Reduced Hypoxia Tolerance and Altered Gill Morphology at Elevated Temperatures May Limit the Survival of Tilapia (GIFT, Oreochromis niloticus) under Global Warming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Fish Stock and Rearing Conditions
2.3. Respiratory Metabolism Experiment
2.4. Acute Hypoxia Trial
2.5. Histological Analysis
2.6. Transcriptome Analysis
2.7. Reverse-Transcription Quantitative Real-Time PCR (RT-qPCR)
2.8. Statistical Analyses
3. Results
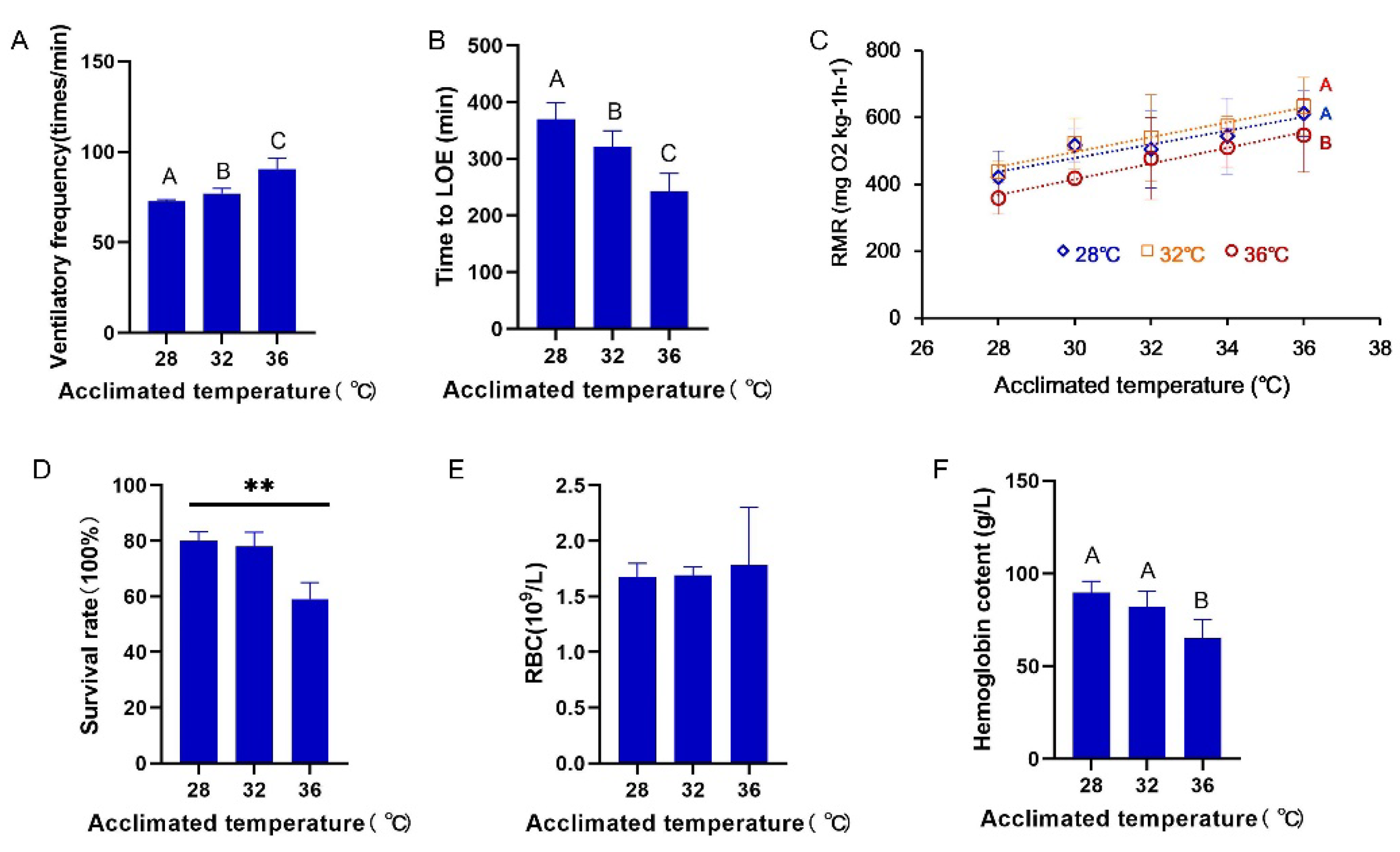
3.1. Ventilatory Frequency, Routine Metabolism, and Survival
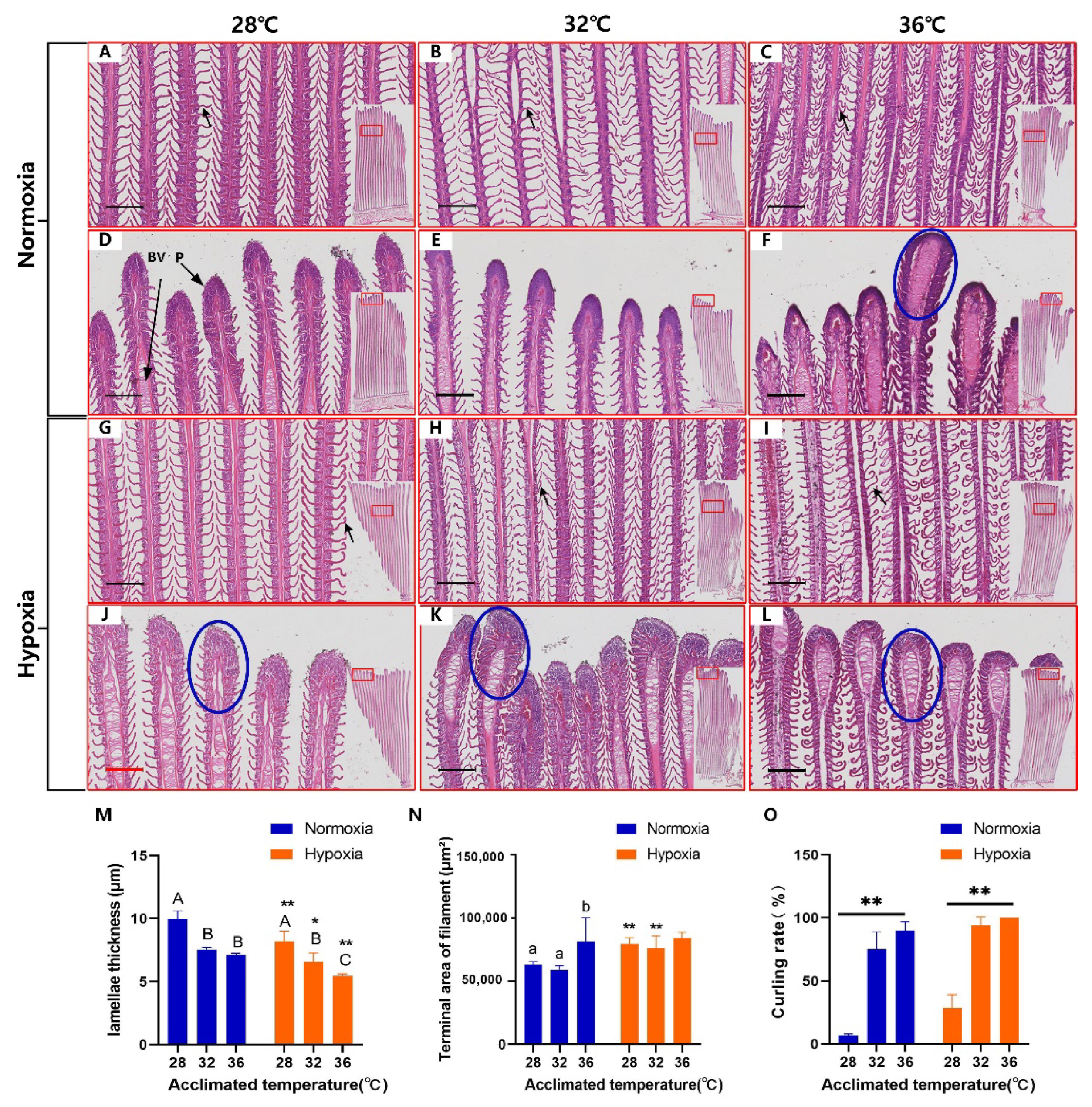
3.2. Gill Deformation in Response to High Temperatures
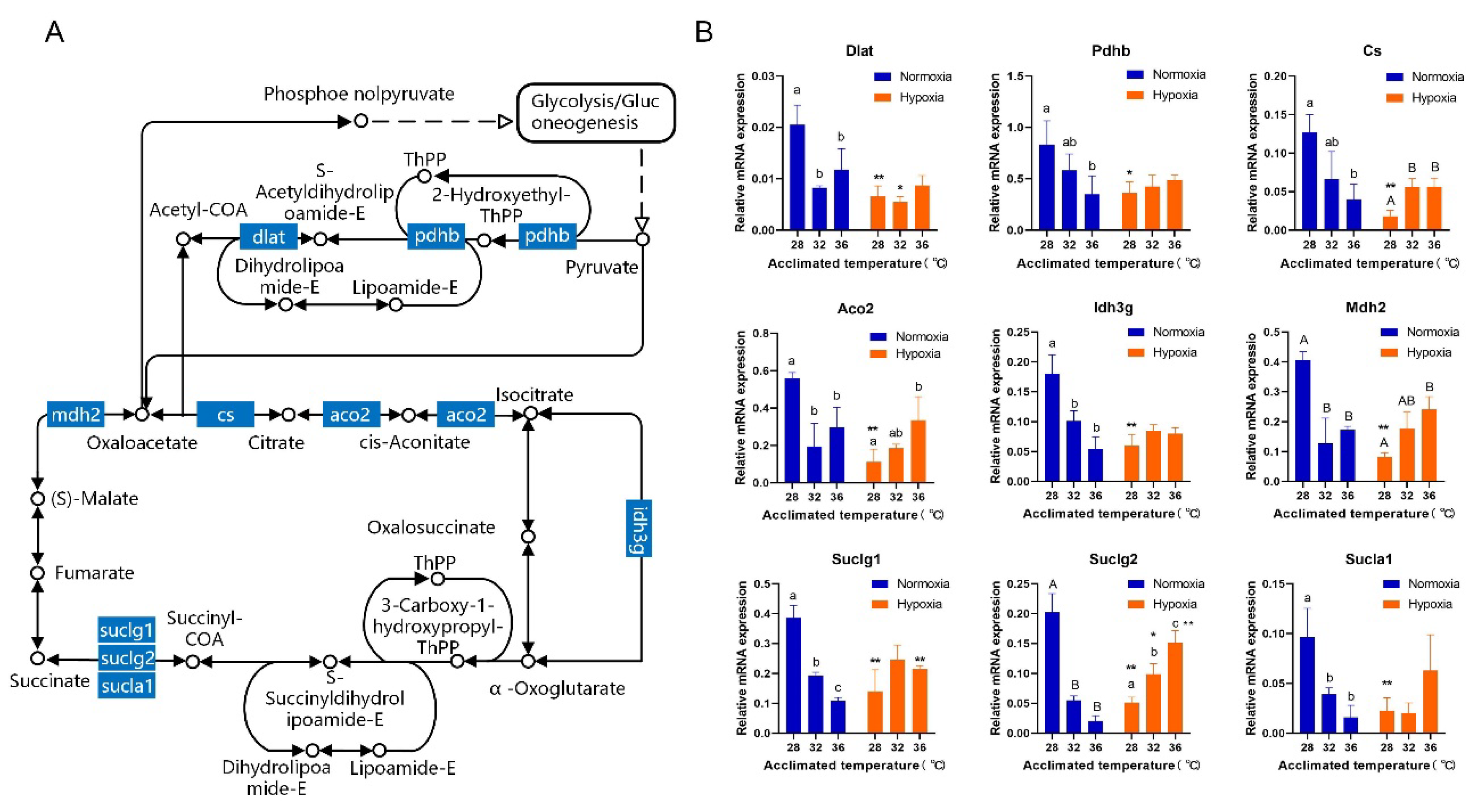
3.3. Gill Transcriptome Analysis
4. Discussion
4.1. Hypoxia Tolerance Is Reduced under Long-Term Exposure to High Temperatures
4.2. Thinning of the Lamellae in Response to High Temperatures
4.3. RMR Is Partially Compensated during Long-Term Warm Exposure
4.4. Threat of Global Warming to Tilapia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO: Fishery and Aquaculture Statistics. Global Production by Production Source 1950–2019 (FishstatJ); FAO Fisheries Division: Rome, Italy, 2021. [Google Scholar]
- Nivelle, R.; Gennotte, V.; Kalala, E.J.K.; Ngoc, N.B.; Muller, M.; Melard, C.; Rougeot, C. Temperature preference of Nile tilapia (Oreochromis niloticus) juveniles induces spontaneous sex reversal. PLoS ONE 2019, 14, e0212504. [Google Scholar]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Bierkens, M.F.P.; Huijbregts, M.A.J.; Schipper, A.M. Threats of global warming to the world’s freshwater fishes. Nat. Commun. 2021, 12, 1701–1710. [Google Scholar] [CrossRef]
- Rubalcaba, J.G.; Verberk, W.C.E.P.; Hendriks, A.J.; Saris, B.; Woods, H.A. Oxygen limitation may affect the temperature and size dependence of metabolism in aquatic ectotherms. Proc. Natl. Acad. Sci. USA 2020, 117, 31963–31968. [Google Scholar] [CrossRef]
