Abundance and Growth of the European Eels (Anguilla anguilla Linnaeus, 1758) in Small Estuarine Habitats from the Eastern English Channel
Abstract
1. Introduction
2. Materials and Methods
2.1. Eels Sampling
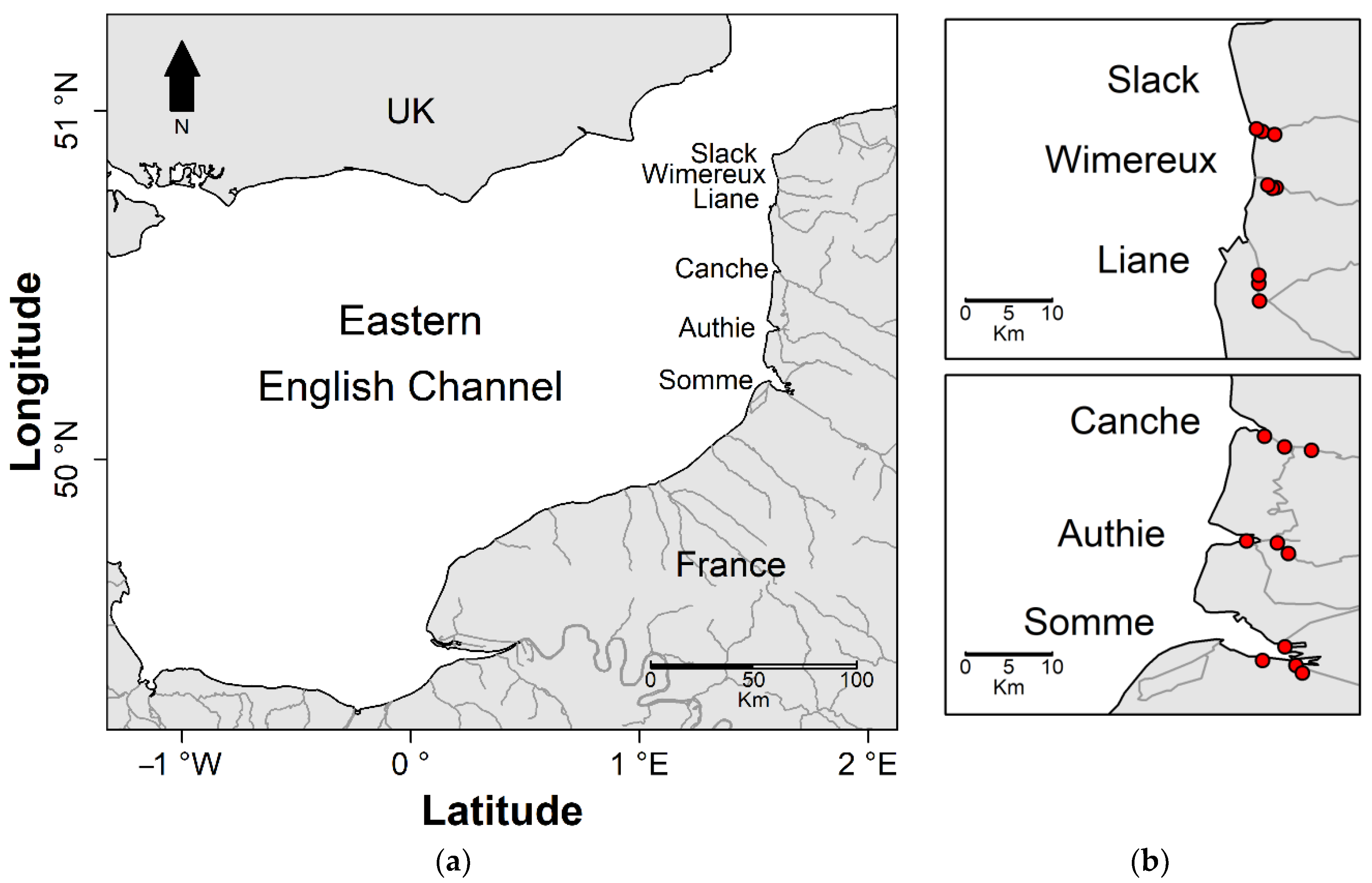
2.2. Sampling Areas Description
2.3. Sample Processing
2.4. Eel Abundance, Total Length and Weight
2.5. Length–Weight Relationship
2.6. Growth Model Selection
2.7. Growth Increments
2.8. PCA Analysis
3. Results
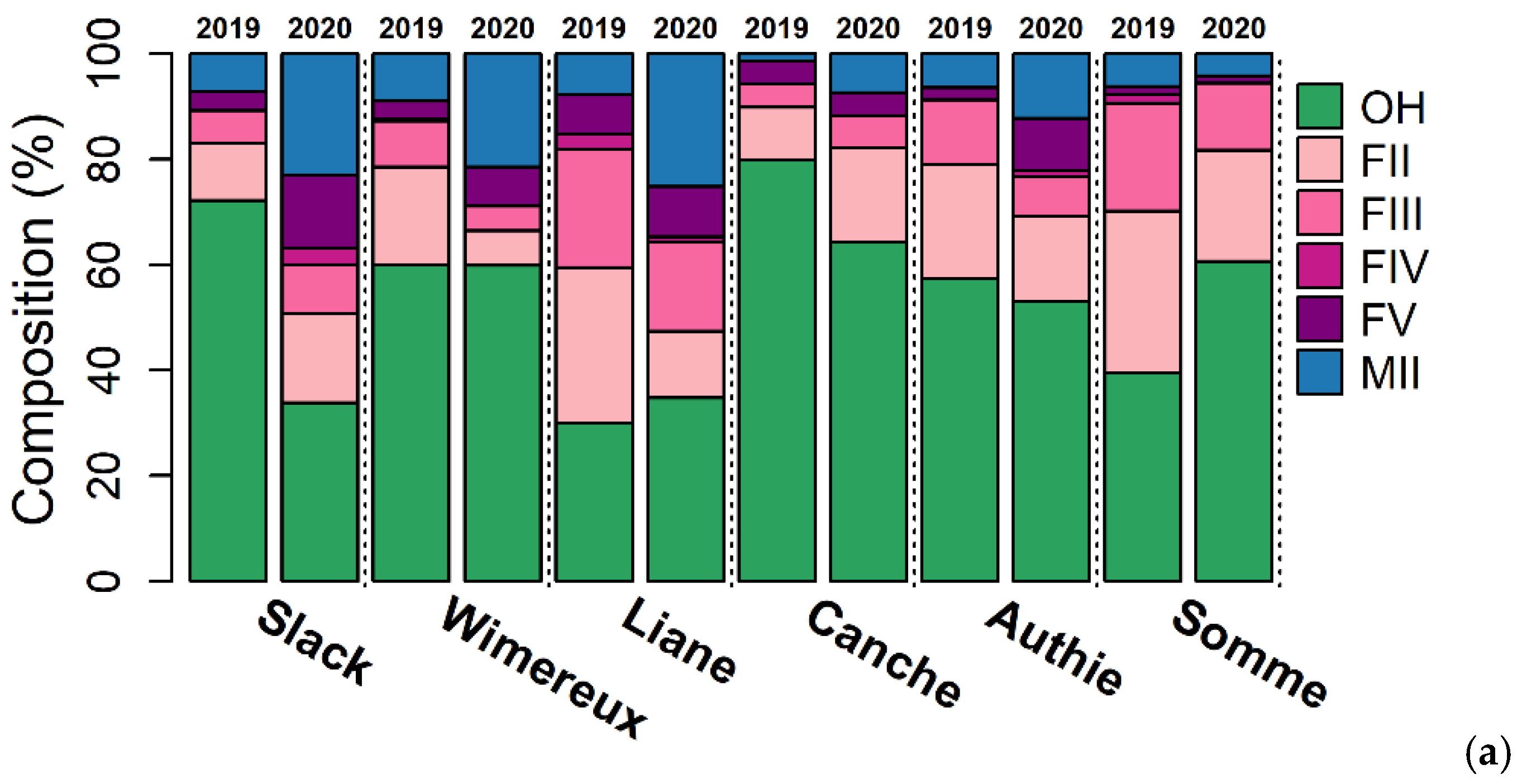
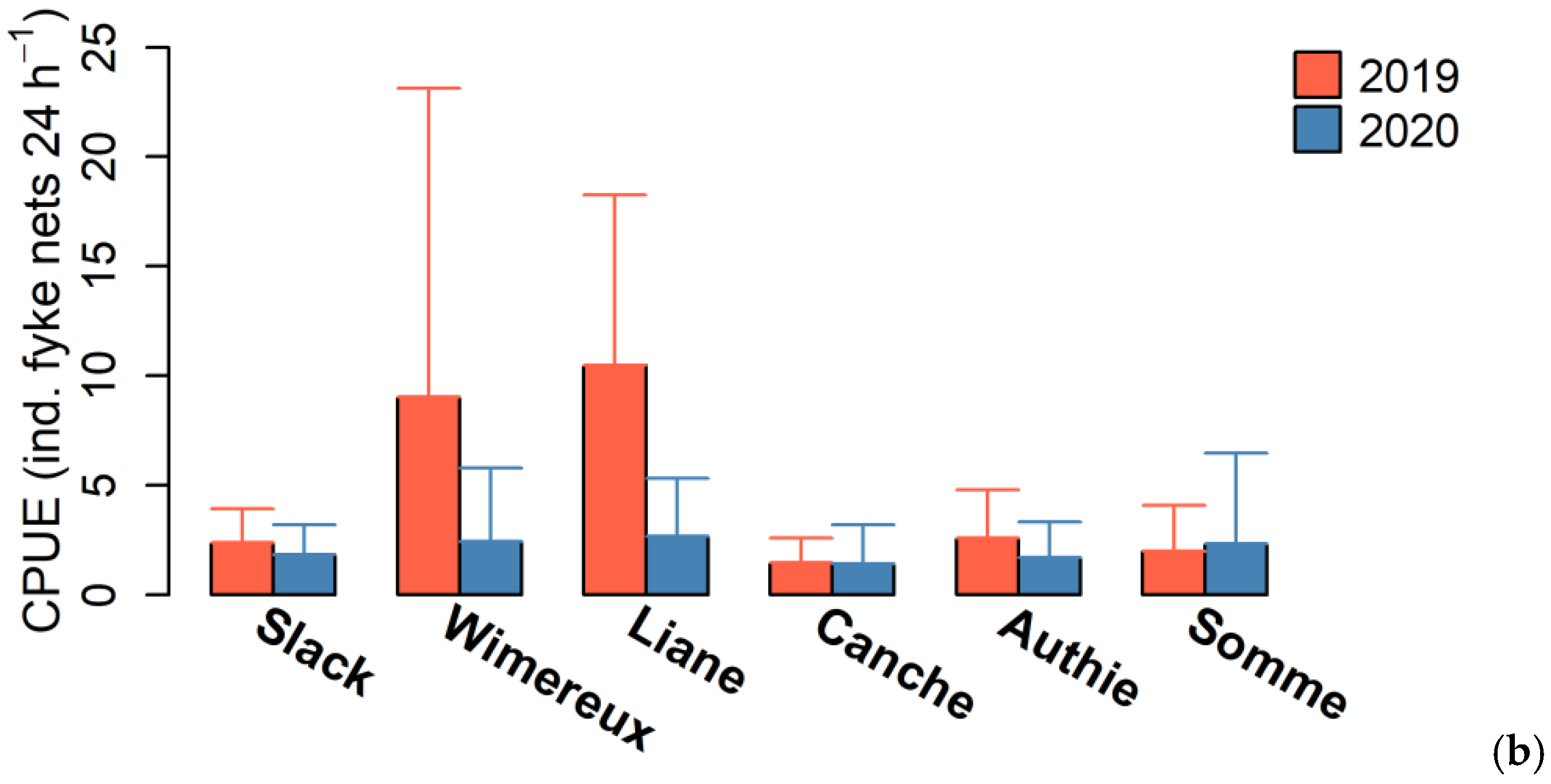
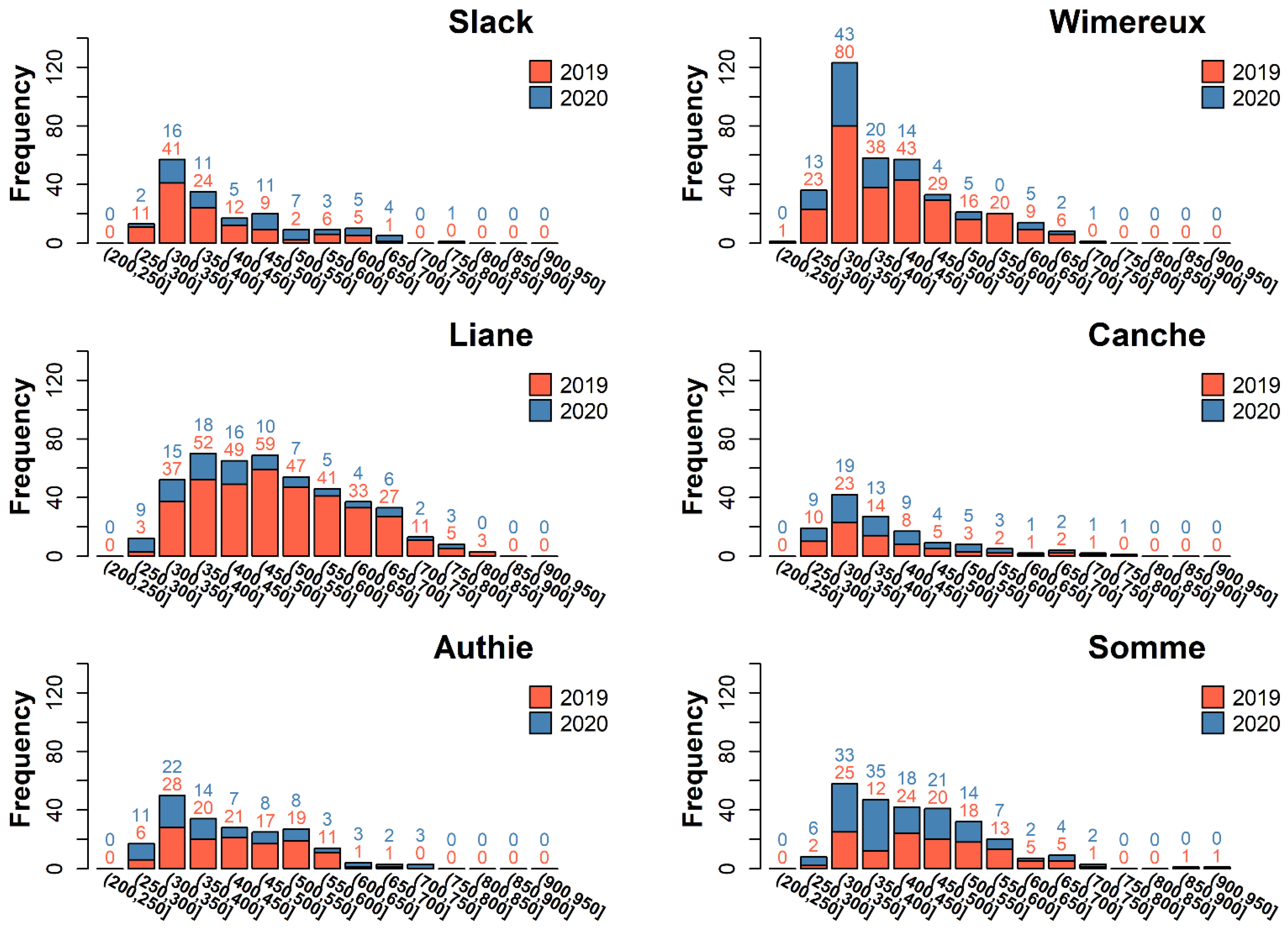
3.1. Eel Population Characteristics
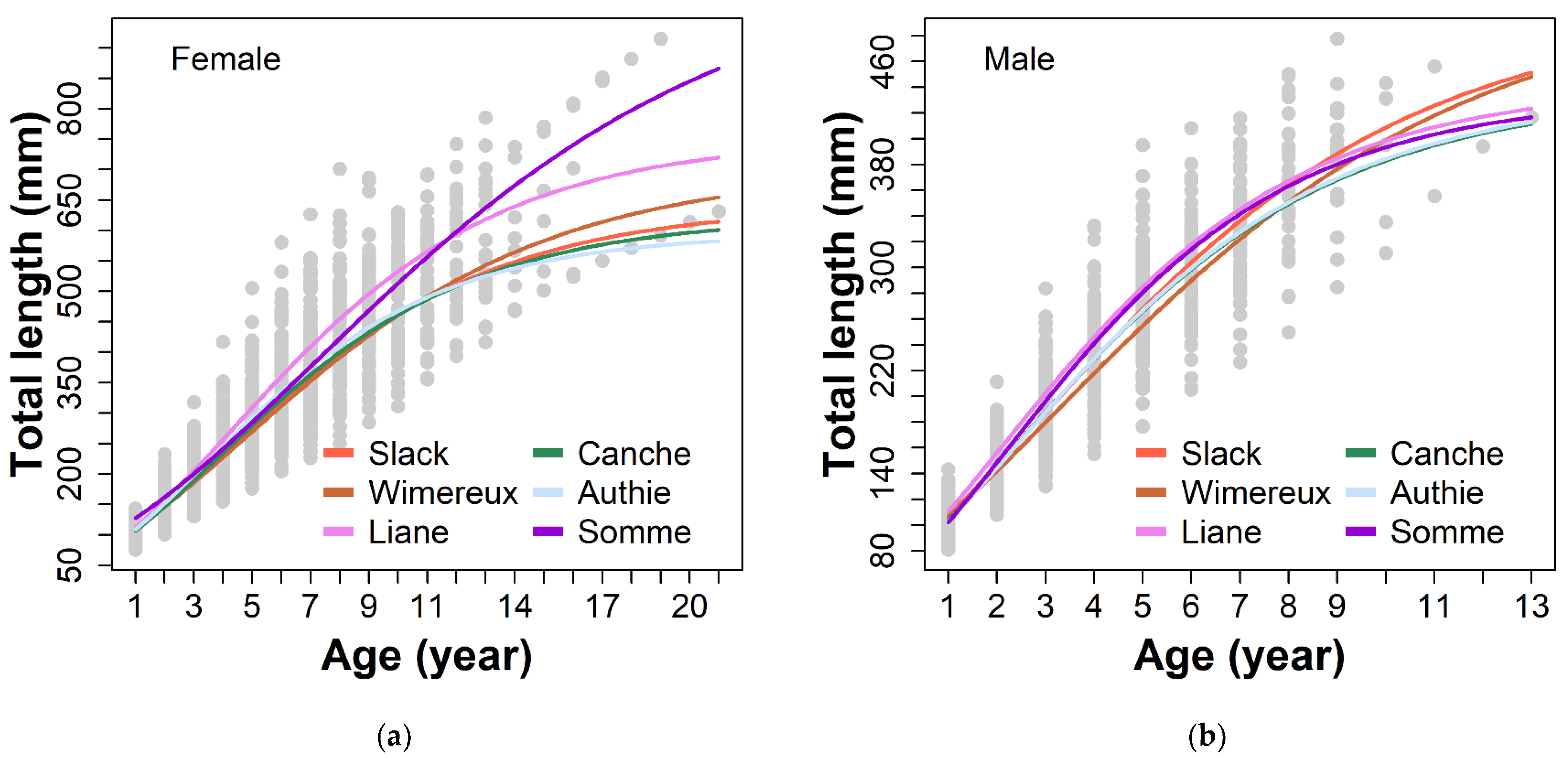
3.2. Growth Model
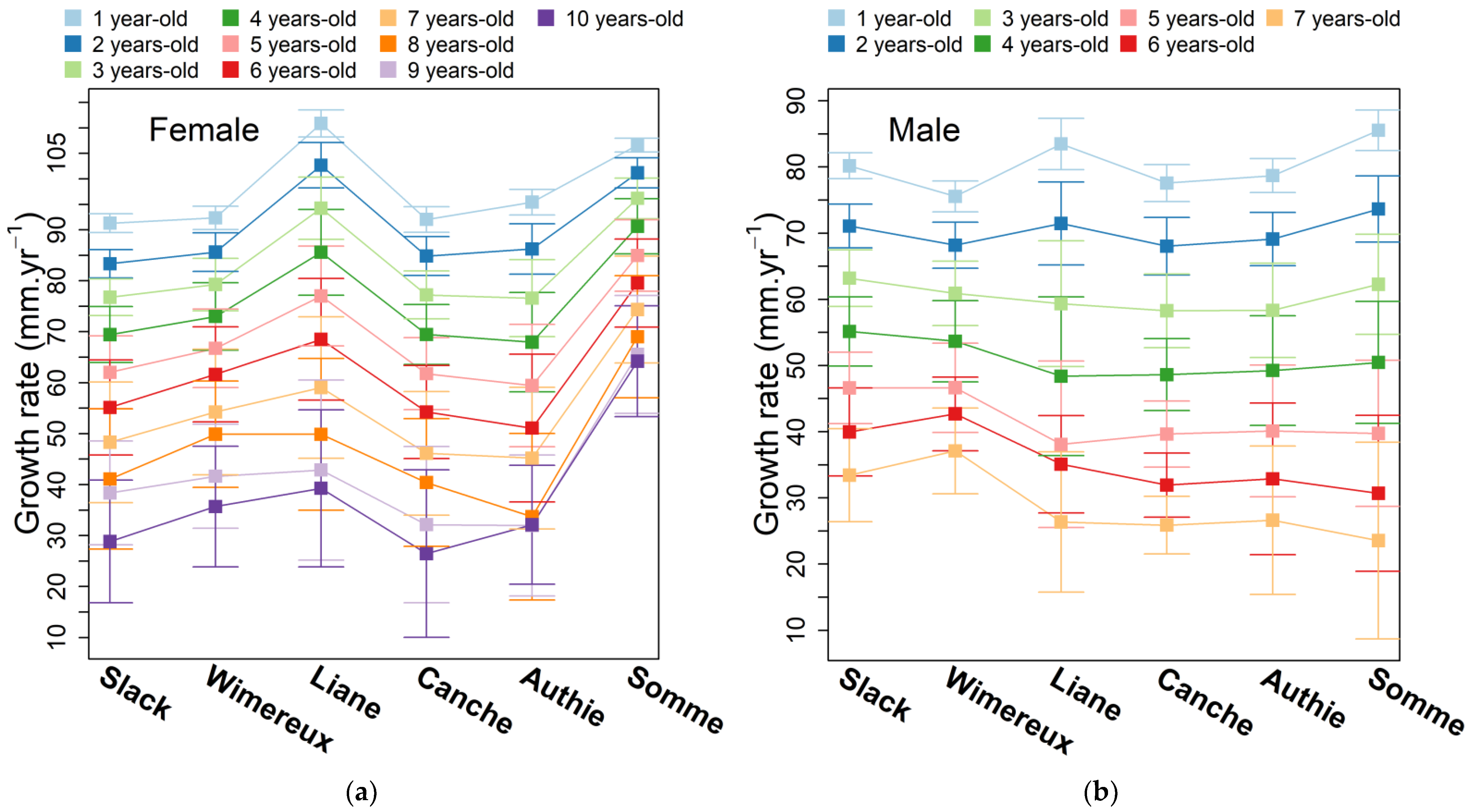
3.3. Annual Growth Rate
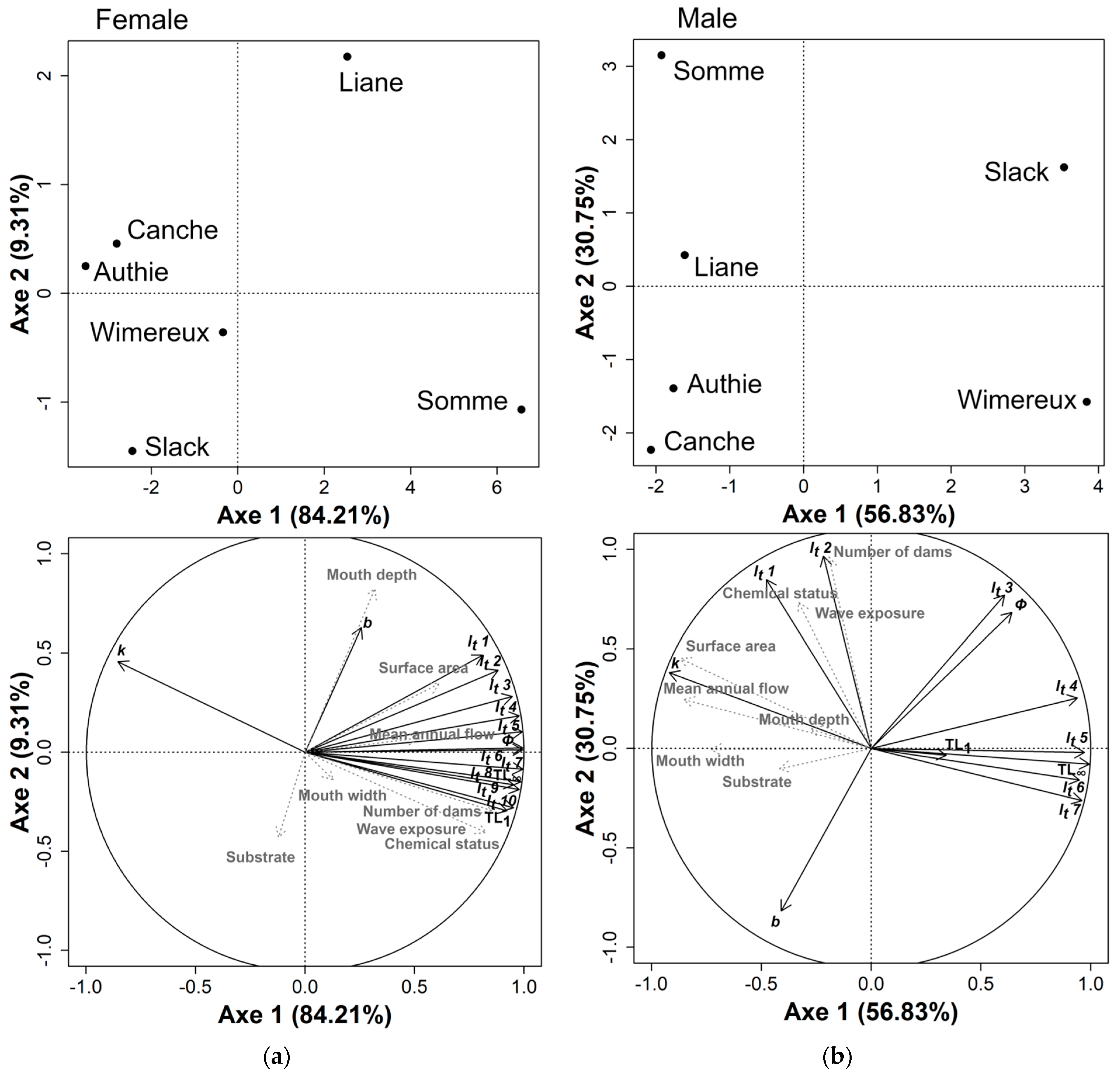
3.4. Local Habitat Characteristics Influence on Eel Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dekker, W. On the Distribution of the European Eel (Anguilla anguilla) and Its Fisheries. Can. J. Fish. Aquat. Sci. 2003, 60, 787–799. [Google Scholar] [CrossRef]
- Belpaire, C.G.J.; Goemans, G.; Geeraerts, C.; Quataert, P.; Parmentier, K.; Hagel, P.; De Boer, J. Decreasing Eel Stocks: Survival of the Fattest? Ecol. Freshw. Fish 2009, 18, 197–214. [Google Scholar] [CrossRef]
- Couillard, C.M.; Verreault, G.; Dumont, P.; Stanley, D.; Threader, R.W. Assessment of Fat Reserves Adequacy in the First Migrant Silver American Eels of a Large-Scale Stocking Experiment. N. Am. J. Fish. Manag. 2014, 34, 802–813. [Google Scholar] [CrossRef]
- van Ginneken, V.J.T.; van den Thillart, G.E.E.J.M. Eel Fat Stores Are Enough to Reach the Sargasso. Nature 2000, 403, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Jessop, B.M. Geographic Effects on American Eel (Anguilla Rostrata) Life History Characteristics and Strategies. Can. J. Fish. Aquat. Sci. 2010, 67, 326–346. [Google Scholar] [CrossRef]
- Vélez-Espino, L.A.; Koops, M.A. A Synthesis of the Ecological Processes Influencing Variation in Life History and Movement Patterns of American Eel: Towards a Global Assessment. Rev. Fish Biol. Fish. 2010, 20, 163–186. [Google Scholar] [CrossRef]
- Daverat, F.; Beaulaton, L.; Poole, R.; Lambert, P.; Wickström, H.; Andersson, J.; Aprahamian, M.; Hizem, B.; Elie, P.; Yalçın-Özdilek, S.; et al. One Century of Eel Growth: Changes and Implications: One Century of Eel Growth. Ecol. Freshw. Fish 2012, 21, 325–336. [Google Scholar] [CrossRef]
- Panfili, J.; Ximénès, M.-C.; Crivelli, A.J. Sources of Variation in Growth of the European Eel (Anguilla anguilla) Estimated from Otoliths. Can. J. Fish. Aquat. Sci. 1994, 51, 506–515. [Google Scholar] [CrossRef]
- Aprahamian, M.W.; Walker, A.M.; Williams, B.; Bark, A.; Knights, B. On the Application of Models of European Eel (Anguilla anguilla) Production and Escapement to the Development of Eel Management Plans: The River Severn. ICES J. Mar. Sci. 2007, 64, 1472–1482. [Google Scholar] [CrossRef]
- Daverat, F.; Tomás, J. Tactics and Demographic Attributes in the European Eel Anguilla anguilla in the Gironde Watershed, SW France. Mar. Ecol. Prog. Ser. 2006, 307, 247–257. [Google Scholar] [CrossRef]
- Tesch, F.W.; Rohlf, N. Migration from Continental Waters to the Spawning Grounds. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 223–234. ISBN 978-4-431-65907-5. [Google Scholar]
