Abstract
Zebrafish (Danio rerio) are becoming one of the most important model organisms in behavioural neuroscience. It has been shown repeatedly that different zebrafish strains show large behavioural differences. These divergent behavioural profiles may have a genetic basis, but environmental factors and previous experience are also known to greatly affect the behavioural phenotype of zebrafish. It could be expected that behavioural differences at the larval stage should be less affected by environmental factors and experience. In the present study, we screened larvae of zebrafish of the AB strain and offspring of wild-caught zebrafish for boldness, using an open field test. In order to follow the behavioural development, we studied larvae at the age of 5-, 7-, 12- and 30-days post fertilization (dpf). Behaviour, as well as behavioural development, clearly differed between the larvae of the different strains. Wild larvae showed larger total distance moved than AB larvae, both at light and dark conditions. These differences were already present at 12 dpf but became more pronounced with age. Wild larvae had a greater variance compared to AB larvae for most of the variables. We have previously shown that bold and shy adult zebrafish differ in the brain expression of dopamine and opioid receptors. The results of the current study show that wild larvae display significantly higher brain expression of drd2b than AB larvae at 30 dpf, a difference that could be related to differences in activity. We did not detect any differences in the expression of opioid receptors.
1. Introduction
Zebrafish (Danio rerio) are one of the most important vertebrate model organisms. From initially being mainly used for studies on developmental biology, zebrafish are now rapidly increasing as a model in all areas of biomedical research [1,2]. Behaviour is usually one of the most important traits when it comes studies on neuroscience and pharmacology. Moreover, zebrafish have been developed as models for studies on affective and neurodegenerative disorders [1,3]. Even though the use of adult zebrafish is increasing, zebrafish larvae are still frequently used in developmental biology, where their transparency, allowing direct in vivo monitoring, is a clear advantage [4]. However, zebrafish larvae are also used for behavioural studies, especially for screening of various pharmaceuticals and xenobiotics; larvae being more cost efficient than adult zebrafish [5]. In addition, the use of larvae has been motivated by ethical reasons [2].
In nature, zebrafish occur in highly diverse environments, ranging from rice paddies to larger streams [6,7]. Not surprisingly, adult zebrafish from these different environments have been shown to differ in behaviour [8]. Moreover, a great number of domesticated laboratory strains of zebrafish are available. Previous studies have shown that fish from these different strains may differ considerably in behaviour [9,10]. The AB strain (ZFIN ID: ZDB-GENO-960809-7) is an often-used lab strain of zebrafish that was established in the 1970s by crossing an A and a B strain of zebrafish available at a pet store in Albany, Oregon, USA [6]. Thus, the AB strain is highly domesticated and may have diverted considerably from wild zebrafish. In fact, Holden and Brown [11] reported considerable differences in gene expression between adults of AB and Wild India Kalkutta (WIK) strains of zebrafish. Similarly, Mustafa et al. [10], showed that adult zebrafish of the AB strain differ in behaviour from offspring of wild caught fish. Overall, as adult AB fish appeared bolder than wild fish, even though behavioural divergence differed depending on behavioural tests.
Inter-strain behavioural differences is a serious problem when comparing results from different studies and makes repetition of studies difficult. In fact, laboratory strains seem to lose natural behavioural responses. For instance, Vossen et al. [12], showed that adult zebrafish of the AB strain did not react to conspecific alarm substance, whereas wild strain fish displayed a clear response to the alarm substance preparation.
Clearly, our knowledge on behavioural differences between different strains/lines of zebrafish is still limited. Intra-specific differences in personality traits, such as boldness, has been extensively studied in teleosts and other vertebrates [13,14,15]. Selection experiments have provided evidence for a genetic component controlling boldness in teleosts [15]. Domestication has also been reported to result in increased boldness [16,17,18]. Still, teleost fish are well known for their large plasticity, and this is especially true when it comes to the development of behavioural phenotypes [19]. Thus, even though behavioural traits, such as boldness, are to some degree heritable they are also most likely to be affected by environmental factors, especially factors related to social interaction. The development of dominance hierarchies, a phenomenon also occurring in zebrafish [20,21], is well known to have large behavioural effects. The ontogenetic development of agonistic behaviour in zebrafish is still not well described. However, Ricci et al. [22] showed that agonistic behaviour increases with age being apparent first from 2 weeks of age. Thus, it could be expected that larvae behaviour will be less affected by social interaction.
The first aim of the present study was to determine if boldness differs between th zebrafish of the AB strain and offspring of wild caught fish at the larval and early juvenile stages also, and to explore behavioural development by testing zebrafish at different ages, i.e., 5, 7, 12 and 30 days post fertilisation (dpf). Boldness refers to risk taking and the willingness to explore novel environments. The open field test is an often-used behavioural assay for screening boldness in fish, as well as rodents. In this test, bold animals are characterized by spending more time in the open area in the centre of the arena, whereas shy animals move along the walls, a behaviour referred to as thigmotaxis [23].