- Nilsson, G.E. Respiratory Physiology of Vertebrates; Cambridge University Press: Cambridge, UK, 2010; p. 96. [Google Scholar]
- Pörtner, H.O.; Farrell, A.P. Ecology Physiology and Climate Change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef]
- Fry, F.E.J.; Hoar, W.S.; Randall, D.J. The Effect of Environmental Factors on the Physiology of Fish, Fish Physiology; Academic Press: New York, NY, USA, 1971; Volume VI, p. 83. [Google Scholar]
- Beuvard, C.; Imsland, A.K.D.; Thorarensen, H. The effect of temperature on growth performance and aerobic metabolic scope in Arctic charr, Salvelinus alpinus. J. Therm. Biol. 2022, 104, 103117. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Bock, C.; Mark, F.C. Oxygen- and capacity-limited thermal tolerance: Bridging ecology and physiology. J. Exp. Biol. 2017, 220, 2685–2696. [Google Scholar] [CrossRef]
- Li, S.N.; Wang, R.J.; Dai, Z.L.; Wang, C.; Wu, Z.L. Dietary supplementation with Yucca schidigera extract alleviated heat stress-induced unfolded protein response and oxidative stress in the intestine of Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2021, 219, 112299. [Google Scholar] [CrossRef]
- Habibah, A.N.; Pfennig, F.; Wilting, J.; Holtz, W.; Hoerstgen-Schwark, G.; Wessels, S. Germline Development of Genetically Female Nile tilapia (Oreochromis niloticus) Reared under Different Temperature Regimes. Sex. Dev. 2017, 11, 217–224. [Google Scholar] [CrossRef]
- Schulte, P.M.; Healy, T.M.; Fangue, N.A. Thermal Performance Curves, Phenotypic Plasticity, and the Time Scales of Temperature Exposure. Integr. Comp. Biol. 2011, 51, 691–702. [Google Scholar] [CrossRef]
