Abstract
Climate change is a growing threat to marine organisms and ecosystems, and it is already modifying ocean properties by, for example, increasing temperature and decreasing pH. Increasing water temperature may also lead to an impairment of primary productivity and an overall depletion of available zooplankton. Understanding how the crossover between warming and zooplankton availability impacts fish populations has paramount implications for conservation and mitigation strategies. Through a cross factorial design to test the effects of ocean temperature and food availability in a temperate marine teleost, Pomatochistus flavescens, we showed that hindered feeding impacted sheltering and avoidance behaviour. Also, low food availability impaired fish reproduction, particularly male reproduction, as the expression of cyp11b1, a gene with a pivotal role in the synthesis of the most important fish androgen, 11-ketotestosterone, was significantly reduced under a low food regime. In contrast, temperature alone did not affect reproductive success, but offspring showed increased saturated fatty acid content (embryos) and increased lipid peroxidation (larvae). Altogether, food availability had a stronger effect on fitness, showing that coping with elevated temperatures, an ability that may be expected in shallow-water fish, can be indirectly impacted, or even overwhelmed, by the effects of ocean warming on primary productivity and downstream ecological processes.
1. Introduction
For ectotherms, temperature is a key environmental factor that influences several fundamental processes such as behaviour [1,2], metabolism [3,4], reproduction [5,6], growth, and development [7,8]. In a world of rapid warming, with ocean temperature projected to increase up to 4 °C until the end of the century [9,10], it is imperative to understand the overall consequences to marine life. In fish, warming has been shown to induce changes in swimming performance [11], activity [12,13], and risk-taking related behaviours such as foraging, predator-prey interactions [14,15], predator escape responses, and boldness [2,16].
Temperature is known to be one of the most important cues for triggering fish reproductive activity [7,17]. Climate change has the potential to induce temperature variations which can have implications on the timing of reproduction, anticipating, delaying, or even interrupting it [18]. Warming can also influence several behavioural and physiological processes related to reproduction, such as parental care [6], spawning frequency, clutch size, egg area, and duration of the embryonic phase [5,19,20]. Moreover, temperature will act on gonads, mainly interfering with steroidogenesis [17], and responsible for the differentiation of gonads, their growth and maturation [21]. Elevated temperatures can also lead to gonadal degeneration and partial or complete sterility [22] and modify gonadal sex in early development [23,24], with further cascading effects on offspring fitness and quality [25].
Ocean warming is also expected to lead to increased metabolism and disrupted metabolic activity in fishes [26,27], triggering an increased production of reactive oxygen species (ROS), such as the singlet oxygen, the superoxide anion, the hydrogen peroxide, and the hydroxyl radical, during the aerobic metabolism [28], causing protein, lipid and DNA damages. Moreover, temperature is responsible for influencing the fatty acid (FA) content in marine fishes [29,30], which are the “building blocks” of lipids, with important roles in cellular membrane formation, protein modification, energy storage, among others [31]. Due to their biosynthesis specificity, FA can act as biological markers of specific taxa [32,33]. In fact, while saturated FA (SFA) and monounsaturated FA (MUFA) are usually synthetized or genetically inherited by most marine organisms [31,34,35], others, such as polyunsaturated FA (PUFA), are usually restricted to phytoplankton [35,36], and are therefore obtained through feeding [37].
As a response to higher temperature, fish will increase their energetic demands [38] and consequently increase food intake [8]. However, in the natural environment, food is never unlimited, and warming is expected to impact plankton communities [39] due to stratification of the water column and the subsequent depletion of nutrients, which result in poor nutritional quality zooplankton [40] with reduced abundance [41], namely a drastically decreased of the production of omega-3 in the phytoplankton that will decrease availability of this fatty acid in the above levels of the trophic chain [42]. Food shortage can affect organisms’ dynamic energy budgets [43], reducing the energy available for key biological processes, such as reproduction, with carry-over effects on number, size, energy and biochemical composition of eggs, and overall quality of offspring (see review by Green [44]). Despite this link between warming and food availability, few studies have addressed their interactive effects on marine organisms’ behaviour and physiology [45,46,47,48], with most experimental studies following an ad libitum feeding regime, which may provide fish with enough energy to compensate for the negative effects of other stressors.
Here, it was proposed to explore the effect of increasing water temperature and low food availability on individual behaviour, metabolism, stress responses and reproduction in temperate coastal teleosts, deploying the two-spotted goby, Pomatochistus flavescens, as a model. This is a semi-pelagic fish that distributes along the European coast and inhabits shallow rocky shores and seaweed beds [49]. Previous studies already demonstrated that the species’ reproductive abilities can be affected by realistically increased water temperature, which led to the hypothesis that fish of temperate waters can be particularly vulnerable to forecasted warming regimes [5], rendering it a promising surrogate for other shallow-water teleosts in this biogeophraphical area.
2. Materials and Methods
2.1. Fish Collection and Experimental Setup
Adult male and female (totaling 150 individuals) two-spotted goby were collected via SCUBA diving at Arrábida Marine Park, Portugal (38°28′ N; 8°59′ W), in February 2019. Upon arrival at the lab facilities, fish were placed in a 140-L tank, with a continuous supply of recirculating seawater, enriched with substrate and artificial algae, with temperature (15 °C) and salinity (35 PSU) matching the conditions at the collection site. The fish photoperiod at the lab facilities varied according to light/dark changes in the wild for the duration of the experiment. Fish were left to acclimate to captivity conditions in this tank for 10 days and were fed daily unto satiation with a mixture of frozen Artemia (Gamma Slice Brine shrimp, Tropical Marine Centre™ Hertfordshire, UK) and Copepods (Gamma Slice Copepods, Tropical Marine Centre™). Subsequently, fish were weighted, measured and randomly assigned to 30 L tanks, at a density of four fish of the same sex per tank. To minimize potential aggressive behaviours, particularly between males, each tank was supplied with artificial nests (pvc tubes) and artificial algae. Water in the 30 L tanks was supplied at a total of eight 200 L sumps (two sumps for each of the four treatments), equipped with biological, mechanical, chemical and ultraviolet filtration. Each sump supplied water to five 30 L tanks. Temperature was controlled using both chillers and heaters. Temperature and salinity were measured daily, and other water quality parameters, such as ammonia, nitrates and nitrites, were monitored weekly and kept below critical levels. Excess feed was removed from tanks daily.
Fish were then assigned to one of four treatments: control temperature (CT) + high ration (HR); control temperature (CT) + low ration (LR); high temperature (HT + 3 °C) + high ration (HR); and high temperature (HT + 3 °C) + low ration (LR). The temperature in HT treatments was increased stepwise at a ratio of about 1 °C per week, using heaters, until +3 °C above the control temperature treatment was reached. As this study involves a prolonged exposure to treatments, temperatures in both control and high temperature conditions varied monthly to match local seasonality (Supplementary Table S1), and the variation followed an average of the last 10 years at the collection site. Once the experimental temperature was reached, the food treatments started (day 0 of treatments) (Figure 1). Fish in the high ration treatments were fed daily with 8 g of frozen Artemia and Copepods, divided by two meals, while fish in low ration treatments were fed the same amount of food once every two days. The choice of the high ration was selected based on preliminary observations that allowed us to conclude that 8 g per day would match fish needs. The choice of low ration was selected while taking into consideration that even lower feeding levels would lead to higher mortality rates within the first few days, which we avoided. The amount of feed was readjusted to mortality.
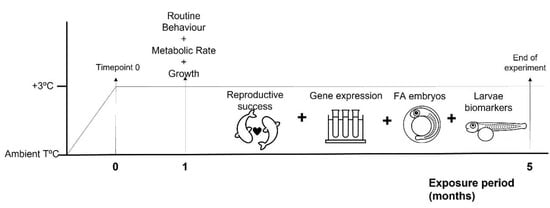
Figure 1.
A diagram of the experimental design. Two-spotted goby, Pomatochistus flavescens were exposed to 4 treatments: CT + HR-control temperature and high ration; CT + LR control temperature and low ration; HT + HR high temperature (+3 °C) and high ration; and HT + LR high temperature (+3 °C) and low ration. At Timepoint 0, temperature in the “High” temperature treatments was reached, and the food treatments started. After 1 month of exposure to treatments, a subset of fish was tested for routine behaviour, metabolic rates and growth. Another subset of fish was exposed to treatments for a total of 5 months, which included the breeding season. During this period, reproductive success and gene expression of reproduction-related genes were determined in adult fish; fatty acid (FA) content was determined in recently fertilized eggs and biomarker analysis determined in recently hatched larvae. Fish under LR treatments did not reproduce, thus FA content and biomarkers were not determined.
2.2. Temperature and Food Availability Effects on Behaviour and Physiology
A total of 48 fish (12 fish per treatment), with 3.7 ± 0.045 cm and 0.675 ± 0.027 g (mean ± s.e.m.), were exposed for a 1-month period to treatment conditions and, subsequently, routine activity, metabolic performance and growth were determined (Figure 1). Fish size (SL) and weight (W) did not differ among the fish randomly assigned to the treatments at the start of the experiment (SL: F(3:36) = 0.063, p = 0.980; W: F(3:35) = 0.409, p = 0.747).