- Thibault, I.; Dodson, J.; Caron, F.; Tzeng, W.; Iizuka, Y.; Shiao, J. Facultative Catadromy in American Eels: Testing the Conditional Strategy Hypothesis. Mar. Ecol. Prog. Ser. 2007, 344, 219–229. [Google Scholar] [CrossRef]
- Daverat, F.; Tomas, J.; Lahaye, M.; Palmer, M.; Elie, P. Tracking Continental Habitat Shifts of Eels Using Otolith Sr/Ca Ratios: Validation and Application to the Coastal, Estuarine and Riverine Eels of the Gironde–Garonne–Dordogne Watershed. Mar. Freshw. Res. 2005, 56, 619–627. [Google Scholar] [CrossRef]
- Shiao, J.C.; Ložys, L.; Iizuka, Y.; Tzeng, W.N. Migratory Patterns and Contribution of Stocking to the Population of European Eel in Lithuanian Waters as Indicated by Otolith Sr:Ca Ratios. J. Fish Biol. 2006, 69, 749–769. [Google Scholar] [CrossRef]
- Tabouret, H.; Bareille, G.; Claverie, F.; Pécheyran, C.; Prouzet, P.; Donard, O.F.X. Simultaneous Use of Strontium:Calcium and Barium:Calcium Ratios in Otoliths as Markers of Habitat: Application to the European Eel (Anguilla anguilla) in the Adour Basin, South West France. Mar. Environ. Res. 2010, 70, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Melià, P.; Bevacqua, D.; Crivelli, A.J.; Panfili, J.; De Leo, G.A.; Gatto, M. Sex Differentiation of the European Eel in Brackish and Freshwater Environments: A Comparative Analysis. J. Fish Biol. 2006, 69, 1228–1235. [Google Scholar] [CrossRef]
- Sadler, K. Effects of Temperature on the Growth and Survival of the European Eel, Anguilla anguilla L. J. Fish Biol. 1979, 15, 499–507. [Google Scholar] [CrossRef]
- Tesch, F.W. The Eel; Blackwell Science: Oxford, UK, 2003; Volume 15, ISBN 0-632-06389-0. [Google Scholar]
- Costa, J.L.; Domingos, I.; Assis, C.A.; Almeida, P.R.; Moreira, F.; Feunteun, E.; Costa, M.J. Comparative Ecology of the European Eel, Anguilla anguilla (L., 1758), in a Large Iberian River. Environ. Biol. Fishes 2008, 81, 421–434. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a Metabolic Theory of Ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Correia, M.J.; Domingos, I.; De Leo, G.A.; Costa, J.L. A Comparative Analysis of European Eel’s Somatic Growth in the Coastal Lagoon Santo André (Portugal) with Growth in Other Estuaries and Freshwater Habitats. Environ. Biol. Fishes 2021, 104, 837–850. [Google Scholar] [CrossRef]
- Fernéndez-Delgado, C.; Hernando, J.A.; Herrera, M.; Bellido, M. Age and Growth of Yellow Eels, Anguilla anguilla, in the Estuary of the Guadalquivir River (South-West Spain). J. Fish Biol. 1989, 34, 561–570. [Google Scholar] [CrossRef]
- Kullmann, B.; Thiel, R. Bigger Is Better in Eel Stocking Measures? Comparison of Growth Performance, Body Condition, and Benefit-Cost Ratio of Simultaneously Stocked Glass and Farmed Eels in a Brackish Fjord. Fish. Res. 2018, 205, 132–140. [Google Scholar] [CrossRef]
- Melia, P.; Bevacqua, D.; Crivelli, A.J.; De Leo, G.A.; Panfili, J.; Gatto, M. Age and Growth of Anguilla anguilla in the Camargue Lagoons. J. Fish Biol. 2006, 68, 876–890. [Google Scholar] [CrossRef]
- Patey, G.; Couillard, C.M.; Drouineau, H.; Verreault, G.; Pierron, F.; Lambert, P.; Baudrimont, M.; Couture, P. Early Back-Calculated Size-at-Age of Atlantic Yellow Eels Sampled along Ecological Gradients in the Gironde and St. Lawrence Hydrographical Systems. Can. J. Fish. Aquat. Sci. 2018, 75, 1270–1279. [Google Scholar] [CrossRef]
