp. Necrosis, epithelial lifting, fusion, hyperplasia and blood congestion were observed in the primary and secondary gill lamellae of 30 mg/L arsenic-treated fish. Cell damages and epithelial cell proliferation were, respectively, noticed in the gill raker and gill filament. Almost similar histopathological changes were documented by some other researchers, where they investigated the effects of different heavy metals such as arsenic, copper, cadmium, mercury and chromium on the histopathology of the gills of tilapia, Catla catla, Cirrhinus mrigala, Ctenopharyngodon idella, etc. [16,24,26,27,66,67,68]. The gill is an important and major organ in fish for respiration, excretion and osmoregulation [6,69]. This organ also plays a vital role in the detoxification of pollutants or toxicants [23]. As a result, it is most susceptible to damage by pollutants. Among different signs, gill edema is the primary structural change caused by the pollution of heavy metals [70]. Therefore, other histopathological changes such as hyperplasia and lifting of epithelia occurred due to the response of the defense system in the bodies of fish.
The present study also revealed that the intestines of arsenic-exposed rohu carp showed severe necrosis, exhaustion and disintegration of intestinal villi, as well as fusion of the muscularis and submucosal layer. An earlier study observed severe histopathological changes including blood congestion, edema and hemorrhage in the intestinal tissue of rohu carp inhabiting industrial-waste-contaminated water in the River Ravi [71]. Metal absorption occurs mostly through the gills, but may also occur through the epithelium of the digestive tract [72]. The intestines of Oreochromis niloticus and Lates niloticus were shown to exhibit severe histopathological alteration, including necrosis and degeneration, due to heavy metal exposure. Toxic metal absorption may cause edema between the mucosa and the submucosal layer [73]. Moreover, the current findings of this experiment are similar to the results of other experiments conducted to observe the impacts of heavy metals on the histology of the fish intestine [73,74,75].
Following 14 days of exposure, the effects of arsenic on MT and HSP 60 gene expression levels were assessed in the present investigation. The level of expression of the MT gene in gill and muscle tissue was increased due to arsenic-induced stress. MT is a thiol-rich low-molecular-weight protein and plays a significant role against the toxicity of heavy metals. Some researchers reported that the level of MT expression is increased due the response to the toxicity of heavy metals as well as environmental stress [37,76,77]. Regarding metal toxicity and MT gene expression in tilapia, it was found that the MT gene was upregulated when fish were exposed to various sublethal concentrations of copper, zinc and cadmium for a period of 21 days [30]. The induction of MT synthesis is related to the increased capacity for binding heavy metals such as zinc, cadmium, lead and copper, as well as guarding against the toxicity of metals. According to a previous study, lead binds to the MT protein and is absorbed and transported to the liver, where it stimulates MT production [78].
The overall level of expression of the HSP 60 gene was increased compared to control fish in this study. However, downregulation was observed in T2 and T3 treatment muscle and gill tissue, respectively. Heat shock protein is a highly conserved cellular protein that is responsible for apoptosis, immunomodulation, proteome preservation and influencing the immune system [17]. The level of expression of some HSPs rises in order to avoid protein aggregation and misfolding caused by stress and, as a result, to reestablish protein homeostasis. A researcher found significant upregulation of HSP 60 and HSP 90 genes in Labeo rohita fingerlings when exposed to arsenic for 12 days [15]. They also observed higher expression of other HSPs, viz. HSP 47, HSP 70, HSP 71 and HSP 78 genes, following arsenic exposure, even at a low level of concentration. The higher HSP expression could be considered a universal response to a variety of stressors, suggesting that it might be a possible mechanism of disease defense and a possible target for new therapies [35,79]. It has also been documented that the expression of HSPs might have two roles depending on the exposure period of arsenic: a defensive or protective function when exposure to arsenic is acute and a deleterious role when the expression is downregulated during continuous exposure [79].
5. Conclusions
The findings of the present study prove that arsenic (As), as a heavy metal pollutant, has strong negative effects on the morphology, behavior, growth, histopathology and gene expression levels of fish. Therefore, the results of the present investigation might be applied as biomonitoring program guidelines for fish populations cultured in heavy-metal-polluted water.