2.2.1. Routine Behaviour
An ethogram was built based on [50,51] to assess routine behavioural activity (Table 1). Behavioural descriptions were made using focal observations (sensu Martin and Bateson [52]), which were conducted between 10:00 and 13:00 a.m., approximately 30 min after feeding, to avoid pre-prandial bias, by a motionless observer standing in front of the aquarium (always the same observer). Time taken by the fish to ignore the observer was registered (determined as the time taken for the fish to turn away from the observer, as initially the fish expected to be fed again and would swim towards the observer). During the focal observations, the behavioural categories displayed by the focal fish were registered. Each focal observation lasted 2 min, and this procedure was repeated for 10 to 12 fish per treatment. Each fish was observed in three sessions throughout a one-week period to confirm consistency in behaviour and outwit punctual behaviours. Fish were not tagged, but small differences in coloration on body and fins of fishes kept in the same tank considerably reduced the risk of misidentifying each individual.

Table 1.
List of behaviours displayed by two-spotted goby, Pomatochistus flavescens during the routine behaviour experiment.
2.2.2. Routine Metabolic Rate
Fish were fasted for 24 h prior to analysis to ensure a post-absorptive state, as metabolic rates tend to increase during digestion [53]. Metabolism was assessed by measuring routine metabolic rate (RMR), using a closed respirometry system, for 11 to 12 fish per treatment. Each individual was tested only in a single assay. One individual was placed into a 100 mL syringe filled with 60 mL of UV filtered aerated water, matching salinity and temperature of the corresponding treatment. All air bubbles were removed from the syringe, parafilm was wrapped around the tip and a cap was used to ensure there were no leaks of water or air from the syringes. The syringe was placed in a water bath to ensure that fish were kept at the same temperature of the treatment and covered with black plastic to avoid external disturbances. After 1 min of acclimation, oxygen levels inside the syringe were measured for 10 min using contactless oxygen sensor spots (OXSP5, Pyroscience, Aachen, Denmark) and monitored using the software Pyro Oxygen Logger (Pyroscience,). The oxygen concentration was maintained above 80% during experimental tests. Four 100 mL syringes were used simultaneously, three with fish, and one without fish, which served as “blank” to account for bacterial respiration. Between trials, all syringes were washed with distilled water and cleaned for particles with a sterilized cotton swab before being filled again for the following fish. After each trial, fish were removed from the syringe, measured and weighted to determine body condition. Experimental trials were conducted over a period of 1 week in a temperature-controlled room to minimize temperature fluctuations during trials. At the end of the 1-month exposure period, all fish were euthanized with an overdose of MS-222 (100 mg L−1).
2.3. Temperature and Food Availability Effects on Reproduction
Another 80 fish were assigned as breeding pairs (male-female), at a density of two breeding pairs per 30 L tank and a total of 10 pairs per treatment. Pairs in each tank were separated by a partition to keep track of the identity of each parental pair. The partitions in the tank allowed constant renewal of the water and the maintenance of water temperature and quality on both sides. Size (3.68 ± 0.029 cm, mean ± s.e.) did not differ between fishes assigned to the different treatments (F(3:80) = 1.369, p = 0.258). Weight (0.633 ± 0.016 g, mean ± s.e.) was also not different at the start of the experiment for fish assigned to the treatments (F(3:80) = 0.716, p = 0.545). These pairs were exposed to treatment conditions for a 5-month period, which included the breeding season. Reproductive success was evaluated by measuring the number of clutches produced by breeding pairs, the average number of eggs, average egg area, and larval size at hatching and adult body condition. Additionally, the expression of reproduction related genes was measured in adults’ gonads, fatty acid content was measured in recently fertilized eggs and oxidative stress and energy metabolism-related biomarkers were measured in recently hatched larvae (Figure 1).
2.3.1. Reproductive Success
Breeding couples were provided with a pvc tube (10 cm, Ø1.3 cm) for shelter and nesting. Each pipe was lined inside with a removable acetate sheet where the spawning females could attach their eggs. Egg clutch presence was checked daily, and when present, the acetate sheet was carefully removed and placed in a Petri dish filled with seawater from the respective tank and photographed for egg counting. Approximately 10 eggs were removed from the clutch and placed in a smaller Petri dish, filled with seawater and photographed under a stereomicroscope for measurements of egg area. The first clutch from each breeding pair was entirely sampled and stored in a 2 mL cryotube at −80 °C for the determination of fatty acid content (see below). Pairs were not affected by the brief removal of the acetate sheet, as fish resumed normal activity after the acetate sheet was put back in place. Reproductive output was determined by multiplying the total number of eggs per breeding pair by the average egg area (mm2). Close to hatch date, the acetate sheet was removed and placed in a maternity cage (16.5 × 12.5 × 11.5 cm) inside a 30 L tank, matching the parental treatment. A total of 10–15 larvae were collected at hatching, euthanized using an overdose of MS-222 and immediately photographed under a stereomicroscope to determine morphometric traits (mm): standard length (SL), head height (HH), pre-caudal body length (BL), and muscle mass depth at vent (MDV). Approximately 150 larvae were collected at hatching and stored in 2 mL cryotubes at −80 °C, in pools of 50 larvae per tube, for a biomarker analysis (see below).
At the end of the experiment, adults were measured, weighted and euthanized with an overdose of MS-222 (100 mg L−1). Fish were dissected, gonads were removed, weighted to determine gonadosomatic index (GSI) and stored at −80 °C for quantification of expression of reproduction related genes. Gonadosomatic index was measured using the formula GSI = (gonad mass (g)/fish mass (g)) × 100. If a fish died in the tank during the experimental period, no morphometric traits were determined, and gonads were not dissected, as the time of death was not possible to determine. This occurred in 22 out of the 80 fishes (3 in the Control, 7 in the Low ration, 2 in the High T°C and 10 in the High T°C × Low ration treatments).
2.3.2. Gene Expression of Reproduction-Related Genes
Total RNA was extracted using the RNeasy Lipid Tissue Mini Kit (Qiagen, Germantown, ML, USA), according to manufacturer instructions, with minor adaptations. A DNase digestion step was included to prevent DNA contamination. Yield was assessed using a NanoDrop® spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA), and RNA integrity was confirmed with Bioanalyzer (Agilent). RNA was stored at −80 °C and then reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Heracles, CA, USA).
mRNA levels of genes encoding the steroidogenic enzymes P450 11b hydroxylase (cyp11b1) were quantified in males and both P450 aromatase (cyp19a1) and carbonyl reductase/20α-hydroxysteroid dehydrogenase (20α-hsd) for females. The selected housekeeping gene was β-actin. Primers used for β-actin are already described in the literature [54]. For the other genes, we obtained cDNA sequences for some teleost fish at the National Center for Biotechnology Information (NCBI) (cyp11b1: XM_029128166.2; cyp19a1: AY684254.1; 20α-hsd: KT932711.1) and searched for homologous sequences in a non-annotated transcriptome of a very closed species (Pomatoschistus minutus, the sand goby [55]). For 20α-hsd, no blast result on the goby transcriptome was obtained, so we considered the above-mentioned NCBI sequence for the following step. Primers were designed with Primer3 software [56,57] using the sequences obtained earlier, and possible primer dimer formation was checked with FastPCR v5.4 software [58]. Primers were tested with a pool of cDNA in a qRT-PCR, and PCR products were confirmed via sequencing. Primer sequences and the size of amplification products are available in Supplementary Table S2. qRT- PCR was performed using the QuantStudio 7 Real-Time PCR Systems (ThermoFisher Scientific). The reaction mix included Power SYBR Green PCR Master Mix (ThermoFisher Scientific), 50 µM of each primer and 2 µL of cDNA in an 8 µL reaction volume. Cycling parameters were: (i) denaturation: 5 min at 95 °C; (ii) amplification and quantification: 40 cycles (30 s at 95 °C, 30 s at the annealing temperature of 56 °C, 30 s at 72 °C); and (iii) dissociation curve assessment (30 s at 95 °C, 30 s at 55 °C, 30 s at 95 °C). The dissociation curve was performed to confirm a single melting curve, proving the inexistence of primer-dimer formation and/or plate contamination. All samples were run in triplicate and no-template controls were also included in each assay. The relative initial template concentration normalized to the housekeeping gene β-actin was determined from the equation: 2^(Ct housekeeping gene − Ct target gene), assuming that the efficiency of the genes was approximately 100%. To validate this assumption, the efficiency of each gene was determined using serial dilutions of stock cDNA and only primers with efficiencies above 90% were used. Mean values for gene β-actin did not differ between treatments, thus confirming its suitability to be used as a reference gene in this study.
2.3.3. Fatty Acids Content in Recently Fertilized Eggs
FA profile of egg clutches, produced by adults exposed to the above-mentioned conditions, was determined byfreeze-drying the samples for 24 h at −50 °C under low pressure (~10–1 atm), after which they were grinded and weighted. Subsequently, 10–50 µL of C21:0 internal standard with a concentration of 10 mg/mL was added according to each sample weight (10–60 mg of dry mass per sample). Afterwards, samples were dissolved in 5 mL of acetyl chloride/methanol (1:19 v/v; Merck), shaken and heated at 80 °C for 1 h, after which they were left to cool on ice. After cooling, we added 1 mL of Milli-Q distilled water and 2 mL of n-heptane pro-analysis (Merck) to each sample. Following a 5 min centrifugation process at 2300× g, we collected the upper phase content and added it to an anhydrous sodium sulphate column (Panreac) to remove the moisture content. Finally, 2 µL of filtered sample was injected in a gas chromatograph (Varian Star 3800 Cp, Walnut Creek, CA, USA) equipped with an auto-sampler and fitted with a flame ionization detector at 250 °C for fatty acid methyl ester (FAME) analysis. The separation was carried out with helium as the carrier gas at a flow rate of 1 mL min−1 in a capillary column DB-WAX (30 m length × 0.32 mm internal diameter; 0.25 mm film thickness; Hewlett-Packard, Minneapolis, MN, USA) programmed at 180 °C for 5 min, raised to 220 °C at 4 °C/min and maintained at 220 °C for 5 min with the injector at 250 °C. FAME identification (% total FA) was accomplished through comparison of retention times with those of Sigma, Nu Check Preap and Larodan Fine Chemicals standards.