Behavioural phenotypes are known to be modified by multiple neurotransmitter/neuromodulatory systems, including brain monoaminergic systems and endogenous peptides [19,24]. In a previous study, we showed that in adult zebrafish of the AB strain, bold fish express higher levels of dopamine D2 receptors (drd2a and drd2b) and delta opioid receptors (oprd1b) than shy fish [25]. The dopaminergic system is known to be important in shaping bold and shy personality traits, as well as being involved in reward and stress responses [24,26,27,28]. The knowledge on the role of endogenous peptides in controlling teleost behaviour is limited. However, these systems appear to be evolutionary conserved and in rodents opioids are known to be important in shaping behavioural profiles, in part by interacting with the dopaminergic system [29,30]. Serotonin (5-hydroxytryptamine, 5-HT) is another neuromodulator that appears to play a key role in shaping behavioural profiles, as well as mediating behavioural effects of stress and social interaction [19]. Multiple 5-HT receptors subtypes are expressed in the vertebrate brain but, in particular, the expression of 5HT1A receptors has previously been related to personality traits in teleosts [25]. Spexin (spx) is a 14 amino acid peptide, also known as neuropeptide Q, which is highly conserved across the vertebrate subphylum [31]. In zebrafish, spexin occurs in two different forms, spx1 and spx2, with different expression in the brain. It has been shown that both spx1 and spx2 activates galanin receptor 2a (galr2a) and 2b (galr2b) in zebrafish [31]. Spexin has been purported to be involved in anxiety and stress responses and to interact with the brain 5-HT system [32].
A second aim of the present study was to compare the expression of drd2a, drd2b, oprd1b, 5ht1aa, spx1, galr2a and galr2b in the brain of wild and AB larvae, in order to clarify possible neuroendocrine mechanisms that may mediate divergent behavioural profiles.
2. Material and Methods
2.1. Animals and Housing
Zebrafish (Danio rerio) of the AB strain were obtained from SciLifeLab, Evolutionary Biology Centre, Uppsala University, a local zebrafish facility that regularly obtains AB strain zebrafish from the Zebrafish International Resource Center (ZIRC at the University of Oregon Eugene). The wild strain used was a fifth-generation offspring of wild-caught zebrafish from West Bengal, India. The wild-caught fish were allowed to reproduce on site and 1000 fertilized eggs were transported to the Norwegian Technical University, Trondheim, Norway in 2016. Offspring (1000 third generation fish) of these fish were transported to Uppsala University in November 2018 (courtesy of Dr. Fredrik Jutfält, Norwegian Technical University, Trondheim, Norway). For breeding, 125–300 parental fish were used to generate the following generations. Animals were kept in mixed-sex groups in a stand-alone system (AquaNeering, San Diego, CA 92126, USA) in 9 L tanks, supplied with recirculating copper-free Uppsala municipal tap water (10% daily exchange). Temperature was maintained at 27 ± 1.5 °C, and the photoperiod was 14 L:10 D (lights on at 07:00 AM). Animals were fed twice a day with a combination of granulated food (Sparos I&D, Olhao, Portugal) and rotifer culture. Embryos were collected from separate spawning containers provided inside the rearing tanks. In short, these modified glass containers (L: 15 cm, W: 15 cm and H: 7 cm) were covered in mesh with plastic plants attached, which allowed the eggs to fall through the mesh preventing the adults access. The eggs were harvested the next day and age was set as post fertilization day (dpf) 1. The eggs were cleaned, separated from nonfertilized eggs and were transferred to cylinders (Ø: 8 cm) with mesh bottom suitable to fit into 1.8 L tanks in the rearing system. Eggs/embryos were grown at a density of ~50 per cylinder. The eggs remained in free-flowing system water until they were hatched and had consumed their yolk sac (5 dpf). Then, they were transferred to an algae bath, which consisted of 400 mL rack water and 100 ml rotifer culture/50 larvae, for the duration of 5 dpf to10 dpf. During this time, they were given 100 mL fresh rack water every day, otherwise no free-flowing water. Dead embryos and eggs were removed every other day until the day of behavioural analysis at 5 dpf. After 10 dpf the algae bath was terminated, and the larvae placed in a 9-litre tanks with 50 individuals per tank supplied with a slow drip off free-flowing system water. Naïve larvae were used for each trial.