- Simon, J.; Ubl, C.; Dorow, M. Growth of European Eel Anguilla anguilla along the Southern Baltic Coast of Germany and Implication for the Eel Management. Environ. Biol. Fishes 2013, 96, 1073–1086. [Google Scholar] [CrossRef]
- Vaughan, L.; Brophy, D.; O’Toole, C.; Graham, C.; Ó Maoiléidigh, N.; Poole, R. Growth Rates in a European Eel Anguilla anguilla (L., 1758) Population Show a Complex Relationship with Temperature over a Seven-Decade Otolith Biochronology. ICES J. Mar. Sci. 2021, 78, 994–1009. [Google Scholar] [CrossRef]
- De Leo, G.A.; Gatto, M. A Size and Age-Structured Model of the European Eel (Anguilla anguilla L.). Can. J. Fish. Aquat. Sci. 1995, 52, 1351–1367. [Google Scholar] [CrossRef]
- Rossi, R.; Colombo, G. Sex Ratio, Age and Growth of Silver Eels, in Two Brackish Lagoons in the Northern Adriatic (Valli of Comacchio and Valle Nuova). Arch. Oceanogr. Limnol. 1976, 18, 227–310. [Google Scholar]
- Bird, D.J.; Rotchell, J.M.; Hesp, S.A.; Newton, L.C.; Hall, N.G.; Potter, I.C. To What Extent Are Hepatic Concentrations of Heavy Metals in Anguilla anguilla at a Site in a Contaminated Estuary Related to Body Size and Age and Reflected in the Metallothionein Concentrations? Environ. Pollut. 2008, 151, 641–651. [Google Scholar] [CrossRef]
- Teichert, N.; Carassou, L.; Sahraoui, Y.; Lobry, J.; Lepage, M. Influence of Intertidal Seascape on the Functional Structure of Fish Assemblages: Implications for Habitat Conservation in Estuarine Ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 798–809. [Google Scholar] [CrossRef]
- Nicolas, D.; Lobry, J.; Le Pape, O.; Boët, P. Functional Diversity in European Estuaries: Relating the Composition of Fish Assemblages to the Abiotic Environment. Estuar. Coast. Shelf Sci. 2010, 88, 329–338. [Google Scholar] [CrossRef]
- Basilone, G.; Guisande, C.; Patti, B.; Mazzola, S.; Cuttitta, A.; Bonanno, A.; Kallianiotis, A. Linking Habitat Conditions and Growth in the European Anchovy (Engraulis encrasicolus). Fish. Res. 2004, 68, 9–19. [Google Scholar] [CrossRef]
- Hayes, D.B.; Ferreri, C.P.; Taylor, W.W. Linking Fish Habitat to Their Population Dynamics. Can. J. Fish. Aquat. Sci. 1996, 53, 383–390. [Google Scholar] [CrossRef]
- Sogard, S. Variability in Growth Rates of Juvenile Fishes in Different Estuarine Habitats. Mar. Ecol. Prog. Ser. 1992, 85, 35–53. [Google Scholar] [CrossRef]
- Copp, G.H.; Daverat, F.; Bašić, T. The Potential Contribution of Small Coastal Streams to the Conservation of Declining and Threatened Diadromous Fishes, Especially the European Eel. River Res. Appl. 2021, 37, 111–115. [Google Scholar] [CrossRef]
- Cucherousset, J.; Paillisson, J.-M.; Carpentier, A.; Thoby, V.; Damien, J.-P.; Eybert, M.-C.; Feunteun, E.; Robinet, T. Freshwater Protected Areas: An Effective Measure to Reconcile Conservation and Exploitation of the Threatened European Eels (Anguilla anguilla)? Ecol. Freshw. Fish 2007, 16, 528–538. [Google Scholar] [CrossRef]
- Selleslagh, J.; Lesourd, S.; Amara, R. Comparison of Macrobenthic Assemblages of Three Fish Estuarine Nurseries and Their Importance as Foraging Grounds. J. Mar. Biol. Assoc. UK 2011, 92, 85–97. [Google Scholar] [CrossRef]