2.3.4. Biomarker Analysis in Recently Hatched Larvae
Biomarkers were selected based on their molecular roles in thermal and oxidative stress responses: (i) Hsp70 is a marker of reversible thermal damage to proteins (e.g., protein unfolding), acting as a chaperone [59,60,61], (ii) TAC measures the concentration of antioxidants in tissues, including both enzymatic (e.g., catalase, superoxide dismutase, glutathione peroxidase, etc.) and non-enzymatic antioxidants (e.g., vitamins and pigments) [62] and (iii) LPO is a marker of oxidative damage to cell membranes that arises from the interaction of reactive oxygen species with unsaturated fatty acids, producing lipid radicals and reactive aldehydes such as malondialdehyde [63].
Samples from whole body larvae were homogenised in 1 mL of Phosphate Buffer Saline solution (PBS, pH 7.4, 140 mM NaCl, 3 mM KCl, 10 mM NA2HPO4, 2 mM KH2PO4) using an electric homogenizer (Fisherbrand 150 Handheld Homogenizer 220V EU cord, 150 W) to extract soluble proteins. The homogenates were centrifuged at 4 °C for 10 min at 10,000× g, and the supernatant fraction was collected and transferred to new microtubes (1.5 mL) and immediately frozen at −80 °C for later analysis.
Total protein in samples was quantified using a NanoDrop® ND-1000 spectrophotometer (ThermoFisher Scientific, by measuring sample absorbance at 280 nm and calculating protein concentration based on the Beer–Lambert equation, using the molar extinction coefficient of BSA (bovine serum albumin) 43,824 cm−1M−1 and a path length of 10 mm. Total proteins were used to normalize all biomarker results.
Heat Shock Protein 70 (HSP70) was quantified using an indirect Enzyme Linked Immunosorbent Assay (ELISA). Protein extracts from skeletal muscle were diluted 1:5 in PBS to optimize the signal-to-noise ratio, and duplicates of 50 µL of each sample were pipetted to the microplates (96-well flat bottom high-binding plates from Greiner, Greinerstraße, Kremsmünster Austria) and then incubated overnight at 4 °C. The microplates were washed (3 × 200 μL PBS 0.05% Tween-20), and then blocked by adding 200 μL of 1% BSA (Albumin Bovine Fraction V (BSA), Nzytech, Lisbon, Portugal) in PBS. The microplates were incubated at 37 °C for 90 min and washed again. Fifty μL of primary antibody (Mouse monoclonal Hsp70/Hsc70 Antibody, ref #TA326357, OriGene Technologies, Inc., Rockville, ML, USA), diluted to 1 μg.mL−1 in a solution of 1% BSA in PBS were added to each microplate wells and incubated at 37 °C for 90 min and washed. Fifty µL of secondary antibody (Anti-mouse IGG (FC fragment) alkaline, ref # A1418, Sigma-Aldrich, Taufkirchen, Germany) was diluted to 1 μg mL−1 in a solution of 1% BSA in PBS and added to the microplate and at 37 °C for 90 min and washed. After the final washing stage, 100 μL of alkaline-phosphatase substrate (100 mM Tris-HCl, 100 mM NaCl, 0.5 mM MgCl2, 27 mM PnPP, pH 8.5) was added to each microplate well and incubated for 30 min at room temperature. Fifty μL of STOP solution (3M NaOH) was added to each well and the absorbance was read in the microplate reader (Multiskan Sky Thermoscientific, Massachusetts, USA) at 405 nm. Quantification was done through a calibration curve constructed using serial dilutions of HSP70 standards (Recombinant Human HSP70 (active), ref #AR03018PU-S, OriGene Technologies, Inc., USA) in order to obtain a protein concentration, range from 0 to 2 μg mL−1. Hsp70 concentrations were normalized per total proteins in each sample (given in µg mg−1 of total protein).
The Total Antioxidant Capacity (TAC) assay was adapted from [62,64], for 96 well-microplates, according to manufacture instructions. Duplicates of 10 µL were pipetted from each sample (protein extracts) into the wells, 10 μL of 90 μM myoglobin solution (lyophilized myoglobin from equine heart, Sigma-Aldrich, in 5 mM phosphate potassium, 0.9% NaCl, pH 7.4) and 150 μL of 600 μM ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) were added the wells. The reaction was started by adding 40 μL of 500 mM hydrogen peroxide (H2O2) followed by an incubation at room temperature for 5 min. The absorbance was read in the microplate reader (Multisky Scan, Thermoscientific) at 405 nm. To determine the concentration of total antioxidants (enzymatic and non-enzymatic) present in the samples, a calibration curve was constructed using serial dilutions of Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) standards to obtain a concentration range from 0 to 0.330 mM. Total antioxidants concentration was normalized per total proteins in each sample (given in mM mg−1 of total protein).
The Lipid Peroxidation assay was adapted from the thiobarbituric acid reactive substances (TBARS) protocol [65]. Five μL of each sample was added to 45 μL of 50 mM Phosphate buffer (pH 7.0–7.4). 12.5 μL of SDS 8.1% and 93.5 μL of trichloroacetic acid (TCA 20%) was added for deproteination of the samples. For LPO detection, 93.5 μL of thiobarbituric acid (TBA 1%) and 50 µL of MQ-water were added to the microtubes. After samples were mixed in the vortex, the microtubes were incubated in boiling water for 10 min and placed on ice to cool down right after. 62.5 µL of ultra pure water was added, duplicates of 150 µL of each sample were pipetted in the microplate and the absorbance was read at 532 nm. To quantify the lipid peroxides, an eight-point calibration curve (0–0.3 μM) was constructed using malondialdehyde (MDA) as standard. MDA concentration in samples was normalized per total proteins in each sample (given in nmol mg−1 of total protein).
2.4. Statistical Analysis
Data normality was assessed using Shapiro–Wilk’s test and homogeneity of variance using Bartlett’s and Levene’s tests. Routine activity was assessed using a generalized mixed model “glmm” with Temperature, Food and Day of observation as fixed factors and ID of individual as a random factor to account for the repeated measures, following a Poisson distribution. Data was tested for over dispersion, and if a significant over or under dispersion was found, a negative binomial distribution was applied. A specific generalized mix model “glmmTMB” was used to explore possible relations between behaviours and treatments, if zero inflation was detected the model would be adjusted with ziformula = ~1. All behaviours followed the negative binomial distribution except Locomotion, which showed a gaussian distribution. For the observed behaviours, different models were fitted and compared, and the best model was selected based on the lowest AIC value (Supplementary Table S3). Impacts of temperature and food availability were assessed on RMR, using a 2-factor “glm”. Reproductive success, gonadosomatic index, fatty acid content on the eggs and biomarkers on the larvae were assessed using a “glm” with temperature as the fixed factor, following a normal distribution. The number of clutches laid and successfully hatched were also measured with a glm but followed a Poisson and negative binomial distribution, respectively. Differences in the larvae morphometric measures were also accessed with a “glm”, with temperature as the fixed factor. For the gene expression analysis, outlier observations were identified and replaced by missing values using a generalized extreme studentized deviate procedure (e.g., Jain [66]), with a p-value of 0.05 and a maximum number of outliers set at 20% of the sample size. Gene expression levels were logarithmically transformed [log10 (x + 1)] to meet parametric test assumptions. A generalized linear model “glm” was used to check for differences between treatments with temperature and food availability as fixed factors. Size and weight of the fish measured in the different time points were assessed with a 2 factor “glm”, and survival was assessed with a χ2 test. All statistical analyses were performed with R [67]. Results were considered statistically significant at p ≤ 0.05.
3. Results
3.1. Temperature and Food Availability Effects on Behaviour and Physiology
3.1.1. Routine Activity
The time it took for the fish to ignore the observer was significantly lower for fish kept under higher temperatures (Table 2); throughout the 3 days of observations, fish also took less time to ignore the observer (Table 2).

Table 2.
Model coefficients for the generalized mixed model: time (in s) two-spotted goby, Pomatochistus flavescens took to ignore the observer (Time to Ignore), spent moving in the aquarium (Locomotion), or were resting, not swimming (Rest); frequency the fish hide in the aquarium (Hide), or showed aggressive behaviours (Aggression), during the observation period for the treatment conditions (temperature and ration) and day of observation. Bold text indicates significant treatment effects (p ≤ 0.05) as compared to CT + HR.