Ethical approval for the use of animals was given by the Uppsala Regional Animal Ethical Committee (permit C55/13), following the guidelines of the Swedish Legislation on Animal Experimentation (Animal Welfare Act SFS1998:56) and the European Union Directive on the Protection of Animals Used for Scientific Purposes (Directive 2010/63/EU).
2.2. Experimental Procedure
The age of the zebrafish used were 5 dpf, 7 dpf, 12 dpf and 30 dpf. At 30 dpf, zebrafish may be referred to as juveniles, but for simplicity they will be referred to as larvae. The 24-well plates (5, 7 and 12 dpf) were filled with 2 mL of system water per well, and wells of the 12-well plates (30 dpf) with 3 mL of system water (Figure 1). One larva per well and 12 (5 dpf, 7 dpf, 12 dpf) or 6 (30 dpf) wells per strain (AB/Wild) were used for each trial. In total 3 trials with 12-well plates (5 dpf, 7 dpf, 12 dpf) and 6 trials with 6-well plates (30 dpf) were performed, resulting in the analysis of 72 individuals at each age. The experiment was carried out using Daniovision and Ethovision® XT 15 (Noldus Information Technology, Wageningen, The Netherlands).
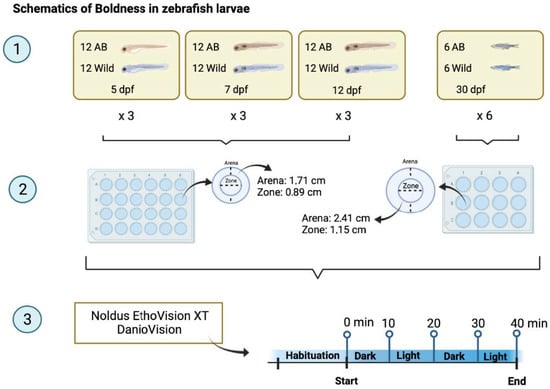
Figure 1.
Schematics of the outline of the experiment. Created with BioRender.com.
2.3. Daniovision and EthoVision
EthoVision settings were optimised using a test plate with larvae of an equivalent life stage, which helped to establish the threshold for tracking. For the 24-well plate, each arena (well) had a diameter of 1.7 cm with a centre zone diameter of 0.9 cm, whereas for the 12-well plate the arena diameter was 2.4 cm, with a centre zone diameter of 1.2 cm.
The Daniovision was connected to a temperature control unit, which kept the temperature of the water constant at 28 °C. After placing the larvae into the wells, they had a 30-min habituation period in dim light outside the DanioVision unit. The total test duration was 40 min with two dark and two light intervals of 10 min each. EthoVision was set to track parameters, such as distance moved (DM in cm), mean velocity (MV in cm/s), latency to first (LTF in s), frequency in zone (F) and time in zone (T in min).
2.4. Larvae Body Length
Screen dumps were generated from video recordings and from the images generated larvae length were measured using imageJ (ver. 1.53k, NIH, http://imagej.nih.gov/ij, accessed on 6 June 2022). Well diameter of the 12- and 24-well plates were used for converting pixels to mm.
2.5. Brain Sampling and qPCR
Whole brains were sampled from wild and AB larvae at the age of 30 dpf. These larvae were sampled directly from the holding tanks, i.e., these larvae were not used in behavioural tests but they were offspring from the same parental groups as the ones used for behavioural studies.
Extraction of RNA from individual brains was performed using the method by Eyster and Brannian [33] with small modifications. The tissues were homogenized in 300 µL TRIzol reagent (ambion by life technologies, Carlsbad, California, USA) and all following volumes were scaled down accordingly. GenElute mammalian total RNA mini prep kit (Sigma, RTN70-1KT) was used together with a DNAse 1 digestion kit (TURBO DNA-free Kit, Applied Biosystems) according to the manufacturer’s instructions. For quality and quantity measures, the total RNA was measured using spectrophotometry (Nanodrop, Thermo Scientific). The cDNA was prepared from 0.8 μg total RNA (Maxima First Strand cDNA Synthesis Kit for RT-qPCR, K1641, Thermo Scientific) according to the manufacturer’s instructions. After cDNA synthesis, the reaction volume of 20 μL was diluted to 800 μL, divided into aliquots, and 4 μL of diluted cDNA was used in each qPCR reaction. Primers were 19–24 nucleotides in length with a melting point around 60 °C and formed products in the range 100–251 bp. From a set of seven reference genes, the four reference genes that displayed the smallest variation were selected, peptidylprolyl isomerase A (ppia, Accession number (ACCN; Genebank, NCBI), NM_212758.1 forward primer GTTTTTCGATCTGACCGCCG reverse primer CACCTCCCTGGCACATGAAA), elongation factor 1 α (ef1α, ACCN, NM_131263.1 forward primer CCCATGT GTGTGGAGAGCTT reverse primer CTTTGTGACCTTGCCAGCAC), hypoxanthine phosphoribosyltransferase 1 (hprt1 ACCN, NM_212986.1 forward primer ATGGACCGAACTGAACGTCT reverse primer CTGTCA TGGGAATGGAGCGA), ribosomal protein L13a (rpl13a ACCN, NM_ 212784.1 forward primer TGACAAGAGAAAGCGCATGGT reverse primer CTCTTCTCCTCCAGTGTGGC) and used for subsequent normalization of qPCR data using geNorm [34]. Seven genes were selected for expression studies htr1aa, drd2a, drd2b, garl2a, galr2b, oprd and spx1 (Table S3).