- Durif, C.; Dufour, S.; Elie, P. The Silvering Process of Anguilla anguilla: A New Classification from the Yellow Resident to the Silver Migrating Stage. J. Fish Biol. 2005, 66, 1025–1043. [Google Scholar] [CrossRef]
- Kushnirov, D.; Degani, G. Sexual Dimorphism in Yellow European Eels, Anguilla anguilla (L.). Aquac. Res. 1995, 26, 409–414. [Google Scholar] [CrossRef]
- Sahyoun, R.; Claudet, J.; Fazio, G.; Silva, C.D.; Lecomte-Finiger, R. The Otolith as Stress Indicator of Parasitism on European Eel. Vie Milieu 2007, 57, 193–200. [Google Scholar]
- ICES. Workshop on Age Reading of European and American Eel (WKAREA). In Proceedings of the Workshop on Age Reading of European and American Eel (WKAREA), Bordeaux, France, 20–24 April 2009. [Google Scholar]
- Meunier, F.J. Données sur la croissance de l’anguille (Anguilla anguilla L.) dans le cours moyen du Rhin, région alsacienne. Bull. Fr. Pêche Piscic. 1994, 335, 133–144. [Google Scholar] [CrossRef][Green Version]
- Poole, W.R.; Reynolds, J.D. Age and Growth of Yellow Eel, Anguilla anguilla (L.), Determined by Two Different Methods. Ecol. Freshw. Fish 1996, 5, 86–95. [Google Scholar] [CrossRef]
- Froese, R. Cube Law, Condition Factor and Weight–Length Relationships: History, Meta-Analysis and Recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Le Cren, E.D. The Length-Weight Relationship and Seasonal Cycle in Gonad Weight and Condition in the Perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Ricker, W. Computation and Interpretation of Biological Statistics of Fish Populations. Bul. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Francis, R.I.C.C. Back-Calculation of Fish Length: A Critical Review. J. Fish Biol. 1990, 36, 883–902. [Google Scholar] [CrossRef]
- Campana, S.E. How Reliable Are Growth Back-Calculations Based on Otoliths? Can. J. Fish. Aquat. Sci. 1990, 47, 2219–2227. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. A Quantitative Theory of Organic Growth (Inquiries on Growth Laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Gompertz, B., XXIV. On the Nature of the Function Expressive of the Law of Human Mortality, and on a New Mode of Determining the Value of Life Contingencies. In a Letter to Francis Baily, Esq. F. R. S. &c. Philos. Trans. R. Soc. Lond. 1825, 115, 513–583. [Google Scholar] [CrossRef]
- Verhulst, P.F. Notice on the Law That a Population Follows in Its Growth. Corr. Math. Phys. 1838, 10, 113–121. [Google Scholar]
- Knight, W. Asymptotic Growth: An Example of Nonsense Disguised as Mathematics. J. Fish. Res. Board Can. 1968, 25, 1303–1307. [Google Scholar] [CrossRef]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ishiguro, M.; Kitagawa, G. Akaike Information Criterion Statistics; D. Reidel: Dordrecht, The Netherlands, 1986; Volume 81, p. 26853. [Google Scholar]
- Pauly, D. On the Interrelationships between Natural Mortality, Growth Parameters, and Mean Environmental Temperature in 175 Fish Stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Pauly, D. Gill Size and Temperature as Governing Factors in Fish Growth: A Generalization of von Bertalanffy’s Growth Formula. Ph.D. Thesis, University of Kiel, Kiel, Germany, Institut für Meereskunde, Hamburg, Germany, 1979. [Google Scholar]
- Pauly, D.; Munro, J.L. Once More on the Comparison of Growth in Fish and Invertebrates. Fishbyte 1984, 2, 1–21. [Google Scholar]