- Bennett, J.M.; Sunday, J.; Calosi, P.; Villalobos, F.; Martinez, B.; Molina-Venegas, R.; Araujo, M.B.; Algar, A.C.; Clusella-Trullas, S.; Hawkins, B.A.; et al. The evolution of critical thermal limits of life on Earth. Nat. Commun. 2021, 12, 1198–1206. [Google Scholar] [CrossRef]
- Qu, Y.F.; Wiens, J.J. Higher temperatures lower rates of physiological and niche evolution. Proc. Biol. Sci. 2020, 287, 823–831. [Google Scholar] [CrossRef]
- Whalen, M.A.; Whippo, R.D.B.; Stachowicz, J.J.; York, P.H.; Aiello, E.; Alcoverro, T.; Altieri, A.H.; Benedetti-Cecchi, L.; Bertolini, C.; Bresch, M.; et al. Climate drives the geography of marine consumption by changing predator communities. Proc. Natl. Acad. Sci. USA 2020, 117, 28160–28166. [Google Scholar] [CrossRef]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Munoz-Cueto, J.A. Climate change impacts on fish reproduction are mediated at multiple levels of the brain-pituitary-gonad axis. Gen. Comp. Endocrinol. 2020, 291, 113439–113453. [Google Scholar] [CrossRef]
- Seebacher, F.; Brand, M.D.; Else, P.L.; Guderley, H.; Hulbert, A.J.; Moyes, C.D. Plasticity of oxidative metabolism in variable climates: Molecular mechanisms. Physiol. Biochem. Zool 2010, 83, 721–732. [Google Scholar] [CrossRef]
- Sandblom, E.; Clark, T.D.; Grans, A.; Ekstrom, A.; Brijs, J.; Sundstrom, L.F.; Odelstrom, A.; Adill, A.; Aho, T.; Jutfelt, F. Physiological constraints to climate warming in fish follow principles of plastic floors and concrete ceilings. Nat. Commun. 2016, 7, 11447. [Google Scholar] [CrossRef]
- Pörtner, H.O. Oxygen- and capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef]
- Jung, E.H.; Brix, K.V.; Richards, J.G.; Val, A.L.; Brauner, C.J. Reduced hypoxia tolerance and survival at elevated temperatures may limit the ability of Amazonian fishes to survive in a warming world. Sci. Total Environ. 2020, 748, 141349. [Google Scholar] [CrossRef]