2.6. Statistical Analysis
Statistical analyses were performed using SAS software (version 9.4). Prior to analyses, the variables were checked for normality. All variables were found to be normally distributed, except MVLat1 where normality was achieved after log-transformation. After analyses, data was extracted and transferred to SigmaPlot (version 14.5) for making the graphs. The pooled-within class correlations were achieved by using the SAS procedure PROC CANDISC. Body length was found to be correlated with several behavioural variables. Thus, length was used as a covariate in the analyses, where the two strains were compared. Pair-wise comparisons were made using t-test on least-square means (PROC GLM in SAS). The two groups were tested for differences in variance simply by dividing the larger variance with the smaller variance. Prior to those analyses, the behavioural (dependent) variables were adjusted by adding the residual of the variable on the independent variable (length) with the mean of the dependent variable. This resulted in negative values in a few cases. Differences in slope between groups (day post-fertilization being the independent variable) were done by using PROC GLM in SAS. Probabilities have been adjusted using Sidak correction (Sidak 1967).
Differences in gene expressions (relative mRNA levels) were analysed using t-test and the p-values obtained were adjusted using Bonferroni correction (m = 7; c.f. Dunn 1961).
3. Results
3.1. Behavioural Development with Age
Pooling within class correlations showed that there were significant differences in behavioural development (Table 1). Distance moved during dark and light conditions increased with body length and this increase was significantly more pronounced in wild larvae than in AB larvae (Table 1). Angular velocity decreased with body length, as indicated with a negative slope (Table 1), and there was no difference between the strains. Time in zone during the dark period decreased with body length, whereas during the light period time in zone increased with body length. However, for this relationship, there was no difference in slope between wild and AB larvae during either light or dark periods (Table 1).

Table 1.
Behavioural variables in light and dark regimes compared between the two strains. This table shows that there are no overall differences between the strains concerning the level of the variables (distance moved, angular velocity and time in zone). Values for body length were log-transformed prior to analysis, except for time in zone.
3.2. Differences in Total Body Length
There was no difference in total body length between AB and wild larvae at 5, 7 or 12 dpf. However, at 30 dpf, AB larvae were significantly larger than wild larvae (Figure 2).
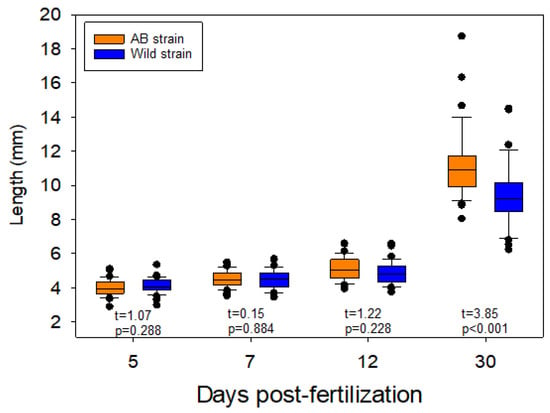
Figure 2.
Boxplots showing the length for the larvae/juveniles for the different days post fertilization.
3.3. Strain Differences in Behavioural Variables
Behavioural divergence increased with age. At 5 and 7 dpf, there were no significant differences in either distance moved, time in zone or angular velocity (Table 2). However, at 12 dpf, wild larvae showed higher distance moved than AB larvae but only during light periods, whereas at 30 dpf, wild larvae showed higher distance moved than AB larvae at both light and dark testing conditions (Table 2, Figure 3A). Similarly, at 12 dpf, AB larvae showed longer time in the zone than wild larvae during light conditions and again at 30 dpf AB larvae showed longer time in zone than wild larvae at both light and dark conditions (Table 2, Figure 3B). Angular velocity did not differ between the strains at any age tested neither in light nor in dark testing conditions (Table 2, Figure 3C).

Table 2.
Differences between AB and wild strain in distance moved, time in zone and angular velocity during light and dark periods. The number of observations in each group was 36 in all cases. The level of significance was adjusted using Sidak method. The means are least square means, using fish length as a covariate. Estimates are given as mean ± S.E.