- Husson, F.; Josse, J.; Le, S.; Mazet, J.; Husson, M.F. Package ‘FactoMineR.’ Multivariate Exploratory Data Analysis and Data Mining. 2017. Available online: http://cran.r-project.org/web/packages/FactoMineR/FactoMineR.pdf (accessed on 10 August 2022).
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL). ICES Sci. Rep. 2020, 2, 223. [Google Scholar] [CrossRef]
- Bark, A.; Williams, B.; Knights, B. Current Status and Temporal Trends in Stocks of European Eel in England and Wales. ICES J. Mar. Sci. 2007, 64, 1368–1378. [Google Scholar] [CrossRef]
- Feunteun, E.; Acou, A.; Guillouët, J.; Laffaille, P.; Legault, A. Spatial Distribution of an Eel Population (Anguilla anguilla L.) in a Small Coastal Catchment of Northern Brittany (France). Consequences of Hydraulic Works. Bull. Fr. Pêche Piscic. 1998, 349, 129–139. [Google Scholar] [CrossRef]
- Laffaille, P.; Feunteun, E.; Baisez, A.; Robinet, T.; Acou, A.; Legault, A.; Lek, S. Spatial Organisation of European Eel (Anguilla anguilla L.) in a Small Catchment. Ecol. Freshw. Fish 2003, 12, 254–264. [Google Scholar] [CrossRef]
- Davey, A.J.H.; Jellyman, D.J. Sex Determination in Freshwater Eels and Management Options for Manipulation of Sex. Rev. Fish Biol. Fish. 2005, 15, 37–52. [Google Scholar] [CrossRef]
- Geffroy, B.; Bardonnet, A. Sex Differentiation and Sex Determination in Eels: Consequences for Management. Fish Fish. 2016, 17, 375–398. [Google Scholar] [CrossRef]
- Drouineau, H.; Durif, C.; Castonguay, M.; Mateo, M.; Rochard, E.; Verreault, G.; Yokouchi, K.; Lambert, P. Freshwater Eels: A Symbol of the Effects of Global Change. Fish Fish. 2018, 19, 903–930. [Google Scholar] [CrossRef]
- Laffaille, P.; Acou, A.; Guillouët, J.; Mounaix, B.; Legault, A. Patterns of Silver Eel (Anguilla anguilla L.) Sex Ratio in a Catchment. Ecol. Freshw. Fish 2006, 15, 583–588. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Schiavina, M.; Crivelli, A.J.; De Leo, G.A.; Gatto, M. A Demographic Model for the Conservation and Management of the European Eel: An Application to a Mediterranean Coastal Lagoon. ICES J. Mar. Sci. 2019, 76, 2164–2178. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Gatto, M.; De Leo, G.A. A Global Viability Assessment of the European Eel. Glob. Chang. Biol. 2015, 21, 3323–3335. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-S.; Tzeng, W.-N. Use of the Sex Ratio as a Means of Resource Assessment for the Japanese Eel Anguilla japonica: A Case Study in the Kaoping River, Taiwan. Zool. Stud. 2006, 45, 255–263. [Google Scholar]
- Jones, V.R.P.; Sinha, W.J. On the Sex and Distribution of the Freshwater Eel (Anguilla anguilla). J. Zool. 1966, 150, 371–385. [Google Scholar] [CrossRef]
- Boulenger, C.; Acou, A.; Trancart, T.; Crivelli, A.J.; Feunteun, E. Length-Weight Relationships of the Silver European Eel, Anguilla anguilla (Linnaeus, 1758), across Its Geographic Range. J. Appl. Ichthyol. 2015, 31, 427–430. [Google Scholar] [CrossRef]
- Barcala, E.; Romero, D.; Bulto, C.; Boza, C.; Peñalver, J.; María-Dolores, E.; Muñoz, P. An Endangered Species Living in an Endangered Ecosystem: Population Structure and Growth of European Eel Anguilla anguilla in a Mediterranean Coastal Lagoon. Reg. Stud. Mar. Sci. 2022, 50, 102163. [Google Scholar] [CrossRef]