- Eliason, E.J.; Anttila, K. Temperature and the Cardiovascular System. Fish Physiol. 2017, 36, 235–297. [Google Scholar]
- Jorgensen, S.M.; Castro, V.; Krasnov, A.; Torgersen, J.; Timmerhaus, G.; Hevroy, E.M.; Hansen, T.J.; Susort, S.; Breck, O.; Takle, H. Cardiac responses to elevated seawater temperature in Atlantic salmon. BMC Physiol. 2014, 14, 2–12. [Google Scholar] [CrossRef]
- Nyboer, E.A.; Chapman, L.J. Cardiac plasticity influences aerobic performance and thermal tolerance in a tropical, freshwater fish at elevated temperatures. J. Exp. Biol. 2018, 221, jeb178087. [Google Scholar] [CrossRef]
- Hu, P.; Liu, M.; Zhang, D.; Wang, J.; Niu, H.; Liu, Y.; Wu, Z.; Han, B.; Zhai, W.; Shen, Y.; et al. Global identification of the genetic networks and cis-regulatory elements of the cold response in zebrafish. Nucleic Acids Res. 2015, 43, 9198–9213. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liu, M.; Liu, Y.; Wang, J.; Zhang, D.; Niu, H.; Jiang, S.; Wang, J.; Zhang, D.; Han, B.; et al. Transcriptome comparison reveals a genetic network regulating the lower temperature limit in fish. Sci. Rep. 2016, 6, 28952. [Google Scholar] [CrossRef] [PubMed]
- Khaw, H.L.; Ponzoni, R.W.; Danting, M.J.C. Estimation of genetic change in the GIFT strain of Nile tilapia (Oreochromis niloticus) by comparing contemporary progeny produced by males born in 1991 or in 2003. Aquaculture 2008, 275, 64–69. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Lavender, E.; Fox, C.J.; Burrows, M.T. Modelling the impacts of climate change on thermal habitat suitability for shallow-water marine fish at a global scale. PLoS ONE 2021, 16, e0258184. [Google Scholar]