During the observation period, fish were found either actively swimming in the tank or resting on the bottom; however, neither behaviour (locomotion nor rest) was statistically significant in the different treatments (Table 2). The frequency of hiding behaviours, observed when fish hide in or below the nests, or between algae, was significantly affected by exposure to low rations, with fish spending less time hiding when under food shortage (Table 2). Contrarily, fish exposed to the treatment interaction (high temperature x low ration) spent significantly more time hiding (Table 2). The frequency of aggressive behaviours during the observational period did not differ among treatments (Table 2).
3.1.2. Metabolic Rate
Routine metabolic rate was not affected by 1 month of exposure to treatments (Table 3), although average oxygen consumption levels tended to be lower under high temperature treatments (MO2Ambient = 507.57 ± 108.10 mg−1 kg−1 h−1; MO2Low-ration = 441.89 ± 75.48 mg−1 kg−1 h−1; MO2HighT°C = 304.08 ± 85.13 mg−1 kg−1 h−1; MO2HighT°CxLow-ration = 386.35 ± 111.96 mg−1 kg−1 h−1).

Table 3.
Effect of temperature and food availability on two-spotted goby, Pomatochistus flavescens routine metabolic rate (MO2 mg−1 kg−1 h−1).
At the end of 1 month of exposure to treatments, there was no difference in the size and weight of the fish, among treatments (Table 4).

Table 4.
Effect of temperature (CT, HT) and food availability (HR, LR) on two-spotted goby, Pomatochistus flavescens size (SL) and weight (W) after one month in the treatments.
3.2. Temperature and Food Availability Effects on Reproduction
3.2.1. Reproductive Success
Couples under high ration reproduced throughout the entire breeding season, regardless of the temperature. A total of 65 clutches were produced by 20 breeding pairs: 9 out of the 10 couples in the ambient temperature and high ration treatment laid 32 clutches, of which 10 successfully hatched; 10 out of 10 couples in the high temperature and high ration treatment laid 31 clutches, of which 7 successfully hatched. High temperature did not affect the number of laid clutches (Z(1,20) = −0.083, p = 0.935) or hatched clutches (Z(1,20) = −0.466, p = 0.641). Low ration treatments, though, had detrimental impacts on reproductive activity of the two-spotted goby, as fish under these conditions did not reproduce throughout the experimental period. The average number of eggs per clutch did not differ between ambient and high temperature (t(1,56) = 0.869, p = 0.389, Figure 2), however, eggs were significantly smaller under high temperature (t(1,56) = −2.008, p = 0.050 Figure 2). Nevertheless, the overall reproductive output (number of eggs × mean egg area) was not affected by high temperatures (t(1,56) = 0.217 p = 0.829). Embryonic development lasted for 8–10 days, regardless of the treatment. Despite smaller eggs, larval size at hatch did not differ between treatments, as well as all the other morphometric traits (SL: t(1,157) = −0.332, p = 0.74; HH: t(1,157) = −0.755, p = 0.451; BL: t(1,157) = 0.055, p = 0.956; MDV: t(1,157) = −0.653, p = 0.515), although there was a tendency for smaller larvae under high temperature treatment (Figure 2). GSI was significantly affected by elevated temperatures and low rations on males of two-spotted gobies (Supplementary Table S4, Figure 3), but no effect of the treatments was found on the females (Supplementary Table S4, Figure 3).
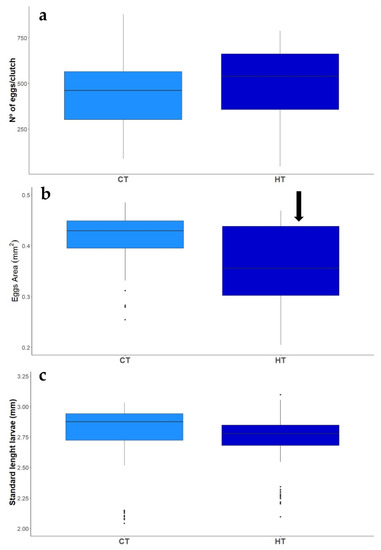
Figure 2.
Effect of exposure to Control (CT) and High (HT) temperature treatments on the average number of eggs per clutch (a), egg area (b) and the size of larvae at hatch (c) of two-spotted goby, Pomatochistus flavescens. Black arrow indicates significant treatment effects (p ≤ 0.05), as compared to CT + HR treatment.
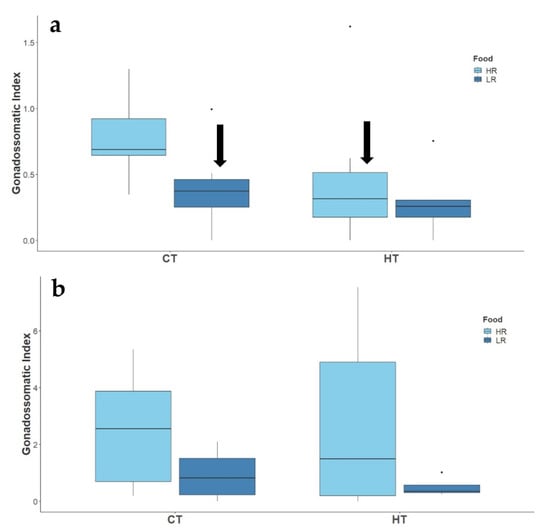
Figure 3.
Effect of temperature (CT and HT) and food availability (HR, LR) on the gonadosomatic index of males (a), and females (b), of adult two-spotted goby, Pomatochistus flavescens. Black arrows indicate significant treatment effects (p ≤ 0.05) as compared to CT + HR treatment.
3.2.2. Expression of Reproduction-Related Genes
Significant differences on gene expression were only detected for males. The levels of cyp11b1 changed significantly with food availability (t(2,26) = −3.527, p = 0.001) and temperature (t(2,26) = −2.479, p = 0.020) but were not significantly affected by the interaction between these factors (t(2,26) = 1.896, p = 0.069). The levels of female genes, cyp19a1 and 20α-hsd, did not change with food availability (cyp19a1: t(2,18) = −1.562, p = 0.136; 20α-hsd: t(2,18) = −0.07, p = 0.939; Figure 4) or temperature (cyp19a1: t(2,18) = −1.193, p = 0.248; 20α-hsd: t(2,18) = −0.895, p = 0.383; Figure 4). The interaction between these factors was also non-significant (cyp19a1: t(2,18) = 0.988, p = 0.336; 20α-hsd: t(2,18) = −0.146, p = 0.886; Figure 4).
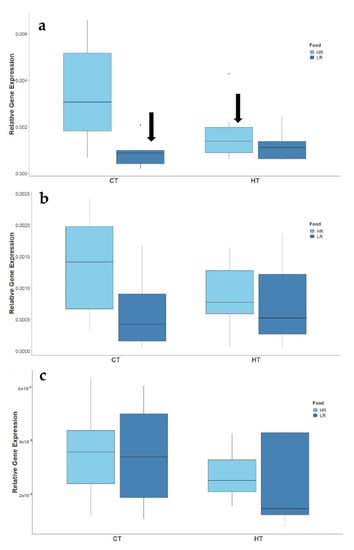
Figure 4.
Effect of temperature (CT and HT) and food availability (HR, LR) on the expression of gonadal steroidogenic enzymes cyp11b1 (a); cyp19a1 (b); 20β-hsd (c) of two-spotted goby, Pomatochistus flavescens. Black arrows indicate significant treatment effects (p ≤ 0.05) as compared to CT + HR treatment.
By the end of the exposure period, fish under the low ration treatment were significantly smaller and leaner than controls (Figure 5). Fish under the interaction of high temperature and low ration treatment, however, did not show statistical significance when compared to fish in control (Supplementary Table S5), possibly due to the several outliers found on their size and weight (Figure 5). At the end of the experiment, the survival of fish on control and high temperature treatments was 85% and 90%, respectively. Fish under the low ration and high temperature × low ration treatment had a survival of 65% and 50%, respectively; however, these values were not statistically significant. (χ2 = 2.828, df = 3, p = 0.419).
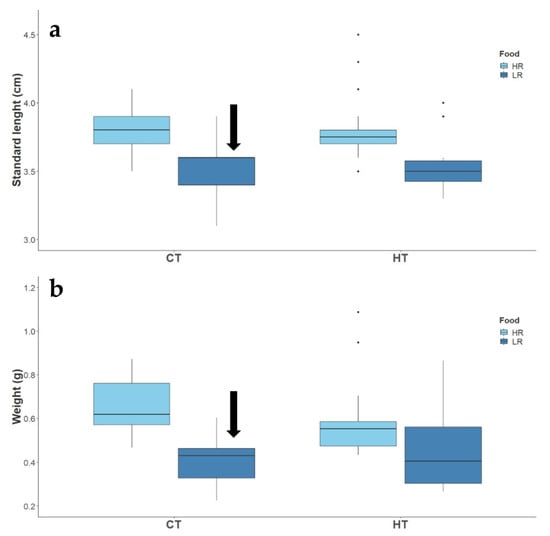
Figure 5.
Effect of temperature (CT and HT) and food availability (HR, LR) on the standard length (a), and weight (b), of adult two-spotted goby, Pomatochistus flavescens, at the end of the exposure period. Black arrows indicate significant treatment effects (p ≤ 0.05) as compared to CT + HR treatment.