Author Contributions
M.G.R. performed this experiment, prepared the manuscript and analyzed data; M.G.M. supervised and critically revised the manuscript; M.A.K. revised and edited; and M.H.-A.-M. partially supervised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financially supported by the University Grant Commission of Bangladesh.
Institutional Review Board Statement
The work was approved by the ethical committee of the Faculty of Biological sciences, University of Dhaka, Bangladesh (Ref. No. 157/Bio.Scs).
Data Availability Statement
The data obtained from the current investigation are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank Anwar Hossain for providing the Horiba U-50 series multiparameter. They are also grateful to Invent Technologies Ltd. for their support during the molecular analysis.
Conflicts of Interest
The authors declare that they have no financial or non-financial interests that are directly or indirectly related to this research work.
References
- Dai, Y.-J.; Jia, Y.-F.; Chen, N.; Bian, W.-P.; Li, Q.-K.; Ma, Y.-B.; Chen, Y.-L.; Pei, D.-S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2008, 35, 625–640. [Google Scholar] [CrossRef]
- Jezierska, B.; Witeska, M. Metal Toxicity to Fish; Wydawnictwo Akademii Podlaskiej: Siedlce, Poland, 2001; 318p. [Google Scholar]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S.; Stolz, J.F. The Ecology of Arsenic. Science 2003, 300, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Kumar, V.; Sinha, A.K.; Ahsan, J.; Ghosh, A.; Wang, H.; De Boeck, G. Toxicology of arsenic in fish and aquatic systems. Environ. Chem. Lett. 2016, 15, 43–64. [Google Scholar] [CrossRef]
- Babich, R.; Van Beneden, R.J. Effect of arsenic exposure on early eye development in zebrafish (Danio rerio). J. Appl. Toxicol. 2019, 39, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Nordsborg, R.B.; Andersen, Z.J.; Tjønneland, A.; Loft, S.; Raaschou-Nielsen, O. Long-Term Exposure to Low-Level Arsenic in Drinking Water and Diabetes Incidence: A Prospective Study of the Diet, Cancer and Health Cohort. Environ. Health Perspect. 2014, 122, 1059–1065. [Google Scholar] [CrossRef]
- Gong, G.; O’Bryant, S.E. Low-level arsenic exposure, AS3MT gene polymorphism and cardiovascular diseases in rural Texas counties. Environ. Res. 2012, 113, 52–57. [Google Scholar] [CrossRef]
- Kundu, M.; Ghosh, P.; Mitra, S.; Das, J.; Sau, T.; Banerjee, S.; States, J.C.; Giri, A.K. Precancerous and non-cancer disease endpoints of chronic arsenic exposure: The level of chromosomal damage and XRCC3 T241M polymorphism. Mutat. Res. Mol. Mech. Mutagen. 2011, 706, 7–12. [Google Scholar] [CrossRef]
- Liu, J.; Waalkes, M.P. Liver is a Target of Arsenic Carcinogenesis. Toxicol. Sci. 2008, 105, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Javed, M.; Razzaq, S. Growth performance of metal stressed major carps viz. Catla catla, Labeo rohita and Cirrhina mrigala reared under semi-intensive culture system. Pak. Vet. J. 2007, 27, 8–12. [Google Scholar]
- Han, J.-M.; Park, H.-J.; Kim, J.-H.; Jeong, D.-S.; Kang, J.-C. Toxic effects of arsenic on growth, hematological parameters, and plasma components of starry flounder, Platichthys stellatus, at two water temperature conditions. Fish. Aquat. Sci. 2019, 22, 3. [Google Scholar] [CrossRef]
- Foley, C.J.; Bradley, D.L.; Höök, T.O. A review and assessment of the potential use of RNA:DNA ratios to assess the condition of entrained fish larvae. Ecol. Indic. 2016, 60, 346–357. [Google Scholar] [CrossRef]