- Carilo Filho, L.M.; de Carvalho, B.T.; Azevedo, B.K.A.; Gutierrez-Pesquera, L.M.; Mira-Mendes, C.V.; Sole, M.; Orrico, V.G.D. Natural history predicts patterns of thermal vulnerability in amphibians from the Atlantic Rainforest of Brazil. Ecol. Evol. 2021, 11, 16462–16472. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Morley, S.; Peck, L.; Sunday, J.; Heiser, S.; Bates, A. Physiological acclimation and persistence of ectothermic species under extreme heat events. Global Ecol. Biogeogr. 2019, 28, 1018–1037. [Google Scholar] [CrossRef]
- McArley, T.J.; Hickey, A.J.R.; Herbert, N.A. Hyperoxia increases maximum oxygen consumption and aerobic scope of intertidal fish facing acutely high temperatures. J. Exp. Biol. 2018, 221, jeb189993. [Google Scholar] [CrossRef] [PubMed]
- Giomi, F.; Portner, H.O. A role for haemolymph oxygen capacity in heat tolerance of eurythermal crabs. Front Physiol. 2013, 4, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Shuang, L.; Su, X.L.; Zheng, G.D.; Zou, S.M. Effects of hypoxia and reoxygenation on gill remodeling, apoptosis, and oxidative stress in hypoxia-tolerant new variety blunt snout bream (Megalobrama amblycephala). Fish Physiol. Biochem. 2022, 48, 263–274. [Google Scholar] [CrossRef]
- Sandersfeld, T.; Davison, W.; Lamare, M.D.; Knust, R.; Richter, C. Elevated temperature causes metabolic trade-offs at the whole-organism level in the Antarctic fish Trematomus bernacchii. J. Exp. Biol. 2015, 218, 2373–2381. [Google Scholar] [CrossRef]
- Strobel, A.; Bennecke, S.; Leo, E.; Mintenbeck, K.; Portner, H.O.; Mark, F.C. Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and PCO2. Front Zool 2012, 9, 28–42. [Google Scholar] [CrossRef]
- Auer, S.K.; Agreda, E.; Chen, A.H.; Irshad, M.; Solowey, J. Late-stage pregnancy reduces upper thermal tolerance in a live-bearing fish. J. Therm. Biol. 2021, 99, 103022. [Google Scholar] [CrossRef]