3.2.3. Fatty Acid Content
Eggs exposed and descended from couples at higher temperature treatment presented a significantly higher percentage of SFAs than the ones at the control temperature (25% against 24%, respectively; p = 0.027), as shown in Table 5 (see also Supplementary Table S6). The higher level of SFAs under the high temperature scenario was mainly related to the higher levels of palmitic acid (16:0; 12% against 13%, in control and high temperature, respectively; p = 0.012, Supplementary Table S6). Regarding monounsaturated fatty acids (MUFA), no significant differences were found with temperature, with higher levels for oleic and vaccenic acids (18:1 n-9/n-7; 20% for both temperatures), and lower for 7-hexadecenoic and palmitoleic acids (16:1 n-9/n-7; 5% for both temperatures). We only found significant differences in polyunsaturated fatty acids (PUFA) with temperature for the eicosapentaenoic acid (EPA, 20:5 n-3) with higher levels in the high temperature scenario, 6% compared with 5% under control temperature (p = 0.013, Supplementary Table S6). Nonetheless, and even though not statistically different (p < 0.05), there is also a higher level of total PUFA with temperature, 41% against 37% under control, representing a p-value of 0.09. The same trend is found for alpha linolenic acid (18:3 n-3; p = 0.058, Supplementary Table S6), total n-3 (24% versus 29%, p = 0.063, Supplementary Table S6), n-3/n-6 ratio (2% versus 3%, p = 0.063, Supplementary Table S6) and unknown FAs (13% versus 8%, p = 0.101, Supplementary Table S6).

Table 5.
Fatty acid composition (% of total FA) on eggs of two-spotted goby, Pomatochistus flavescens in Control (CT) and High (CT) temperature treatments (mean ± s.e.). Fish under low ration did not reproduce. Bold text indicates significant differences between temperatures in fatty acid composition.
3.2.4. Biomarkers
Total antioxidant capacity (TAC) levels in recently hatched larvae did not differ between temperature treatments, with average levels of 0.38 ± 0.14 mM mg−1 total protein under control temperature and 0.38 ± 0.11 mM mg−1 total protein for the high temperature treatment (Table 6, Figure 6). Regarding HSP70 levels, temperature did not increase HSP70 levels, 0.59 ± 0.30 µg mg−1 total protein and 0.50 ± 0.25 µg mg−1 total protein, under control and high temperature treatments, respectively (Table 6, Figure 6). Lipid peroxidation levels, measured as MDA levels, however, significantly increased with temperature, from an average of 0.01± 0.006 nmol mg−1 total protein under control temperature, to an average of 0.02 ± 0.009 nmol mg−1 total protein in the high temperature treatment (Table 6, Figure 6).

Table 6.
Effect of Control (CT) and High (HT) temperature treatments on larvae of two-spotted goby, Pomatochistus flavescensMDA (nmol mg−1 total protein), TAC (mM mg−1 total protein) and HSP70 (µg mg−1 total protein). Bold text indicates significant treatment effects (p ≤ 0.05) as compared to CT + HR treatment.
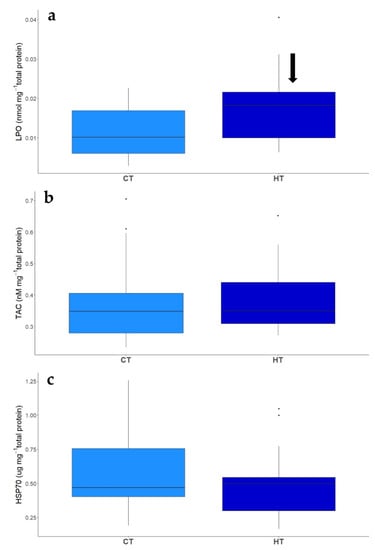
Figure 6.
Effect of exposure to Control (CT) and High (HT) temperature treatments on LPO (a), TAC (b) and HSP70 (c) of larvae of two-spotted goby, Pomatochitus flavescens, at hatching. Black arrows indicate significant treatment effects (p ≤ 0.05) as compared to CT + HR treatment.
4. Discussion
The current results demonstrate that food availability played a stronger influence on two-spotted goby behaviour and physiology than temperature itself. Fish under LR showed poor body condition and increased mortality. Moreover, reproduction was completely impaired in fish under LR treatments, regardless of temperature conditions, which indicates that changes in food availability have paramount implications for populations. Contrarily, exposure to HT, per se, did not induce changes in adults’ behaviour, metabolism, or reproductive performance, but it did affect egg area, with smaller eggs under higher temperatures.
Individual variation in routine behaviour is important in a species as it allows for populations to solve challenges posed by their complex environments differently [68]. In this study, assessing fish activity after the first month of exposure to the treatments allowed us to understand how fish would be affected by temperature and food availability in routine behaviours like swimming, resting, hiding, as well as assessing their aggressiveness, which is higher during the reproductive season [49]. Swimming and resting activities were not influenced by exposure to high temperature, low food availability, or the interaction of stressors. Different studies suggest that temperature can impact locomotion and activity of fish differently. Elevated temperature was shown to negatively influence swimming activity in juvenile Salmo trutta, brown trout [69]. In [26] while testing two species of minnows, Chrosomus erythrogaste, demonstrated strikingly opposite trends of activity rates. Although it was hypothesized that food availability would limit fish activity, as a strategy for saving energy, this was not the case. Ref. [70] showed an effect of low food ration on swimming activity of brown trout fry, but the effects were opposite to our hypothesis, as fry tended to be more active. Likewise, aggressiveness in the two-spotted goby was also not influenced by any of the treatments. Exposure to elevated temperatures can increase aggressive behaviours towards conspecifics, as described in Pomacentrus moluccensis, lemon damselfish, after 4 days of exposure to the temperatures, but a prolonged exposure to elevated temperature decreased aggressiveness to control levels [71], which the authors justify as an acclimation response. Exposure to food shortage conditions leads to an increase in aggressiveness in brown trout fry [70]. Interestingly, time spent by two-spotted goby hiding in the nests or algae was significantly lower in the LR treatment, which might suggest higher propensity to take risks when seeking for prey options. Hiding can be a response to predators’ presence [72] and can be related to the fish willingness to take risks or be averse to them. A study on juvenile Dicentrarchus labrax, seabass, showed fish under a food-deprived regime for 7 days increased their risk-taking behaviour [73]. A different study on juveniles of the same species however, showed that after a 3-week period of food deprivation, the seabass, did not show any differences in risk-taking and exploratory patterns [74]. Interestingly, and opposite to what was found under LR, under the combination of HT × LR, two-spotted goby spent more time hiding. Increasing temperatures usually increase energetic demands [75], and since these fish had lower access to food, this might suggest a strategy to conserve energy, particularly because they were in their reproductive season. Contrarily to our results, a study on juveniles of Pomacentrus chrysurus, whitetail damselfish, exposed to different temperatures and food availability conditions, showed the interaction of these two factors influence their risk assessment behaviour, making the fish more risk-prone in their foraging behaviour [45]. Risk-prone individuals accept the risk to gain, in return of important information and higher rewards, while risk-averse individuals, avoid risking situation accepting that lower risk will also lower the gains [74].
Metabolic performance of the two-spotted goby was not affected by exposure to elevated temperature or low food availability, which indicates an import degree of plasticity towards thermal challenge. Although we expected higher temperatures to increase metabolic and energetic demands [75,76], the high temperatures tested fell within the two-spotted goby temperature range of variability. It is therefore possible that fish successfully acclimated and can manage their metabolic demands in these higher temperatures. A study on the effects of temperature and food availability on larvae of Amphiprion percula, the anemonefish, showed routine oxygen consumption was higher at elevated temperatures [77], however food availability did not impact the oxygen consumption, as the method to calculate metabolic rates accounts for individuals’ weight. A different study in the white seabream, Diplodus sargus, showed that the interaction of elevated temperatures and low food availability decreases RMR [48]. In our study, the lack of an effect on temperature and food availability in the RMR of the fish can be related with the exposure time. Fish were only exposed to the treatments for one month when they were tested, and this might have not been enough time to induce metabolic changes. More so, as mentioned before, two-spotted goby specimens were tested for elevated temperatures within their temperature range of variability, and it might be possible they are already acclimated to these temperatures. Finally, our experiment was done in fully grown adults, which have wider thermal windows and aerobic scope than early life stages [13,78] and are therefore less likely to be negatively affected by environmental stressors. Future studies should look to the impacts of temperature and food availability on behaviour and metabolism for longer periods of time, and also test different levels of temperature, especially outside this species variability range, like extreme climatic events (marine heat waves).
Temperature is one of the most important cues for the onset of the reproductive season. Changes in temperature can delay or even interrupt the reproductive process, affecting the long-term survival of the species [79]. Reproduction of the two spotted goby was shown to be impaired by high temperature. In a different study fish from the same species (different population) were exposed to ambient and +3 °C for a 3-month period, coincident with the breeding season, and showed reproduction failure in couples under high temperature, as well as decreased egg size [5]. Although temperature in the current study did not affect reproductive performance of the fish, it also led to smaller eggs. We argue that these seemingly contradictory results, on the same species, might reflect a population dependent effect, as the author of the study tested fish from the Swedish population of two-spotted goby, which inhabit a fjord [5]. The Portuguese population of the present study, on the other hand, inhabits the rocky shores of the Atlantic Ocean coast, the different habitats can have a strong influence in the fish plasticity to temperature variability.