- Ahmed, K.; Mamun, H.A.; Parvin, E.; Akter, M.S.; Khan, M.S. Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus). Exp. Toxicol. Pathol. 2013, 65, 903–909. [Google Scholar] [CrossRef]
- Banerjee, S.; Mitra, T.; Purohit, G.K.; Mohanty, S.; Mohanty, B.P. Immunomodulatory effect of arsenic on cytokine and HSP gene expression in Labeo rohita fingerlings. Fish Shellfish. Immunol. 2015, 44, 43–49. [Google Scholar] [CrossRef]
- Minatel, B.C.; Sage, A.P.; Anderson, C.; Hubaux, R.; Marshall, E.A.; Lam, W.L.; Martinez, V.D. Environmental arsenic exposure: From genetic susceptibility to pathogenesis. Environ. Int. 2017, 112, 183–197. [Google Scholar] [CrossRef]
- Beyers, D.W.; Rice, J.A.; Clements, W.H.; Henry, C.J. Estimating physiological cost of chemical exposure: Integrating energetics and stress to quantify toxic effects in fish. Can. J. Fish. Aquat. Sci. 1999, 56, 814–822. [Google Scholar] [CrossRef]
- Pedlar, R.M.; Ptashynski, M.D.; Evans, R.; Klaverkamp, J.F. Toxicological effects of dietary arsenic exposure in lake whitefish (Coregonus clupeaformis). Aquat. Toxicol. 2002, 57, 167–189. [Google Scholar] [CrossRef]
- Farag, A.M.; Stansbury, M.A.; Bergman, H.L.; Hogstrand, C.; MacConnell, E. The physiological impairment of free-ranging brown trout exposed to metals in the Clark Fork River, Montana. Can. J. Fish. Aquat. Sci. 1995, 52, 2038–2050. [Google Scholar] [CrossRef]
- Datta, S.; Ghosh, D.; Saha, D.R.; Bhattacharaya, S.; Mazumder, S. Chronic exposure to low concentration of arsenic is immunotoxic to fish: Role of head kidney macrophages as biomarkers of arsenic toxicity to Clarias batrachus. Aquat. Toxicol. 2009, 92, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Usmani, N. An Overview of the Adverse Effects of Heavy Metal Contamination on Fish Health. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2017, 89, 389–403. [Google Scholar] [CrossRef]
- Bose, M.J.; Ilavazhahan, M.; Tamilselvi, R.; Viswanathan, M. Effect of Heavy Metals on the Histopathology of Gills and Brain of Fresh Water Fish Catla catla. Biomed. Pharmacol. J. 2013, 6, 99–105. [Google Scholar] [CrossRef]
- Selvi, R.; Ilavazhahan, M. Histopathological Changes in Gill Tissue of the Fish Catla catla Exposed to Sublethal Concentration of Pesticide Methyl Parathion and a Heavy Metal Ferous Sulphate. Biomed. Pharmacol. J. 2012, 5, 305–312. [Google Scholar] [CrossRef]
- Chavan, V.R.; Muley, D.V. Effect of heavy metals on liver and gill of fish Cirrhinus mrigala. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 277–288. [Google Scholar]
- Padhy, G.; Mishra, C.; Barik, B.P. Histological alterations in gill tissues of Anabas testiduneus on exposure to heavy metal CdCl2. RJLBPCS 2018, 4, 328–334. [Google Scholar] [CrossRef]
- Gürcü, B.; Yildiz, S.; Koca, Y.B.G.; Koca, S. Investigation of Histopathological and Cytogenetic Effects of Heavy Metals Pollution on Cyprinus carpio (Linneaus, 1758) in the Gölmarmara Lake, Turkey. J. Anim. Vet. Adv. 2010, 9, 798–808. [Google Scholar] [CrossRef]
- Yildiz, S.; Gürcü, B.; Koca, Y.B.; Koca, S. Histopathological and Genotoxic Effects of Pollution on Anguilla anguilla in the Gediz River (Turkey). J. Anim. Vet. Adv. 2010, 9, 2890–2899. [Google Scholar] [CrossRef]
- Lam, K.L.; Ko, P.W.; Wong, J.K.-Y.; Chan, K.M. Metal toxicity and metallothionein gene expression studies in common carp and tilapia. Mar. Environ. Res. 1998, 46, 563–566. [Google Scholar] [CrossRef]