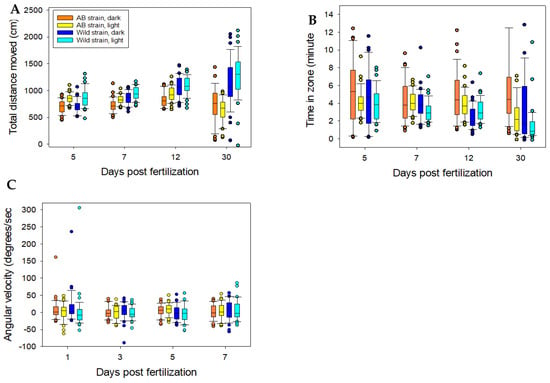
Figure 3.
Behavioural variables from EthoVision, (A) total distance moved (cm), (B) time in zone (s) (C) angular velocity (degrees sec−1). Data for zebrafish larvae at different days post fertilization. The centre line in the boxes represent the median value, the upper and lower end of the boxes third and first quartile, respectively. The bars represent the 5th and the 95th percentile and the circles outliers. For statistics see Table 2.
At 12 dpf, there was a significant difference in variance of distance moved with wild larvae showing larger variance in light conditions than AB larvae (Table 3). Similarly, at 12 dpf, there was a significant difference in variance of time in zone, again only in light conditions, but with AB larvae showing larger variance than wild larvae (Table 3). For values of angular velocity, there were significant differences in variance between AB and wild larvae at 5 and 7 dpf with wild larvae showing larger variance (Table 3).

Table 3.
Means and standard deviation for corrected values of; time in zone, distance moved and angular velocity for dark and light period (using residuals of response variable on length) and analyses of difference between strains in variance of response variable.
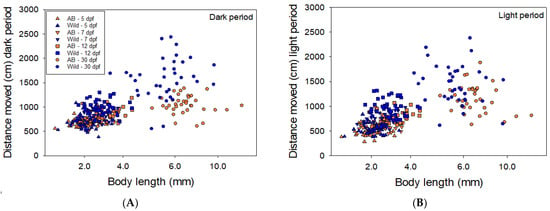
As expected, we found a clear relationship between body length and distance moved, the larger the larvae the longer distance moved (Figure 4). In both wild and AB larvae, the larger the larvae the longer distance moved and the larger the variance. However, differences in activity appear even when distance moved is plotted against body length, with wild larvae showing higher activity and larger variance (Figure 4A,B).
3.4. Brain Gene Expression
Wild larvae showed significantly higher brain expression of drd2b than AB larvae at 30 dpf (Table 4). There was no significant difference between AB and wild larvae in the expression of drd2a, 5ht1aa, galr2a, galr2b, oprd1b or spx1 (Table 4).

Table 4.
Brain gene expression in 30 dpf zebrafish larvae of the strains AB and wild (see Materials and Methods). Values indicate relative mRNA levels ± SEM.
4. Discussion
The results of the current study clearly show that wild and AB zebrafish differ in behaviour even at the larval and early juvenile (30 dpf) stage. The most obvious behavioural difference was the difference in activity, as shown by differences in distance moved, with wild larvae being more active. This behavioural divergence became evident at 12 dpf. As has been shown previously [35], distance moved was longer during darkness but similar differences between wild and AB larvae were observed during both dark and light testing conditions. However, the difference in activity between wild and AB larvae occurred at an earlier age at light conditions. Moreover, we observed a striking difference in behavioural development from 5 to 30 dpf. In both wild and AB larvae, activity increased with age and body length, even though this increase was much more pronounced in wild larvae. They also showed larger variance in travelled distance, as compared to AB larvae, and the variance increased with age. Another behavioural difference is that AB larvae spent more time in the central zone than wild larva. In an open field test, such as the one applied in the current study, the central zone is considered risky and spending more time in this zone is usually interpreted as bold behaviour. Shy, “anxious” and risk aversive animals avoid the central zone, staying close to the walls of the arena, a behaviour referred to as thigomotaxis [23]. Still, this interpretation is complicated by the observation that wild fish showed longer distance moved than AB larvae. Longer distance moved could either reflect exploration, i.e., boldness, or panicking, i.e., anxiety-like behaviour and low boldness [36]. However, distance moved and time in zone may reflect different aspects of the behavioural profile, e.g., distance moved reflecting activity, whereas time in zone may be more related to boldness and risk taking.