In this study it was also investigated how the regulatory molecular mechanisms underlying reproduction were affected by temperature and food availability. It was showed that the pattern of expression of the gene cyp11b1 was influenced by low food availability (LR) and high temperatures (HT). LR is responsible for significantly decreasing the expression of cyp11b1 in males, which can explain why male fish in this condition had lower GSI and did not show courtship behaviour (observation only). In the case of male fish exposed to HT there is a decrease in their GSI in accordance with a previous study [5], and also a decrease in the expression of cyp11b1. However, GSI and cyp11b1 on males exposed to higher temperatures and low food availability (HT + LR) was not affected. This unexpected result might be related to low statistical power of the data on the males GSI and gene expression of cyp11b1 for HT + LR, since at the end of the experiment the sample size of this group was low. Together, these results suggest that low food availability has impaired male reproduction, given the pivotal role of cyp11b1 in the synthesis of the most important fish androgen, 11-ketotestosterone, which displays higher expression during the pre-spawning phase of the testicular cycle [80], consequently affecting the regulation of testis growth and maturation [81,82,83] and reproductive behaviour (e.g., [84]). Thus, though females were not seen to lay eggs, even if they did males were probably not able to fertilize them. Still, further studies should measure 11-ketotestosterone blood levels and analyse gonads histology to confirm this hypothesis. Interestingly, we detected no differences in females’ GSI and ovaries’ gene expression, for all the treated groups in comparison to the control group, even though we reported an impairment of the reproductive output in the short fed groups. Thus, it was hypothesized that, in these groups, other mechanisms and/or molecular pathways are being affected, since steroidogenic enzymes are putatively present (because gene expression occurs), but still, females did not lay eggs nor were seen courting. It seems like females continue investing in functionally reproductive ovaries even in food shortage conditions, contrary to males that stop investing in mature testis in these stressful situations. Moreso, LR treated females may be saving energy through a different mechanism, such as skip spawning. This is a phenomenon that has been reported in many fish species and is linked to deficient feeding conditions and energy accumulation (reviewed in [85,86]). For instance, it has been reported that in fish may occur retaining (i.e., fully ripened eggs are never released) or reabsorbing (vitellogenic oocytes are reabsorbed via follicular atresia) processes (e.g., [87]) leading to spawning omission. In the two-spotted goby, food demanding conditions seem to induce different energy allocation strategies between males and females, as males seem to invest in their physical condition while females continue allocating energy in gonad maturation but skip spawning. Sex differences in energy allocation have already been reported, in a study with Salmo salar L., the Atlantic salmon. In this species, researchers showed that during the reproduction season, males spend more energy investing in somatic tissue while females’ energetic changes occur preferentially at the gonadal tissue [88].
The FA composition of fish eggs is often used to evaluate their quality, as they must provide the required nutritional needs to endure embryonic and larval development, which will have direct consequences on hatching, survival and growth of fish larvae [89]. In the present study, the FA profile of eggs of the two-spotted goby, showed similar contents to the ones found in eggs of Solea solea, common sole, [90] and Acipenser baerii, Siberian sturgeon, [91], showing high levels of oleic and vaccenic acids (18:1 n-9/n-7), followed by palmitic acid (16:0), 18:3 n-3, linoleic acid (18:2 n-6), stearic acid (18:0), docosahexaenoic acid (DHA, 22:6 n-3) and eicosapentaenoic acid (EPA, 20:5 n-3). Differences in eggs FA profiles observed between the two different temperature regimes were minimal and mostly underpinned by higher levels of SFA, mainly related to higher levels of palmitic acid (16:0), and eicosapentaenoic acid (EPA, 20:5 n-3) at the higher temperature. Nonetheless it is important to mention that this is the first report on the FA profile of two-spotted goby embryos, and that comparisons between different populations, or even different life stages, were impossible to make.
Temperature is known to affect the FA profile of marine organisms, specifically due to lipids role in maintaining cell membrane integrity maintenance, and both palmitic acid and EPA are important for this task [89,92]. However, in the present study, we obtained different results from those already described in literature [93], as the proportion of unsaturated FA tend to increase at lower temperatures rather than at higher temperatures, in a process called homeoviscous adaptation that helps organisms to maintain the correct membrane fluidity at low temperatures. Nonetheless, the FA analysis in the present study refers to the total FA proportion in the two-spotted goby eggs and not only on the phospholipidic fraction. Therefore, high levels of PUFAs, such as EPA at higher temperatures could be a sign of maternal investment on the success of progeny, as this essential fatty acid (EPA) is not synthetized by the fish and must be provided via diet [94,95]. EPA has been proven essential not only for reproduction but also for embryo/larval development in many fish species [96].
Despite this possible increase in FA content in eggs due to maternal investment, we did observe smaller eggs under HT treatment, as well as an increase in lipid peroxidation levels in larvae from this exposure treatment. Early life stages are known to be particularly sensitive to environmental fluctuations and in this study, it was not an exception. The rise in temperature did not cause changes in the antioxidant capacity (TAC) or HSP70 levels, which concomitantly leads to lipid damage. Previous studies have also showed an increase in MDA levels with temperature in Solea Senegalensis, Senegalese sole, larvae after 30 days post-hatch [97].
5. Conclusions
Overall, the findings of the present study suggest a pronounced effect of low food availability on fish reproductive performance, as reproduction was completely cancelled. In a different study, it was found that Acanthochromis polycanthus, spiny chromis, reproduction failed completely when fish were under higher temperatures and low food availability [47]. Although lack of breading in some species may represent only a delay, the two-spotted goby life cycle is of 1 to 1.5 years [49], and they have only one breading season. The success or failure of the breeding season of the two-spotted goby will certainly affect the species’ long-term survival. Moreover, the fitness of fish under the low ration treatment after 5 months of exposure decreased significantly. Low condition fish presented a pronounced curvature in the spine that increased with exposure time, which translated in smaller sized fish by the end of the experimental period. Unexpectedly, fish under high temperature and low ration treatment did not present significant differences compared to control conditions, which might be attributed to outliers, and also to the fact that half of the fish under this treatment died before the end of the experiment, which might mean the fish in the worst body condition did not make it and thus the remaining fish had better, even if slightly, conditions. Exposure to warming conditions, per se, did not induce significant changes in reproductive performance; however, there seem to be costs at the offspring level, with smaller eggs, altered FA contents, and increased lipid peroxidation levels in recently hatched larvae. Whether or not these changes at the offspring level will have consequences later in life remains to be tested and should be a priority for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes7040194/s1, Table S1. Temperature (°C) (mean ± s.d.) over the experimental period (April–August 2019) in the four treatments: the two levels of temperature control (CT) and high (HT), and the two levels of food availability, high ration (HR), and low ration (LR); Table S2. Primer sequences and amplicon size for the genes studied; Table S3. Aikake’s Information Criteria (AIC) model selection for “glmm” with: (1) negative binomial distribution for Time to Ignore the observer; (2) gaussian distribution for duration of Locomotion behaviours; (3) negative binomial distribution for duration of Rest behaviours; (4) negative binomial distribution for the frequency fish Hide; (5) negative binomial distribution for the frequency fish show Aggression. Temperature refers to Control (CT) or High T°C (HT). Food refers to high ration (HR) or low ration (LR) treatments, day refers to the day of observation (1 to 3). The selected model is shown in bold; Table S4. Effect of temperature (control, CT, and high, HT) and food availability (high ration, HR, and low ration, LR) on the gonadosomatic index of males (♂) and females (♀) of two-spotted gobies, Pomatochistus flavescens. Bold text indicates significant treatment effects (p ≤ 0.05) as compared to CT + HR; Table S5. Effect of temperature (control, CT, and high, HT) and food availability (high ration, HR, and low ration, LR) on two-spotted goby, Pomatochistus flavescens size (SL) and weight (W), after 4 months of exposure to the treatments. Bold text indicates significant treatment effects (p ≤ 0.05) as compared to CT + HR; Table S6. Effect of Control (CT) and High (HT) temperature treatments on fatty acid profile (% of total FA) of two-spotted goby, Pomatochistus flavescens. Bold text indicates significant treatment effects (p ≤ 0.05) as compared to CT + HR treatment.
Author Contributions
Conceptualization, A.M.F. and E.J.G.; methodology, A.F.L., R.M., S.M.-C. and C.V.; samples analysis, C.M., P.M.C., A.R.L., A.S.F., R.F.O. and N.M.B.; resources, A.M.F., P.M.C., R.F.O., E.J.G., N.M.B. and C.V.; writing—original draft preparation, A.F.L. and A.M.F.; writing—review and editing, all authors; supervision, A.M.F., R.F.O., P.M.C., C.M. and N.M.B.; project administration, A.M.F.; funding acquisition, A.M.F., P.M.C., R.F.O. and N.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the PhD grant of Ana F. Lopes (SFRH/BD/131592/2017), the strategic Project MARE/UIDB/MAR/04292/2020, UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020 and the Project NextGen (PTDC/CTA-AMB/31532/2017), co-financed by FCT through national funds and by the European Union through FEDER (European Regional Development Fund). The research leading to these results received partial funding from the European Union’s Horizon 2020 research and innovation program. This work was also financed by national funds from FCT in the scope of the project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences-UCIBIO and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy-i4HB. Carolina Madeira was supported by FCT through a research grant CEECIND/01526/2018, and Ana Rita Lopes was supported by FCT through a research grant CEECIND/00067/2018.