- Olsson, P.-E.; Kille, P. Functional comparison of the metal-regulated transcriptional control regions of metallothionein genes from cadmium-sensitive and tolerant fish species. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1997, 1350, 325–334. [Google Scholar] [CrossRef]
- Kakkar, V.; Meister-Broekema, M.; Minoia, M.; Carra, S.; Kampinga, H.H. Barcoding heat shock proteins to human diseases: Looking beyond the heat shock response. Dis. Model. Mech. 2014, 7, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2002, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef]
- Purohit, G.K.; Mahanty, A.; Suar, M.; Sharma, A.P.; Mohanty, B.P.; Mohanty, S. Investigating hsp gene expression in liver of Channa striatus under heat stress for understanding the upper thermal acclimation. Biomed. Res. Int. 2014, 2014, 381719. [Google Scholar] [CrossRef]
- Rabbane, G.; Ali, Y.; Zahid, A.; Hossain, J. Diet Effects on Growth, Mortality, RNA: DNA Ratio and Gene Expression of Zebrafish Danio rerio. Genet. Aquat. Org. 2020, 4, 19–27. [Google Scholar] [CrossRef]
- Pandi Prabha, S.; Rajkumar, J.; Karthik, C. Hepatotoxic effect of lead and hepatoprotective effect of Hydrilla verticillata on hepatic transcriptional and physiological response in edible fish Labeo rohita. Drug Chem. Toxicol. 2020, 45, 1276–1283. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR) 2007. Toxicological Profile for Arsenic. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. Available online: https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=3 (accessed on 30 May 2021).
- Sfakianakis, D.; Renieri, E.; Kentouri, M.; Tsatsakis, A. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- Lavanya, S.; Ramesh, M.; Kavitha, C.; Malarvizhi, A. Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere 2011, 82, 977–985. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharya, S.; Mazumder, S. Perturbations in the catfish immune responses by arsenic: Organ and cell specific effects. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2006, 143, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Bhattacharya, S. Induction of oxidative stress by arsenic in Clarias batrachus: Involvement of peroxisomes. Ecotoxicol. Environ. Saf. 2007, 66, 178–187. [Google Scholar] [CrossRef]
- Roy, S.; Bhattacharya, S. Arsenic-induced histopathology and synthesis of stress proteins in liver and kidney of Channa punctatus. Ecotoxicol. Environ. Saf. 2006, 65, 218–229. [Google Scholar] [CrossRef]
- Akter, M.S.; Ahmed, M.K.; Akhand, M.A.A.; Islam, M.M. Acute toxicity of arsenic and mercury to fresh water climbing perch, Anabas testudineus (Bloch). World J. Zool. 2008, 3, 13–18. [Google Scholar]
- Agusa, T.; Takagi, K.; Kubota, R.; Anan, Y.; Iwata, H.; Tanabe, S. Specific accumulation of arsenic compounds in green turtles (Chelonia mydas) and hawksbill turtles (Eretmochelys imbricata) from Ishigaki Island, Japan. Environ. Pollut. 2008, 153, 127–136. [Google Scholar] [CrossRef]
- Cheng, S.H.; Wai, A.W.K.; So, C.H.; Wu, R.S.S. Cellular and molecular basis of cadmium-induced deformities in zebrafish embryos. Environ. Toxicol. Chem. 2000, 19, 3024–3031. [Google Scholar] [CrossRef]
- Hallare, A.; Schirling, M.; Luckenbach, T.; Köhler, H.-R.; Triebskorn, R. Combined effects of temperature and cadmium on developmental parameters and biomarker responses in zebrafish (Danio rerio) embryos. J. Therm. Biol. 2005, 30, 7–17. [Google Scholar] [CrossRef]
