1. Introduction
Photovoltaic (PV) power generation, which can replace fossil energy, is essential to mitigate climate change. According to reports, the global annual PV generation level will reach 6970 TWh in 2030 from 820 TWh in 2020, the average generation growth needs 24% during 2020–2030 [
1]. By the end of 2021, the installed capacity of PV power generation in China had reached 306 million KW, ranking first in the world for seven consecutive years [
2]. However, the assertive growth of photovoltaics will occupy a lot of land resources [
3]. This is in contradiction with agricultural development and food production. As an important economic crab, the Chinese mitten crab (
Eriocheir sinensis) is widely cultured in China, and the pond culture of
E. sinensis has developed rapidly in the past years. There is also a need to expand the culturing area. However, in recent years, the government has controlled the excavation of culturing ponds in China. Therefore, the aquavoltaic systems, which integrate aquaculture and PV power generation, offer a potential solution to this problem [
4,
5]. In recent years, more and more individuals have considered using aquaculture ponds to carry out PV power generation. For example, the floating photovoltaic (FPV) systems on aquaculture ponds could promote the production of the giant freshwater prawn (
Macrobrachium rosenbergii), tilapia (
Oreochromis niloticus) and milkfish (
Chanos chanos) [
6]. However, there is a lack of research on the feasibility of
E. sinensis culturing under PV panels.
As we know, the PV panels covering the pond will affect the light intensity and the air circulation on the surface of the water body and, in addition, the water temperature and dissolved oxygen (DO) can be influenced by the PV panels [
6,
7]. The light intensity, temperature, and DO have a direct impact on the growth and development of animals [
8,
9,
10]. For example, Tigan et al. (2020) confirmed that the larval Delta Smelt (
Hypomesus transpacificus) has a minimum threshold of light to generate its exogenous feeding response [
11]. However, this did not mean that the higher the light intensity, the better the growth of animals. Another study showed that the peak value of weight gain (WG) and specific growth rate (SGR) of juvenile mud crab (
Scylla paramamosain) is 10 W m
−2 rather than the higher light intensity [
9]. In the study, the WG and SGR of
S. paramamosain displayed a curvilinear response to light treatments [
9]. In addition, there is a positive correlation between water temperature and light intensity and the high temperature was not conducive to animal feeding and growth [
12]. In the hot summer season, PV panels could block sunlight and reduce the intensity of light and temperature rise [
13,
14]. However, we also need to consider the possible adverse effects of PV panels on animal growth in the spring and continuous rainy weather. After all, the low water temperature will also affect the growth of animals [
15]. Moreover, the PV panels may affect the photosynthesis of aquatic plants, resulting in the reduction of DO [
7]. In aquaculture, yield is an important measure of the success of aquavoltaic systems. Therefore, we must pay attention to the impact of PV panels on the growth of
E. sinensis.
The edible tissues (muscle, gonad, and hepatopancreas) of
E. sinensis usually attract consumers with their unique flavor, delicious taste, and rich nutrition. The nutrients, aromatic flavor, and umami flavor of cooked crabs are mainly derived from a large number of enriched amino acids and fatty acids [
16,
17]. Therefore, the amino acid composition, fatty acid composition, and proximate composition in edible tissues are important indicators to judge the quality of crabs. According to one report, light intensity could affect the biosynthesis of amino acids and unsaturated fatty acids in
S. paramamosain [
18]. Liu et al. (2020) also suggested that the amino acid composition and polyunsaturated fatty acids of muscles are influenced by the light intensity in giant clams (
Tridacna crocea) [
19]. Considering the potential impact of PV panels, we needed to analyze the amino acid, fatty acid, and proximate composition of the crabs, to ensure the final crab quality.
Moreover, the carapace, hepatopancreas, and ovaries color of crustaceans is also an important factor to attract customers [
20]. For example, consumers usually prefer the light grey and grey raw shrimp (
Litopenaeus vannamei), and orange and intense orange cooked shrimp [
21]. Therefore, the color of crabs directly affects consumers’ purchase decisions and becomes one of the important indicators to evaluate the value of crabs [
20]. According to the report, constant darkness treatment darkened the body color of
L. vannamei [
22]. Another study also confirmed that light intensity with 434 lux which is the highest in the study can darken the skin color of catfish (
Leiocassis longirostris Gunther) [
23]. The mentioned studies suggested that changes in light intensity may affect the color of crustaceans. Therefore, the effect of PV panels on the crabs’ color is needed to be further evaluated.