The adult zebrafish of the AB strain has previously been reported to be bolder than adult offspring of wild zebrafish [10]. In fact, in the study by Mustafa et al. [36], fish of the AB strain were also compared to the spiegeldanio, a zebrafish strain carrying a mutation in the fibroblast growth factor receptor 1a (fgrf1a) gene. The fgrf1a−/− mutation has been reported to result in increased boldness and aggression in mirror tests, as compared to zebrafish of the Tubingen strain, which was used to generate the fgrf1a−/− mutant [37]. However, Mustafa et al. [36] showed that AB fish were equally bold as spiegeldanio. Moreover, even though spiegeldanio were more aggressive than AB fish in mirror tests, there was no difference in aggression when studied in staged dyadic interactions [36]. Thus, adult AB zebrafish appear to be bold and aggressive, behavioural traits that characterize a proactive stress-coping style [38]. Moreover, AB larvae showed a faster growth rate than wild larvae at 30 dpf, being significantly longer than the wild larvae. Proactive coping has been linked to faster growth and development at conditions where growth is not limited by food availability [39,40,41]. Taken together, the results from the current study suggest that zebrafish of the AB strain are bolder than offspring of wild zebrafish even at the larval stage.
The AB strain was originally created from two pet store strain and has kept in the lab for five decades [6]. Thus, the AB strain can be expected to be highly domesticated, and domestication has been suggested to result in a shift towards a more proactive coping style, including stress resilience, boldness and aggression, even though the effects of domestication on aggression is somewhat ambiguous [16,17,18].
Lab strains, such as AB, are also inbred, since they are often generated from a relatively small number of fish. Moreover, over time these strains may have gone through additional genetic bottlenecks. Lab strains do not only differ from wild zebrafish, they also differ from each other [42,43]. The results of the current study clearly show that wild larvae display considerably larger variance in behaviour than AB larvae. Moreover, this variance increased with age and body length, suggesting large intra-strain divergence in developmental trajectories. The AB strain appear more homogenous in behaviour and development, a difference that could be related to inbreeding in AB.
We observed significantly higher brain expression of drd2b in wild as compared to AB larvae (30 dpf). Thörnqvist et al. (2019) reported higher expression of drd2b and also a small but significant upregulation of drd2a in bold as compared shy adult AB males. Moreover, they also reported a small but significantly higher expression of oprd1b in the brains of bold adult males. The upregulation of drd2b expression that we observed in the current study was relatively large and of the same magnitude as the one observed in bold adult AB males [25]. However, we did not find any difference in the expression of drd2a or oprd1b; neither showed any difference in the expression of 5ht1aa, galr2a, galr2b or spx1, in the brain of wild and AB larvae (30 dpf). Another difference is that, in the current study, wild larvae were the ones showing higher expression of drd2b, even though according to the behaviour they appeared shyer than AB, as discussed above. It is difficult to speculate on the cause of this opposite relationship to boldness. However, in the present study, AB larvae appeared bolder than wild larvae since they spent more time in the central zone. Still, at the same time, wild larvae were more active, showing longer distance travelled than AB larvae. In the study by Thörnqvist et al. [25], the fish classified as bold showed a longer distance travelled and higher mean velocity than those classified as shy. Thus, differences in the brain expression of drd2b may be more related to activity [44]. D2 receptors occur both as pre-synaptic autoreceptors and post-synaptic receptors [45]. Thus, it is also difficult to speculate on the relationship between an upregulation of drd2b and the dopaminergic tone.
5. Conclusions
The results of the present study show that AB and the offspring of the wild caught zebrafish clearly differ in behaviour, even at the larval and early juvenile stage. The behavioural divergence is obvious from 12 dpf and becomes more pronounced with age and size. Moreover, wild larvae show much larger behavioural variance, and the variance is also increasing with age. It appears likely that these behavioural differences are caused by domestication and inbreeding in the AB strain. Larvae of these two strains also differ in brain expression of drd2b receptors, a difference that could be related to differences in activity.
Author Contributions
H.J. and N.F. performed the experiment, P.-O.T. performed the wet lab qPCR part of the study, E.P. validated, curated and visualized the data and conducted the statistical analysis. J.A. wrote the first draft of the manuscript and carried out visual illustrations. S.W. provided supervision and resources and conceptualized and designed the study. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the support of the Facias Foundation (J.A., S.W.) and the Swedish Research Council (VR-NT11 2017-03779 to S.W.).