- Piria, M.; Šprem, N.; Tomljanović, T.; Slišković, M.; Jelić Mrčelić, G.; Treer, T. Length Weight Relationships of the European Eel Anguilla anguilla (Linnaeus, 1758) from Six Karst Catchments of the Adriatic Basin, Croatia. Croat. J. Fish. 2014, 72, 32–35. [Google Scholar] [CrossRef]
- Vøllestad, L.A. Geographic Variation in Age and Length at Metamorphosis of Maturing European Eel: Environmental Effects and Phenotypic Plasticity. Br. Ecol. Soc. 1992, 61, 9. [Google Scholar] [CrossRef]
- Robinet, T.; Feunteun, E. Sublethal Effects of Exposure to Chemical Compounds: A Cause for the Decline in Atlantic Eels? Ecotoxicology 2002, 11, 265–277. [Google Scholar] [CrossRef]
- Acou, A.; Robinet, T.; Lance, E.; Gerard, C.; Mounaix, B.; Brient, L.; Le Rouzic, B.; Feunteun, E. Evidence of Silver Eels Contamination by Microcystin-LR at the Onset of Their Seaward Migration: What Consequences for Breeding Potential? J. Fish Biol. 2008, 72, 753–762. [Google Scholar] [CrossRef]
- Moriarty, C. Age Determination and Growth Rate of Eels, Anguilla anguilla (L). J. Fish Biol. 1983, 23, 257–264. [Google Scholar] [CrossRef]
- Morais, R.A.; Bellwood, D.R. Global Drivers of Reef Fish Growth. Fish Fish. 2018, 19, 874–889. [Google Scholar] [CrossRef]
- Munro, J.L.; Pauly, D. A Simple Method for Comparing the Growth of Fishes and Invertebrates. Fishbyte 1983, 1, 5–6. [Google Scholar]
- Kullmann, B.; Pohlmann, J.-D.; Freese, M.; Keth, A.; Wichmann, L.; Neukamm, R.; Thiel, R. Age-Based Stock Assessment of the European Eel (Anguilla anguilla) Is Heavily Biased by Stocking of Unmarked Farmed Eels. Fish. Res. 2018, 208, 258–266. [Google Scholar] [CrossRef]
- Gordo, L.S.; Jorge, M.I. Age and growth of the european eel, Anguilla anguilla (Linnaeus, 1758) in the Aveiro Lagoon, Portugal. Sci. Mar. 1991, 55, 389–395. [Google Scholar]
- O’Connor, W. Biology and Management of European Eel (Anguilla anguilla, L) in the Shannon Estuary, Ireland. Ph.D. Thesis, National University of Ireland, Galway, Ireland, 2003. [Google Scholar]
- Mann, R.H.K.; Blackburn, J.H. The Biology of the Eel Anguilla anguilla (L.) in an English Chalk Stream and Interactions with Juvenile Trout Salmo Trutta L. and Salmon Salmo salar L. Hydrobiologia 1991, 218, 65–76. [Google Scholar] [CrossRef]
- Rasmussen, G.; Therkildsen, B. Food, Growth and Production of Anguilla anguilla in a Small Danish Stream. Rapp. Procès-Verbaux Réun. Cons. Int. Pour Explor. Mer 1979, 174, 32–40. [Google Scholar]
- Vøllestad, L.A.; Jonsson, B. Life-History Characteristics of the European Eel Anguilla anguilla in the Imsa River, Norway. Trans. Am. Fish. Soc. 1986, 115, 864–871. [Google Scholar] [CrossRef]
- Paulovits, G.; Biro, P. Age Determination and Growth of Eel, Anguilla anguilla (L.), in Lake FertS, Hungary. Fish 1986, 4, 101–110. [Google Scholar] [CrossRef]
- Le Pape, O.; Bonhommeau, S. The Food Limitation Hypothesis for Juvenile Marine Fish. Fish Fish. 2015, 16, 373–398. [Google Scholar] [CrossRef]
- Denis, J.; Rabhi, K.; Le Loc’h, F.; Ben Rais Lasram, F.; Boutin, K.; Kazour, M.; Diop, M.; Gruselle, M.-C.; Amara, R. Role of Estuarine Habitats for the Feeding Ecology of the European Eel (Anguilla anguilla L.). PLoS ONE 2022, 17, e0270348. [Google Scholar] [CrossRef] [PubMed]