- Marshall, D.J.; McQuaid, C.D. Warming reduces metabolic rate in marine snails: Adaptation to fluctuating high temperatures challenges the metabolic theory of ecology. Proc. Biol. Sci. 2011, 278, 281–288. [Google Scholar] [CrossRef]

| Treatment Temperature (°C) | PH | Dissolved Oxygen (mg/L) | Total Ammonia (mg/L) |
|---|---|---|---|
| 27.92 ± 0.29 °C | 7.32 ± 0.29 | 4.28 ± 0.56 | 1.57 ± 0.05 |
| 31.93 ± 0.26 °C | 7.60 ± 0.22 | 4.13 ± 0.47 | 1.30 ± 0.22 |
| 35.98 ± 0.34 °C | 7.45 ± 0.11 | 3.97 ± 0.77 | 1.67 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, Y.; Wei, S.; Li, W.; Li, W.; Wu, Z.; Jiang, S.; Lu, Y.; Xu, Q.; Chen, L. Reduced Hypoxia Tolerance and Altered Gill Morphology at Elevated Temperatures May Limit the Survival of Tilapia (GIFT, Oreochromis niloticus) under Global Warming. Fishes 2022, 7, 216. https://doi.org/10.3390/fishes7050216
Zhou Y, Zhang Y, Wei S, Li W, Li W, Wu Z, Jiang S, Lu Y, Xu Q, Chen L. Reduced Hypoxia Tolerance and Altered Gill Morphology at Elevated Temperatures May Limit the Survival of Tilapia (GIFT, Oreochromis niloticus) under Global Warming. Fishes. 2022; 7(5):216. https://doi.org/10.3390/fishes7050216
Chicago/Turabian StyleZhou, Yan, Yanjie Zhang, Shang Wei, Wei Li, Wenhao Li, Zhichao Wu, Shouwen Jiang, Ying Lu, Qianghua Xu, and Liangbiao Chen. 2022. "Reduced Hypoxia Tolerance and Altered Gill Morphology at Elevated Temperatures May Limit the Survival of Tilapia (GIFT, Oreochromis niloticus) under Global Warming" Fishes 7, no. 5: 216. https://doi.org/10.3390/fishes7050216
APA StyleZhou, Y., Zhang, Y., Wei, S., Li, W., Li, W., Wu, Z., Jiang, S., Lu, Y., Xu, Q., & Chen, L. (2022). Reduced Hypoxia Tolerance and Altered Gill Morphology at Elevated Temperatures May Limit the Survival of Tilapia (GIFT, Oreochromis niloticus) under Global Warming. Fishes, 7(5), 216. https://doi.org/10.3390/fishes7050216