Acknowledgments
The authors thank Uppsala University Behavioural Facility (UUBF), Disciplinary Domain of Medicine and Pharmacy, Uppsala University.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, C.; von Keyserlingk, M.A.G.; Franks, B. Zebrafish welfare: Natural history, social motivation and behaviour. Appl. Anim. Behav. Sci. 2018, 200, 13–22. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.-B.; He, K.-J.; Wang, F.; Liu, C.-F. Advances of Zebrafish in Neurodegenerative Disease: From Models to Drug Discovery. Front. Pharmacol. 2021, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 86, 6–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. Camb. Philos. Soc. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Paull, G.C.; Tyler, C.R. Improving zebrafish laboratory welfare and scientific research through understanding their natural history. Biol. Rev. 2022, 97, 1038–1056. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Bhat, A. Population, sex and body size: Determinants of behavioural variations and behavioural correlations among wild zebrafish Danio rerio. R. Soc. Open Sci. 2018, 5, 170978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Séguret, A.; Collignon, B.; Halloy, J. Strain differences in the collective behaviour of zebrafish (Danio rerio) in heterogeneous environment. R. Soc. Open Sci. 2016, 3, 160451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, A.; Roman, E.; Winberg, S. Boldness in Male and Female Zebrafish (Danio rerio) Is Dependent on Strain and Test. Front. Behav. Neurosci. 2019, 13, 248. [Google Scholar] [CrossRef]
- Holden, L.A.; Brown, K.H. Baseline mRNA expression differs widely between common laboratory strains of zebrafish. Sci. Rep. 2018, 8, 4780. [Google Scholar] [CrossRef] [PubMed]
- Vossen, L.E.; Červenỳ, D.; Sarma, O.S.; Thörnqvist, P.-O.; Jutfelt, F.; Fick, J.; Brodin, T.; Winberg, S. Low Concentrations of the Benzodiazepine Drug Oxazepam Induce Anxiolytic Effects in Wild-Caught but Not in Laboratory Zebrafish. Sci. Total Environ. 2020, 703, 134701. [Google Scholar] [CrossRef] [PubMed]
- Coppens, C.M.; de Boer, S.F.; Koolhaas, J.M. Coping styles and behavioural flexibility: Towards underlying mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Vindas, M.A.; Gorissen, M.; Höglund, E.; Flik, G.; Tronci, V.; Damsgård, B.; Thörnqvist, P.-O.; Nilsen, T.O.; Winberg, S.; Øverli, Ø.; et al. How do individuals cope with stress? Behavioural, physiological and neuronal differences between proactive and reactive coping styles in fish. J. Exp. Biol. 2017, 220, 1524–1532. [Google Scholar] [CrossRef] [Green Version]
- Øverli, Ø.; Pottinger, T.G.; Carrick, T.R.; Øverli, E.; Winberg, S. Differences in behaviour between rainbow trout selected for high- and low-stress responsiveness. J. Exp. Biol. 2002, 205, 391–395. [Google Scholar] [CrossRef]
- Huntingford, F.A. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J. Fish Biol. 2004, 65, 122–142. [Google Scholar] [CrossRef]
- Huntingford, F.; Adams, C. Behavioural syndromes in farmed fish: Implications for production and welfare. Behaviour 2005, 142, 1207–1221. [Google Scholar] [CrossRef]
- Agnvall, B.; Katajamaa, R.; Altimiras, J.; Jensen, P. Is domestication driven by reduced fear of humans? Boldness, metabolism and serotonin levels in divergently selected red junglefowl (Gallus gallus). Biol. Lett. 2015, 11, 20150509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backström, T.; Winberg, S. Serotonin Coordinates Responses to Social Stress—What We Can Learn from Fish. Front. Neurosci. 2017, 11, 595. [Google Scholar] [CrossRef]
- Larson, E.T.; O’Malley, D.M.; Melloni, R.H. Aggression and vasotocin are associated with dominant–subordinate relationships in zebrafish. Behav. Brain Res. 2006, 167, 94–102. [Google Scholar] [CrossRef]
- Dahlbom, S.J.; Backström, T.; Lundstedt-Enkel, K.; Winberg, S. Aggression and monoamines: Effects of sex and social rank in zebrafish (Danio rerio). Behav. Brain Res. 2012, 228, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Summers, C.H.; Larson, E.T.; O’Malley, D.; Melloni, R.H. Development of aggressive phenotypes in zebrafish: Interactions of age, experience and social status. Anim. Behav. 2013, 86, 245–252. [Google Scholar] [CrossRef]