In this study, we integrated PV power generation and crab culturing for the first time. Previously, we had published some data from this study in another journal, including the effect of PV panels on the light intensity, water temperature, dissolved oxygen, and aquatic plant growth in the ponds culture of
E. sinensis [
24]. In this paper, we mainly focused on the effect of PV panels on the growth performance, amino acid, fatty acid, proximate composition, and tissue color of
E. sinensis. We aimed to evaluate the potential value of the aquavoltaic system to solve the competition for land resources between PV power generation and aquaculture.
4. Discussion
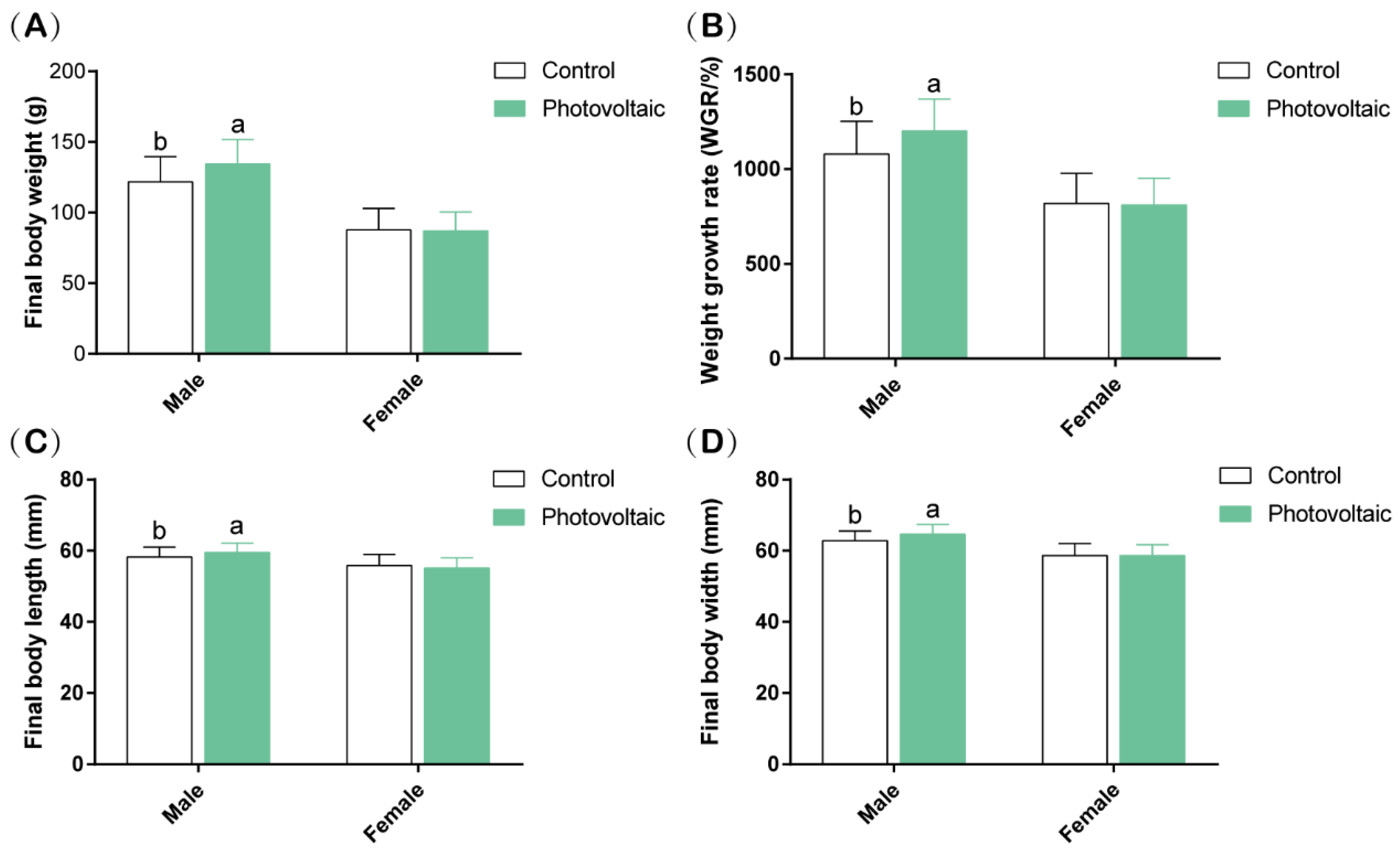
According to our results, the PV panels promoted the growth of
E. sinensis, especially the male crabs. Not coincidentally, Wang et al. (2022) reported that the FPV systems significantly improve the production of
M. rosenbergii,
O. niloticus, and
C. chanos. Moreover, another research also suggested that PV panels do not affect the growth of fish (
Pelteobagrus fulvidraco), and the proportion of 75% panels can increase fish production compared with the unshaded areas [
7]. Therefore, the aquavoltaic system has been proved to be beneficial to the increase of aquaculture production. The demand for light from aquatic animals is generally not high. For example, light (500 lux) lasting for 8 h could significantly improve the survival rate of larval fish (
Argyrosomus regius) rather than stronger light lasting longer [
29]. As for crustaceans, most of them are active at night [
30]. Not only that, Li et al. (2020a) also confirmed that
S. paramamosain has a higher survival under low light (1.43 μmol.m
−2·s
−1, white LED lights). According to our published data, the average underwater light intensity of the photovoltaic group during the daytime is 3255 ± 124 lux in this study [
24]. Therefore, the light intensity under the PV panels could meet the regular needs of
E. sinensis. In addition, the optimum growth temperature of
E. sinensis is generally 22–28 °C [
31]. However, the water temperature in summer is often higher than 30 °C. In this study, the water temperature of the photovoltaic group was significantly lower than that of the control group [