Institutional Review Board Statement
This study was carried out under the approval of Direção-Geral de Alimentação e Veterinária (DGAV, Portuguese Authority for Animal Health, permit 0421/000/000/2020) and according to the ISPA, IU animal ethics guidelines. Fishes were collected under the approval of Instituto da Conservação da Natureza e das Florestas (ICNF, permit 25624/2018/DCNF-LVT/DPAP).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Thanks to António Roleira for the valuable assistance throughout the experimental work, and Henrique Folhas, Gonçalo Ramos, Maria Jiménez, Noélia Rios, Ronnie Pinheiro for the support on the field work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Djurichkovic, L.D.; Donelson, J.M.; Fowler, A.M.; Feary, D.A.; Booth, D.J. The Effects of Water Temperature on the Juvenile Performance of Two Tropical Damselfishes Expatriating to Temperate Reefs. Sci. Rep. 2019, 9, 13937. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.T.; Donelson, J.M.; McCormick, M.I. Extended Exposure to Elevated Temperature Affects Escape Response Behaviour in Coral Reef Fishes. PeerJ 2017, 2017, 5, e3652. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, G.E.; Crawley, N.; Lunde, I.G.; Munday, P.L. Elevated Temperature Reduces the Respiratory Scope of Coral Reef Fishes. Glob. Chang. Biol. 2009, 15, 1405–1412. [Google Scholar] [CrossRef]
- Vinagre, C.; Madeira, D.; Mendonça, V.; Dias, M.; Roma, J.; Diniz, M.S. Effect of Increasing Temperature in the Differential Activity of Oxidative Stress Biomarkers in Various Tissues of the Rock Goby, Gobius Paganellus. Mar. Environ. Res. 2014, 97, 10–14. [Google Scholar] [CrossRef]
- Lopes, A.F.; Faria, A.M.; Dupont, S. Elevated Temperature, but Not Decreased PH, Impairs Reproduction in a Temperate Fish. Sci. Rep. 2020, 10, 20805. [Google Scholar] [CrossRef]
- Hopkins, K.; Moss, B.R.; Gill, A.B. Increased Ambient Temperature Alters the Parental Care Behaviour and Reproductive Success of the Three-Spined Stickleback (Gasterosteus Aculeatus). Environ. Biol. Fishes 2011, 90, 121–129. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Munday, P.L. Effects of Climate Change on Fish Reproduction and Early Life History Stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Sswat, M.; Stiasny, M.H.; Jutfelt, F.; Riebesell, U.; Clemmesen, C. Growth Performance and Survival of Larval Atlantic Herring, under the Combined Effects of Elevated Temperatures and CO2. PLoS ONE 2018, 13, e0191947. [Google Scholar] [CrossRef] [Green Version]
- Fox-Kemper, B.; Hewitt, H.T.; Xiao, C.; Aðalgeirsdóttir, G.; Drijfhout, S.S.; Edwards, T.L.; Golledge, N.R.; Hemer, M.; Kopp, R.E.; Krinner, G.; et al. Ocean, Cryosphere and Sea Level Climate Change 2021: The Physical Science Basis Contribution Working Group I to Sixth Assessment. Rep. Intergov. Panel Clim. 2021, 2018, 1–257. [Google Scholar]
- IPCC. Assessment Report 6 Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; IPCC: Geneva, Switzerland, 2021; In Press. [Google Scholar]
- Deslauriers, D.; Kieffer, J.D. The Effects of Temperature on Swimming Performance of Juvenile Shortnose Sturgeon (Acipenser Brevirostrum). J. Appl. Ichthyol. 2012, 28, 176–181. [Google Scholar] [CrossRef]
- Milazzo, M.; Mirto, S.; Domenici, P.; Gristina, M. Climate Change Exacerbates Interspecific Interactions in Sympatric Coastal Fishes. J. Anim. Ecol. 2013, 82, 468–477. [Google Scholar] [CrossRef]
- Laubenstein, T.D.; Rummer, J.L.; Nicol, S.; Parsons, D.M.; Pether, S.M.J.; Pope, S.; Smith, N.; Munday, P.L. Correlated Effects of Ocean Acidification and Warming on Behavioral and Metabolic Traits of a Large Pelagic Fish. Diversity 2018, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Beck, H.J.; Feary, D.A.; Fowler, A.M.; Madin, E.M.P.; Booth, D.J. Temperate Predators and Seasonal Water Temperatures Impact Feeding of a Range Expanding Tropical Fish. Mar. Biol. 2016, 163, 70. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Munday, P.L.; Rummer, J.L.; McCormick, M.I.; Corkill, K.; Watson, S.-A.; Allan, B.J.M.; Meekan, M.G.; Chivers, D.P. Interactive Effects of Ocean Acidification and Rising Sea Temperatures Alter Predation Rate and Predator Selectivity in Reef Fish Communities. Glob. Chang. Biol. 2015, 21.5, 1848–1855. [Google Scholar] [CrossRef]
- Allan, B.J.M.; Domenici, P.; Watson, S.A.; Munday, P.L.; McCormick, M.I. Warming Has a Greater Effect than Elevated CO2 on Predator–Prey Interactions in Coral Reef Fish. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170784. [Google Scholar] [CrossRef] [Green Version]
- Servili, A.; Canario, A.V.M.; Mouchel, O.; Muñoz-Cueto, J.A. Climate Change Impacts on Fish Reproduction Are Mediated at Multiple Levels of the Brain-Pituitary-Gonad Axis. Gen. Comp. Endocrinol. 2020, 291, 113439. [Google Scholar] [CrossRef]
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From Gametogenesis to Spawning: How Climate-Driven Warming Affects Teleost Reproductive Biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Miller, G.M.; Kroon, F.J.; Metcalfe, S.; Munday, P. Temperature Is the Evil Twin: Effects of Increased Temperature and Ocean Acidification on Reproduction in a Reef Fish. Ecol. Appl. 2015, 25, 603–620. [Google Scholar] [CrossRef]
- Parker, L.M.; Ross, P.M.; O’Connor, W.A. The Effect of Ocean Acidification and Temperature on the Fertilization and Embryonic Development of the Sydney Rock Oyster Saccostrea Glomerata (Gould 1850). Glob. Chang. Biol. 2009, 15, 2123–2136. [Google Scholar] [CrossRef]
- Rajakumar, A.; Senthilkumaran, B. Steroidogenesis and Its Regulation in Teleost-a Review. Fish Physiol. Biochem. 2020, 46, 803–818. [Google Scholar] [CrossRef]
- Strüssmann, C.A.; Conover, D.O.; Somoza, G.M.; Miranda, L.A. Implications of Climate Change for the Reproductive Capacity and Survival of New World Silversides (Family Atherinopsidae). J. Fish Biol. 2010, 77, 1818–1834. [Google Scholar] [CrossRef]
- Sandra, G.E.; Norma, M.M. Sexual Determination and Differentiation in Teleost Fish. Rev. Fish Biol. Fish. 2010, 20, 101–121. [Google Scholar] [CrossRef]
- Díaz, N.; Piferrer, F. Lasting Effects of Early Exposure to Temperature on the Gonadal Transcriptome at the Time of Sex Differentiation in the European Sea Bass, a Fish with Mixed Genetic and Environmental Sex Determination. BMC Genom. 2015, 16. [Google Scholar] [CrossRef] [Green Version]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I. Climate Change May Affect Fish through an Interaction of Parental and Juvenile Environments. Coral Reefs 2012, 31, 753–762. [Google Scholar] [CrossRef]
- Frenette, B.D.; Bruckerhoff, L.A.; Tobler, M.; Gido, K.B. Temperature Effects on Performance and Physiology of Two Prairie Stream Minnows. Conserv. Physiol. 2019, 7, coz063. [Google Scholar] [CrossRef]
- Slesinger, E.; Andres, A.; Young, R.; Seibel, B.; Saba, V.; Phelan, B.; Rosendale, J.; Wieczorek, D.; Saba, G. Correction: The effect of ocean warming on black sea bass (Centropristis striata) aerobic scope and hypoxia tolerance. PLoS ONE 2020, 15, e0244002. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative Stress in Marine Environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [Green Version]
- Imbs, A.B.; Yakovleva, I.M. Dynamics of Lipid and Fatty Acid Composition of Shallow-Water Corals under Thermal Stress: An Experimental Approach. Coral Reefs 2012, 31, 41–53. [Google Scholar] [CrossRef]
- Oku, H.; Yamashiro, H.; Onaga, K.; Sakai, K.; Iwasaki, H. Seasonal Changes in the Content and Composition of Lipids in the Coral Goniastrea Aspera. Coral Reefs 2003, 22, 83–85. [Google Scholar] [CrossRef]
- Parrish, C.C. Lipids in Marine Ecosystems. ISRN Oceanogr. 2013, 2013, 604045. [Google Scholar] [CrossRef] [Green Version]
- Imbs, A.B.; Latyshev, N.A.; Dautova, T.N.; Latypov, Y.Y. Distribution of Lipids and Fatty Acids in Corals by Their Taxonomic Position and Presence of Zooxanthellae. Mar. Ecol. Prog. Ser. 2010, 409, 65–75. [Google Scholar] [CrossRef]