- Baldissarelli, L.A.; Capiotti, K.M.; Bogo, M.R.; Ghisleni, G.; Bonan, C.D. Arsenic alters behavioral parameters and brain ectonucleotidases activities in zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Pepin, P.; Evans, G.T.; Shears, T.H. Patterns of RNA/DNA ratios in larval fish and their relationship to survival in the field. ICES J. Mar. Sci. 1999, 56, 697–706. [Google Scholar] [CrossRef]
- Gwak, W.-S.; Tanaka, Y.; Tominaga, O.; Tsusaki, T.; Tanaka, M. Field evaluation by RNA/DNA ratios on post-release nutritional status of released and wild Japanese flounder Paralichthys olivaceus juveniles. J. Exp. Mar. Biol. Ecol. 2003, 293, 107–124. [Google Scholar] [CrossRef]
- Smith, T.R.; Buckley, L.J. RNA–DNA ratio in scales from juvenile cod pro-vides a nonlethal measure of feeding condition. Trans. Am. Fish. Soc. 2003, 132, 9–17. [Google Scholar] [CrossRef]
- Kyle, M.; Watts, T.; Schade, J.; Elser, J.J. A microfluorometric method for quantifying RNA and DNA in terrestrial insects. J. Insect Sci. 2003, 3, 1. [Google Scholar] [CrossRef]
- Berdalet, E.; Dortch, Q. New double-staining technique for RNA and DNA measurement in marine phytoplankton. Mar. Ecol. Prog. Ser. 1991, 73, 295–305. [Google Scholar] [CrossRef]
- Gorokhova, E.; Kyle, M. Analysis of nucleic acids in Daphnia: Development of methods and ontogenetic variations in RNA-DNA content. J. Plankton Res. 2002, 24, 511–522. [Google Scholar] [CrossRef]
- Gorokhova, E. Relationships between nucleic acid levels and egg production rates in Acartia bifilosa: Implications for growth assessment of copepods in situ. Mar. Ecol. Prog. Ser. 2003, 262, 163–172. [Google Scholar] [CrossRef][Green Version]
- Suthers, I.; Cleary, J.; Battaglene, S.; Evans, R.; Suthers, I. Relative RNA Content as a Measure of Condition in Larval and Juvenile Fish. Mar. Freshw. Res. 1996, 47, 301–307. [Google Scholar] [CrossRef]
- Ferron, A.; Leggett, W. An Appraisal of Condition Measures for Marine Fish Larvae. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 1994; Volume 30, pp. 217–303. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, P.; Shi, Z.-C. Molecular responses in digestive tract of juvenile common carp after chronic exposure to sublethal tributyltin. Ecotoxicol. Environ. Saf. 2014, 109, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Varó, I.; Navarro, J.; Nunes, B.; Guilhermino, L. Effects of dichlorvos aquaculture treatments on selected biomarkers of gilthead sea bream (Sparus aurata L.) fingerlings. Aquaculture 2007, 266, 87–96. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kang, J.-C. Effect of dietary copper exposure on accumulation, growth and hematological parameters of the juvenile rockfish, Sebastes schlegeli. Mar. Environ. Res. 2004, 58, 65–82. [Google Scholar] [CrossRef]
- De Boeck, G.; Borger, R.; Van der Linden, A.; Blust, R. Effects of sublethal copper exposure on muscle energy metabolism of common carp, measured by31P-nuclear magnetic resonance spectroscopy. Environ. Toxicol. Chem. 1997, 16, 676–684. [Google Scholar] [CrossRef]
- Miliou, H.; Zaboukas, N.; Moraitou-Apostolopoulou, M. Biochemical Composition, Growth, and Survival of the Guppy, Poecilia reticulata, During Chronic Sublethal Exposure to Cadmium. Arch. Environ. Contam. Toxicol. 1998, 35, 58–63. [Google Scholar] [CrossRef]
- Hussain, S.M.; Javed, M.; Asghar, S.; Hussain, M.; Abdullah, S.; Raza, S.A.; Javid, A. Studies on growth performance of metals mixture stressed Cirrhina mrigala in earthen ponds. Pak. J. Agric. Sci. 2010, 47, 263–270. [Google Scholar]
- Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef]