24]. That was very helpful for crabs to survive the hot summer season and might also be one of the reasons why the PV panel area is conducive to the growth of crabs. Moreover, the average OD in the PV area was more than 5 mg/L in this study [
24]. Generally, OD more significant than 3 mg/L can meet the average growth of
E. sinensis [
32]. According to this research, we suggested that the PV panels system can keep the regular needs of OD in the ponds culture of the crabs, and the PV panels could improve the animal growth in this study.
We should not only pay attention to the growth of crabs but also focus on whether the PV panels system affects the nutritional composition of edible tissues. A previous study reported that temperature is the strongest influencing factor that affects the fat (R = 0.73;
p < 0.001) and moisture (R = −0.73;
p < 0.001) of rainbow trout (
Oncorhynchus mykiss) [
33]. It could be seen that the temperature has a negative correlation with body moisture and a positive correlation with fat. In our study, the water temperature in the PV group was significantly lower than in the control group [
24]. According to our results, the moisture of muscles, hepatopancreas, and testes in the PV group significantly increased compared with the control group (
Table 1). At the same time, the total lipids of the hepatopancreas and muscles in the PV group significantly decreased. Therefore, we suggested that the PV panels affected the water temperature and then regulated the tissues’ moisture and total lipids. On the other hand, the light intensity could have induced an increase in triacylglycerols to avoid photochemical damage [
34]. Thus, the increase in total lipid might reduce cell damage in the control group. In addition, Güroy et al., (2011) suggested that much of the energy requirement of fish during starvation was satisfied from the fat or protein reserves [
35]. As we described in the previous paragraph that the crabs living in the PV group have a larger physique. Therefore, the lipids of the hepatopancreas and muscles might be more used to support body development in the PV group. In addition, we also found that the ash can be affected by the PV panels. However, there was no regular change in different groups. We needed further to explore the mechanism of the effect of light shading on the ash and other proximate compositions in the crabs.