- Feunteun, E. Management and Restoration of European Eel Population (Anguilla anguilla): An Impossible Bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Haenen, O.L.M.; Mladineo, I.; Konecny, R.; Yoshimizu, M.; Groman, D.; Muñoz, P.; Saraiva, A.; Bergmann, S.M.; van Beurden, S.J. Diseases of Eels in an International Perspective: Workshop on Eel Diseases at the 15th International Conference on Diseases of Fish and Shellfish, Split, Croatia, 2011. Bull. Eur. Assoc. Fish Pathol. 2012, 32, 109–115. [Google Scholar]
- Muñoz, P.; Barcala, E.; Peñalver, J.; Romero, D. Can Inorganic Elements Affect Herpesvirus Infections in European Eels? Environ. Sci. Pollut. Res. 2019, 26, 35266–35269. [Google Scholar] [CrossRef]
- Delrez, N.; Zhang, H.; Lieffrig, F.; Mélard, C.; Farnir, F.; Boutier, M.; Donohoe, O.; Vanderplasschen, A. European Eel Restocking Programs Based on Wild-Caught Glass Eels: Feasibility of Quarantine Stage Compatible with Implementation of Prophylactic Measures Prior to Scheduled Reintroduction to the Wild. J. Nat. Conserv. 2021, 59, 125933. [Google Scholar] [CrossRef]
- Legrand, M.; Briand, C.; Buisson, L.; Artur, G.; Azam, D.; Baisez, A.; Barracou, D.; Bourré, N.; Carry, L.; Caudal, A.-L.; et al. Contrasting Trends between Species and Catchments in Diadromous Fish Counts over the Last 30 Years in France. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 7. [Google Scholar] [CrossRef]
- Waldman, J.; Wilson, K.A.; Mather, M.; Snyder, N.P. A Resilience Approach Can Improve Anadromous Fish Restoration. Fisheries 2016, 41, 116–126. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Brown, C.J.; Dwyer, R.G.; Harding, D.J.; Roberts, D.T.; Fuller, R.A.; Linke, S.; Possingham, H.P. Impacts of Fishing, River Flow and Connectivity Loss on the Conservation of a Migratory Fish Population. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 45–54. [Google Scholar] [CrossRef]
- Baudoin, J.-M.; Burgun, V.; Chanseau, M.; Larinier, M.; Ovidio, M.; Sremski, W.; Steinbach, P.; Voegtle, B. Évaluer le Franchissement des Obstacles par les Poissons: Principes et Méthodes: Informations sur la Continuité Ecologique, ICE; ONEMA: Vincennes, France, 2014; ISBN 979-10-91047-29-6. [Google Scholar]
- Itakura, H.; Kitagawa, T.; Miller, M.J.; Kimura, S. Declines in Catches of Japanese Eels in Rivers and Lakes across Japan: Have River and Lake Modifications Reduced Fishery Catches? Landsc. Ecol. Eng. 2015, 11, 147–160. [Google Scholar] [CrossRef]
- Costa, J.L.; Assis, C.A.; Almeida, P.R.; Moreira, F.M.; Costa, M.J. On the Food of the European Eel, Anguilla anguilla (L.), in the Upper Zone of the Tagus Estuary, Portugal. J. Fish Biol. 1992, 41, 841–850. [Google Scholar] [CrossRef]
| Tested Parameters | Estuary | Female | Male | ||||
|---|---|---|---|---|---|---|---|
| a | b | R2 | a | b | R2 | ||
| %95 CL | %95 CL | %95 CL | %95 CL | ||||
| TL-W | Slack | −12.8 | 2.9 | 0.82 | −11.7 | 2.7 | 0.93 |
| −19.4–6.3 | 1.9–4.0 | −19.4–6.3 | 1.9–4.0 | ||||
| Wimereux | −17.2 | 3.6 | 0.89 | −12.9 | 3.0 | 0.91 | |
| −21.8–12.5 | 2.9–4.4 | −21.8–12.5 | 2.9–4.4 | ||||
| Liane | −17.0 | 3.6 | 0.89 | −13.4 | 3.0 | 0.92 | |
| −20.3–13.7 | 3.1–4.1 | −20.3–13.7 | 3.1–4.1 | ||||
| Canche | −16.7 | 3.5 | 0.91 | −13.5 | 3.0 | 0.87 | |
| −20.4–13.0 | 3.0–4.1 | −20.4–13.0 | 3.0–4.1 | ||||
| Authie | −14.3 | 3.2 | 0.95 | −13.0 | 2.8 | 0.94 | |
| −16.4–12.2 | 2.8–3.5 | −16.4–12.2 | 2.8–3.5 | ||||