- Bervoets, L.; Knapen, D.; De Jonge, M.; Van Campenhout, K.; Blust, R. Differential Hepatic Metal and Metallothionein Levels in Three Feral Fish Species along a Metal Pollution Gradient. PLoS ONE 2013, 8, e60805. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.F.; Brumbaugh, W.G.; DeLonay, A.J.; Little, E.E.; Smith, C.E. Effects on Rainbow Trout Fry of a Metals-Contaminated Diet of Benthic Invertebrates from the Clark Fork River, Montana. Trans. Am. Fish. Soc. 1994, 123, 51–62. [Google Scholar] [CrossRef]
- Carvalho, T.L.A.D.B.; Nascimento, A.A.D.; Gonçalves, C.F.D.S.; Dos Santos, M.A.J.; Sales, A. Assessing the histological changes in fish gills as environmental bioindicators in Paraty and Sepetiba bays in Rio de Janeiro, Brazil. Lat. Am. J. Aquat. Res. 2020, 48, 590–601. [Google Scholar] [CrossRef]
- Shah, N.; Khan, A.; Ali, R.; Marimuthu, K.; Uddin, M.N.; Rizwan, M.; Rahman, K.U.; Alam, M.; Adnan, M.; Jawad, S.M.; et al. Monitoring Bioaccumulation (in Gills and Muscle Tissues), Hematology, and Genotoxic Alteration in Ctenopharyngodon idella Exposed to Selected Heavy Metals. BioMed Res. Int. 2020, 2020, 6185231. [Google Scholar] [CrossRef] [PubMed]
- Javid, A.; Javed, M.; Abdullah, S. Nickel bio-accumulation in the bodies of Catla catla, Labeo rohita and Cirrhina mrigala during 96-hr LC50 exposures. Int. J. Agric. Biol. 2007, 9, 139–142. [Google Scholar]
- Mallatt, J. Fish Gill Structural Changes Induced by Toxicants and Other Irritants: A Statistical Review. Can. J. Fish. Aquat. Sci. 1985, 42, 630–648. [Google Scholar] [CrossRef]
- Sultana, T.; Butt, K.; Sultana, S.; Al-Ghanim, K.A.; Mubashra, R.; Bashir, N.; Ahmed, Z.; Ashraf, A.; Mahboob, S. Histopathological Changes in Liver, Gills and Intestine of Labeo rohita Inhabiting Industrial Waste Contaminated Water of River Ravi. Pak. J. Zool. 2016, 48, 1171–1177. [Google Scholar]
- Mohamed, F.A.S. Bioaccumulation of selected metals and histopathological alterations in tissues of Oreochromis niloticus and Lates niloticus from Lake Nasser, Egypt. Global Vet. 2008, 2, 205–218. [Google Scholar]
- Hanna, M.I.; Shaheed, I.B.; Elias, N.S. A contribution on chromium and lead toxicity in cultured Oreochromis niloticus. Egypt. J. aquat. Biol. Fish. 2005, 9, 177–209. [Google Scholar]
- Salim, F. Histopathological Effect of heavy metal on different organs of fresh water fish tissues from Garmat Ali River adjacent to Al- Najebyia Power Station Kufa. J. Vet. Med. Sci. 2015, 6, 141–153. [Google Scholar]
- Giari, L.; Manera, M.; Simoni, E.; Dezfuli, B. Cellular alterations in different organs of European sea bass Dicentrarchus labrax (L.) exposed to cadmium. Chemosphere 2007, 67, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Durnam, D.M.; Hoffman, J.S.; Quaife, C.J.; Benditt, E.P.; Chen, H.Y.; Brinster, R.L.; Palmiter, R.D. Induction of mouse metallothionein-I mRNA by bacterial endotoxin is independent of metals and glucocorticoid hormones. Proc. Natl. Acad. Sci. USA 1984, 81, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Cousins, R.J. Glucocorticoid independent mediation of interleukin-1 induced changes in serum zinc and liver metallothionein levels. Life Sci. 1984, 35, 2113–2118. [Google Scholar] [CrossRef]
- Swerdel, M.R.; Cousins, R.J. Changes in Rat Liver Metallothionein and Metallothionein mRNA Induced by Isopropanol. Exp. Biol. Med. 1984, 175, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Del Razo, L.M.; Quintanilla-Vega, B.; Brambila-Colombres, E.; Aranda, E.S.C.; Manno, M.; Albores, A. Stress Proteins Induced by Arsenic. Toxicol. Appl. Pharmacol. 2001, 177, 132–148. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).