This study found a significant difference in amino acid composition between males and females. This gender difference was also reflected in the growth performance as described in
Section 3.1. So far, there was no evidence that light intensity can lead to gender differences in animal growth and amino acid composition. However, a study has shown that high water temperature can accelerate the sexual maturation of hermaphrodite compared with male shrimp (
Eulimnadia braueriana Ishikawa) [
36]. In aquaculture, earlier sexual maturity is not conducive to individual growth [
37,
38]. From our results, the water temperature in the control group was significantly higher than in the PV group. Therefore, we conjectured that the effect of PV panels on the growth performance of different sexes may be related to the water temperature. In addition, there was also evidence suggesting that the hepatopancreas and gonads of male and female
E. sinensis have different developmental states in the same periods. For example, the GSI and gonad-hepatopancreas index (100 × Gonad wet weight/Hepatopancreas wet weight) of male
E. sinensis was significantly higher than the female in August–September but lower in October–November [
39]. Therefore, the difference in amino acid composition between males and females might be related to the developmental state of crabs at the sampling point (16 November) in this study. In fact, the amino acid composition in the freedom group has a significant difference compared with the control group or PV group (
Table 2,
Table 3 and
Table 4). According to a report, the Arg, Leu, Met, Pro, and Gly could be influenced by the change of light intensity in giant clams (
Tridacna crocea) [
19]. Li et al. (2020) confirmed that the light promoted the biosynthesis of Cys in
S. paramamosain, and previous studies also suggested that the photoperiod can regulate the content of Tyr in the Malaysian red tilapia and His in the shrimp (
Branchinecta orientalis) [
40,
41]. Our results also showed that the PV panels affected the content of eight amino acids in different tissues of
E. sinensis compared with the control group, including Met, Arg, Cys, Pro, Gly, Leu, Tyr, and His. Among these amino acids, the Gly content in the PV group increased in almost all tissues. As we know, Gly is a generally recognized sweet substance and can mask bitter flavors and saltiness [
42,
43]. The content of ∑EAA, ∑SEAA, ∑NEAA, and TAA had no significant difference between the control and the PV groups. Therefore, our results indicated that the PV panels have little effect on the total amino acid nutrition of
E. sinensis and can even improve the flavor of edible tissues.
In addition, the light intensity and water temperature also can affect the fatty acid composition in animals [
44,
45]. A previous study suggested that organism exposure to overheated water can promote the ∑MUFA content [
46]. In this study, the ∑MUFA in male and female muscles also significantly increased without the PV panels. The theory of homeoviscous adaptation suggests that a lower temperature means a greater degree of unsaturation of lipid [
47,
48], but this theory is still debated [
49]. The effect of environmental temperature on fatty-acid unsaturation may be influenced by the different animal species and tissues [
50]. The lack of significant change in ∑PUFA between the control and PV groups seemed to confirm this point. However, the n-3/n-6 PUFA ratio of muscle in the PV group was lower than in the control group. Speers-Roesch et al. (2008) confirmed that the n-3/n-6 ratios in tropical marine elasmobranchs are significantly higher than in the temperate marine elasmobranchs [
51]. At the same time, the decrease of DHA and EPA in hepatopancreases resulted in the reduction of the DHA/EPA ratio in the PV group. Miliou et al., 2006 also observed that EPA and DHA decrease with increasing body weight at the low temperature. It is not unique, the growth performance of
E. sinensis in the PV group was also better than in the control group (
Figure 1). Therefore, the decreases in DHA and EPA observed in the PV group might reflect higher demand for EPA and DHA at a lower temperature for membrane synthesis of faster crabs rather than the response for maintaining membrane permeability and plasticity [
50]. Generally, an increase in human dietary n-3/n-6 PUFA is beneficial to prevent coronary heart disease [
52,
53]. Therefore, the PV panels reduced the nutritional value of
E. sinensis to a certain extent.
In crustaceans, the coloration is related to the various carotenoids [
54]. According to a report, light intensity can affect carotenoid distribution through hormone regulation [
55]. For example, the low light level (250–500 lux) has the highest carotenoid level and brighter skin color in marine smoke angelfish (
Apolemichthys xanthurus) compared with the high light level (750–1000 or 1500–2000 lux) [
56]. In addition, Pavlidis et al. (2008) proved that the low light intensity induced an increase in skin brightness of red porgy (
Pagrus pagrus). They suggested that the change in skin color is related to melanophore motility and/or skin melanin concentration [
57]. Our study also found that the ovaries (dry) color in the PV group is significantly brighter than in the control group. Therefore, the low light level might be related to the brightness of animals by regulating the carotenoid levels and melanin levels. However, the red (a*) and yellow (b*) color of female crabs’ hepatopancreas (wet) in the control group was lower than in the PV group (
Table 7). According to reports, the carotenoid pigment levels were increased at a higher water temperature in European catfish (
Silurus glanis) and Atlantic salmon (
Salmo salar), and then the coloration of the fish fillets had a more intense increase [
58,
59]. Therefore, the intense yellow and red color of the hepatopancreas might be related to the higher water temperature in the control group.