| Somme | −15.3 | 3.3 | 0.94 | −11.9 | 2.8 | 0.87 | |
| −17.6–12.9 | 2.9–3.7 | −17.6–12.9 | 2.9–3.7 | ||||
| TL-Ro | Slack | 15.0 | 0.27 | 0.76 | 132.0 | 0.17 | 0.73 |
| −92.0–121.9 | 0.21–0.34 | 68.7–195.4 | 0.12–0.22 | ||||
| Wimereux | 41.9 | 0.25 | 0.75 | 209.0 | 0.11 | 0.32 | |
| −42.6–126.4 | 0.20–0.31 | 128.6–289.3 | 0.05–0.17 | ||||
| Liane | −45.6 | 0.33 | 0.67 | 205.5 | 0.12 | 0.23 | |
| −180.5–89.4 | 0.25–0.41 | 65.2–345.8 | 0.02–0.22 | ||||
| Canche | 17.5 | 0.30 | 0.70 | 174.1 | 0.14 | 0.30 | |
| −86.1–121.2 | 0.22–0.37 | 54.6–293.5 | 0.04–0.24 | ||||
| Authie | 27.0 | 0.28 | 0.64 | 47.5 | 0.24 | 0.69 | |
| −78.2–132.1 | 0.21–0.35 | −52.4–147.4 | 0.17–0.31 | ||||
| Somme | 22.4 | 0.30 | 0.71 | 198.6 | 0.13 | 0.43 | |
| −94.8–139.5 | 0.23–0.37 | 88.6–308.5 | 0.06–0.21 | ||||
| Estuary | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| TL∞ %95 CL | TL1% 95 CL | k %95 CL | Φ | TL∞ %95 CL | TL1 %95 CL | k %95 CL | Φ | |
| Slack | 641 | 109 | 0.19 | 57.6 | 500 | 107 | 0.23 | 55.9 |
| 574–708 | 97–122 | 0.15–0.22 | 436–564 | 99–114 | 0.18–0.27 | |||
| Wimereux | 701 | 111 | 0.16 | 57.9 | 516 | 106 | 0.20 | 55.7 |
| 601–800 | 99–123 | 0.13–0.20 | 416–616 | 98–115 | 0.15–0.25 | |||
| Liane | 751 | 112 | 0.19 | 59.2 | 442 | 110 | 0.29 | 55.7 |
| 647–855 | 98–126 | 0.15–0.23 | 367–518 | 96–125 | 0.20–0.38 | |||
| Canche | 621 | 107 | 0.20 | 57.5 | 437 | 104 | 0.27 | 55.2 |
| 576–667 | 96–118 | 0.17–0.22 | 391–482 | 97–111 | 0.22–0.31 | |||
| Authie | 596 | 109 | 0.22 | 57.5 | 437 | 103 | 0.27 | 55.3 |
| 523–669 | 96–122 | 0.17–0.26 | 387–488 | 90–115 | 0.21–0.33 | |||
| Somme | 1047 | 127 | 0.12 | 60.6 | 433 | 102 | 0.30 | 55.7 |
| 873–1221 | 114–141 | 0.10–0.14 | 370–497 | 88–116 | 0.22–0.39 | |||
| Sex | Estuary | Slack | Wimereux | Liane | Canche | Authie |
|---|---|---|---|---|---|---|
| Female | Wimereux | <0.001 | ||||
| Liane | <0.001 | <0.001 | ||||
| Canche | 0.999 | <0.001 | <0.001 | |||
| Authie | 0.721 | <0.001 | <0.001 | 0.906 | ||
| Somme | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Male | Wimereux | 0.706 | ||||
| Liane | <0.001 | 0.056 | ||||
| Canche | <0.001 | <0.001 | 0.315 | |||
| Authie | <0.001 | <0.001 | 0.746 | 0.974 | ||
| Somme | <0.05 | 0.458 | 0.947 | <0.05 | 0.195 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denis, J.; Mahé, K.; Amara, R. Abundance and Growth of the European Eels (Anguilla anguilla Linnaeus, 1758) in Small Estuarine Habitats from the Eastern English Channel. Fishes 2022, 7, 213. https://doi.org/10.3390/fishes7050213
Denis J, Mahé K, Amara R. Abundance and Growth of the European Eels (Anguilla anguilla Linnaeus, 1758) in Small Estuarine Habitats from the Eastern English Channel. Fishes. 2022; 7(5):213. https://doi.org/10.3390/fishes7050213
Chicago/Turabian StyleDenis, Jérémy, Kélig Mahé, and Rachid Amara. 2022. "Abundance and Growth of the European Eels (Anguilla anguilla Linnaeus, 1758) in Small Estuarine Habitats from the Eastern English Channel" Fishes 7, no. 5: 213. https://doi.org/10.3390/fishes7050213
APA StyleDenis, J., Mahé, K., & Amara, R. (2022). Abundance and Growth of the European Eels (Anguilla anguilla Linnaeus, 1758) in Small Estuarine Habitats from the Eastern English Channel. Fishes, 7(5), 213. https://doi.org/10.3390/fishes7050213