- Schnörr, S.; Steenbergen, P.; Richardson, M.; Champagne, D. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Winberg, S.; Nilsson, G.E. Induction of social dominance by L-dopa treatment in Arctic charr. NeuroReport 1992, 3, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Thörnqvist, P.-O.; McCarrick, S.; Ericsson, M.; Roman, E.; Winberg, S. Bold zebrafish (Danio rerio) express higher levels of delta opioid and dopamine D2 receptors in the brain compared to shy fish. Behav. Brain Res. 2019, 359, 927–934. [Google Scholar] [CrossRef]
- Winberg, S.; Nilsson, G.E.; Olsén, K.H. Social rank and brain levels of monoamines and monoamine metabolites in Arctic charr, Salvelinus alpinus (L.). J. Comp. Physiol. A Sensory Neural Behav. Physiol. 1991, 168, 241–246. [Google Scholar] [CrossRef]
- Øverli, Ø.; Harris, C.A.; Winberg, S. Short-Term Effects of Fights for Social Dominance and the Establishment of Dominant-Subordinate Relationships on Brain Monoamines and Cortisol in Rainbow Trout. Brain, Behav. Evol. 1999, 54, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Cabib, S.; Puglisi-Allegra, S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012, 36, 79–89. [Google Scholar] [CrossRef]
- Spanagel, R.; Herz, A.; Shippenberg, T.S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc. Natl. Acad. Sci. USA 1992, 89, 2046–2050. [Google Scholar] [CrossRef] [Green Version]
- Trigo, J.M.; Martin-García, E.; Berrendero, F.; Robledo, P.; Maldonado, R. The endogenous opioid system: A common substrate in drug addiction. Drug Alcohol Depend. 2010, 108, 183–194. [Google Scholar] [CrossRef]
- Kim, D.-K.; Yun, S.; Son, G.H.; Hwang, J.-I.; Park, C.R.; Kim, J.I.; Kim, K.; Vaudry, H.; Seong, J.Y. Coevolution of the Spexin/Galanin/Kisspeptin Family: Spexin Activates Galanin Receptor Type II and III. Endocrinology 2014, 155, 1864–1873. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.H.; Soga, T.; Levavi-Sivan, B.; Parhar, I.S. Chronic Social Defeat Stress Up-Regulates Spexin in the Brain of Nile Tilapia (Oreochromis niloticus). Sci. Rep. 2020, 10, 7666. [Google Scholar] [CrossRef]
- Eyster, K.M.; Brannian, J.D. Gene Expression Profiling in the Aging Ovary. Methods Mol. Biol. 2009, 590, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colwill, R.M.; Creton, R. Imaging escape and avoidance behavior in zebrafish larvae. Rev. Neurosci. 2011, 22, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, A.; Thörnqvist, P.-O.; Roman, E.; Winberg, S. The aggressive spiegeldanio, carrying a mutation in the fgfr1a gene, has no advantage in dyadic fights with zebrafish of the AB strain. Behav. Brain Res. 2019, 370, 111942. [Google Scholar] [CrossRef]
- Norton, W.; Mangoli, M.; Lele, Z.; Pogoda, H.-M.; Diamond, B.; Mercurio, S.; Russell, C.; Teraoka, H.; Stickney, H.L.; Rauch, G.-J.; et al. Monorail/Foxa2 regulates floorplate differentiation and specification of oligodendrocytes, serotonergic raphé; neurones and cranial motoneurones. Development 2005, 132, 645–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Ward, A.J.W.; Thomas, P.; Hart, P.J.B.; Krause, J. Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 2004, 55, 561–568. [Google Scholar] [CrossRef]
- Brown, C.; Jones, F.; Braithwaite, V.A. Correlation between boldness and body mass in natural populations of the poeciliid Brachyrhaphis episcopi. J. Fish Biol. 2007, 71, 1590–1601. [Google Scholar] [CrossRef] [Green Version]
- Mas-Muñoz, J.; Komen, H.; Schneider, O.; Visch, S.W.; Schrama, J.W. Feeding Behaviour, Swimming Activity and Boldness Explain Variation in Feed Intake and Growth of Sole (Solea solea) Reared in Captivity. PLoS ONE 2011, 6, e21393. [Google Scholar] [CrossRef] [PubMed]
- Vignet, C.; Begout, M.-L.; Péan, S.; Lyphout, L.; Leguay, D.; Cousin, X. Systematic Screening of Behavioral Responses in Two Zebrafish Strains. Zebrafish 2013, 10, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, M.; Neuzeret, F.; Fabreges, B.; Froc, C.; Bedu, S.; Bally-Cuif, L.; Norton, W.H.J. Inter-Individual and Inter-Strain Variations in Zebrafish Locomotor Ontogeny. PLoS ONE 2013, 8, e70172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zhao, Y.; Liu, W.; Li, Z.; Souders, C.L.; Martyniuk, C.J. Butylated hydroxytoluene induces hyperactivity and alters dopamine-related gene expression in larval zebrafish (Danio rerio). Environ. Pollut. Barking Essex 1987 2020, 257, 113624. [Google Scholar] [CrossRef]
- Schweitzer, J.; Löhr, H.; Filippi, A.; Driever, W. Dopaminergic and noradrenergic circuit development in zebrafish. Dev. Neurobiol. 2012, 72, 256–268. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).