- Dalsgaard, J.; John, M.S.; Kattner, G.; Müller-Navarra, D.; Hagen, W. Fatty Acid Trophic Markers in the Pelagic Marine Environment. Adv. Mar. Biol. 2003, 46, 225–340. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T. Iso- and Anteiso-Fatty Acids in Bacteria: Biosynthesis, Function, and Taxonomic Significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Mortillaro, J.M.; Pitt, K.A.; Lee, S.Y.; Meziane, T. Light Intensity Influences the Production and Translocation of Fatty Acids by Zooxanthellae in the Jellyfish Cassiopea Sp. J. Exp. Mar. Bio. Ecol. 2009, 378, 22–30. [Google Scholar] [CrossRef]
- Drazen, J.C.; Phleger, C.F.; Guest, M.A.; Nichols, P.D. Lipid, Sterols and Fatty Acid Composition of Abyssal Holothurians and Ophiuroids from the North-East Pacific Ocean: Food Web Implications. Comp. Biochem. Physiol. -B Biochem. Mol. Biol. 2008, 151, 79–87. [Google Scholar] [CrossRef]
- Patton, J.S.; Battey, J.F.; Rigler, M.W.; Porter, J.W.; Black, C.C.; Burris, J.E. A Comparison of the Metabolism of Bicarbonate 14C and Acetate 1-14C and the Variability of Species Lipid Compositions in Reef Corals. Mar. Biol. 1983, 75, 121–130. [Google Scholar] [CrossRef]
- Nyboer, E.A.; Chapman, L.J. Elevated Temperature and Acclimation Time Affect Metabolic Performance in the Heavily Exploited Nile Perch of Lake Victoria. J. Exp. Biol. 2017, 220, 3782–3793. [Google Scholar] [CrossRef] [Green Version]
- Hays, G.C.; Richardson, A.; Robinson, C. Climate Change and Marine Plankton. Trends Ecol. Evol. 2005, 20, 337–344. [Google Scholar] [CrossRef]
- Richardson, A.J. In Hot Water: Zooplankton and Climate Change. ICES J. Mar. Sci. 2008, 65, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Garzke, J.; Ismar, S.M.H.; Sommer, U. Climate Change Affects Low Trophic Level Marine Consumers: Warming Decreases Copepod Size and Abundance. Oecologia 2015, 177, 849–860. [Google Scholar] [CrossRef]
- Hixson, S.M.; Arts, M.T. Climate Warming Is Predicted to Reduce Omega-3, Long-Chain, Polyunsaturated Fatty Acid Production in Phytoplankton. Glob. Chang. Biol. 2016, 22, 2744–2755. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, J. An Introduction to Dynamic Energy Budget (DEB) Models with Special Emphasis on Parameter Estimation. J. Sea Res. 2006, 56, 85–102. [Google Scholar] [CrossRef]
- Green, B.S. Chapter 1 Maternal Effects in Fish Populations. Adv. Mar. Biol. 2008, 54, 1–105. [Google Scholar] [CrossRef]
- Lienart, G.D.H.; Mitchell, M.D.; Ferrari, M.C.O.; McCormick, M.I. Temperature and Food Availability Affect Risk Assessment in an Ectotherm. Anim. Behav. 2014, 89, 199–204. [Google Scholar] [CrossRef]
- Liu, S.; Fu, S.J. Correction: Effects of Food Availability on Metabolism, Behaviour, Growth and Their Relationships in a Triploid Carp. J. Exp. Biol. 2018, 221, jeb187302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donelson, J.; Munday, P.; McCormick, M.; Pankhurst, N.; Pankhurst, P. Effects of Elevated Water Temperature and Food Availability on the Reproductive Performance of a Coral Reef Fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.; Lopes, A.R.; Ribeiro, L.; Castanho, S.; Candeias-mendes, A.; Pousão-ferreira, P.; Faria, A.M. Effects of Exposure to Elevated Temperature and Different Food Levels on the Escape Response and Metabolism of Early Life Stages of White Seabream, Diplodus Sargus. Conserv. Physiol. 2022, 10, coac023. [Google Scholar] [CrossRef]
- Amundsen, T. Sex Roles and Sexual Selection: Lessons from a Dynamic Model System. Curr. Zool. 2018, 64, 363–392. [Google Scholar] [CrossRef]
- Borg, Å.A.; Forsgren, E.; Amundsen, T. Seasonal Change in Female Choice for Male Size in the Two-Spotted Goby. Anim. Behav. 2006, 72, 763–771. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour. In Measuring Behaviour; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar] [CrossRef]
- Killen, S.S.; Mitchell, M.D.; Rummer, J.L.; Chivers, D.P.; Ferrari, M.C.O.; Meekan, M.G.; Mccormick, M.I. Aerobic Scope Predicts Dominance during Early Life in a Tropical Damselfish. Funct. Ecol. 2014, 28, 1367–1376. [Google Scholar] [CrossRef]
- Song, Y.F.; Huang, C.; Shi, X.; Pan, Y.X.; Liu, X.; Luo, Z. Endoplasmic Reticulum Stress and Dysregulation of Calcium Homeostasis Mediate Cu-Induced Alteration in Hepatic Lipid Metabolism of Javelin Goby Synechogobius Hasta. Aquat. Toxicol. 2016, 175, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Leder, E.H.; André, C.; Le Moan, A.; Töpel, M.; Blomberg, A.; Havenhand, J.N.; Lindström, K.; Volckaert, F.A.M.; Kvarnemo, C.; Johannesson, K.; et al. Post-Glacial Establishment of Locally Adapted Fish Populations over a Steep Salinity Gradient. J. Evol. Biol. 2021, 34, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and Modifications of Primer Design Program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [Green Version]
- Kalendar, R.; Khassenov, B.; Ramankulov, Y.; Samuilova, O.; Ivanov, K.I. FastPCR: An in Silico Tool for Fast Primer and Probe Design and Advanced Sequence Analysis. Genomics 2017, 109, 312–319. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. HSP70 Production Patterns in Coastal and Estuarine Organisms Facing Increasing Temperatures. J. Sea Res. 2012, 73, 137–147. [Google Scholar] [CrossRef]
- Mukhopadhyay, I.; Nazir, A.; Saxena, D.K.; Kar Chowdhuri, D. Heat Shock Response: Hsp70 in Environmental Monitoring. J. Biochem. Mol. Toxicol. 2003, 17, 249–254. [Google Scholar] [CrossRef]
- Yamashita, M.; Yabu, T.; Ojima, N. Stress Protein HSP70 in Fish. Aqua-BioScience Monogr. 2010, 3, 111–141. [Google Scholar] [CrossRef]
- Kambayashi, Y.; Binh, N.T.; Asakura, H.W.; Hibino, Y.; Hitomi, Y.; Nakamura, H.; Ogino, K. Efficient Assay for Total Antioxidant Capacity in Human Plasma Using a 96-Well Microplte. J. Clin. Biochem. Nutr. 2009, 44, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J. A New Method for Measuring Antioxidant Activity. Biochem. Soc. Trans. 1993, 21, 95S. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Mihara, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Jain, R.B. A Recursive Version of Grubbs’ Test for Detecting Multiple Outliers in Environmental and Chemical Data. Clin. Biochem. 2010, 43, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Ballew, N.G.; Kjelvik, M.K. Fish Behavioral Types and Their Ecological Consequences. Can. J. Fish. Aquat. Sci. 2014, 71, 927–944. [Google Scholar] [CrossRef]
- Ojanguren, A.F.; Braña, F. Thermal Dependence of Swimming Endurance in Juvenile Brown Trout. J. Fish Biol. 2000, 56, 1342–1347. [Google Scholar] [CrossRef]
- Näslund, J.; Johnsson, J.I. State-Dependent Behavior and Alternative Behavioral Strategies in Brown Trout (Salmo trutta L.) Fry. Behav. Ecol. Sociobiol. 2016, 70, 2111–2125. [Google Scholar] [CrossRef] [Green Version]
- Warren, D.T.; Donelson, J.M.; McCormick, M.I.; Ferrari, M.C.O.; Munday, P.L. Duration of Exposure to Elevated Temperature Affects Competitive Interactions in Juvenile Reef Fishes. PLoS ONE 2016, 11, e0164505. [Google Scholar] [CrossRef] [Green Version]
- Lehtiniemi, M. Swim or Hide: Predator Cues Cause Species Specific Reactions in Young Fish Larvae. J. Fish Biol. 2005, 66, 1285–1299. [Google Scholar] [CrossRef]
- Killen, S.S.; Marras, S.; Mckenzie, D.J. Fuel, Fasting, Fear: Routine Metabolic Rate and Food Deprivation Exert Synergistic Effects on Risk-Taking in Individual Juvenile European Sea Bass. J. Anim. Ecol. 2011, 80, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Aimon, C.; Le Bayon, N.; Le Floch, S.; Claireaux, G. Food Deprivation Reduces Social Interest in the European Sea Bass Dicentrarchus Labrax. J. Exp. Biol. 2019, 222, jeb190553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, R.E.; Johnson, D.W. Metabolic Responses to Temperature in a Sedentary Reef Fish, the Bluebanded Goby (Lythrypnus dalli, Gilbert). J. Exp. Mar. Bio. Ecol. 2018, 501, 83–89. [Google Scholar] [CrossRef]
- Di Santo, V.; Bennett, W.A. Effect of Rapid Temperature Change on Resting Routine Metabolic Rates of Two Benthic Elasmobranchs. Fish Physiol. Biochem. 2011, 37, 929–934. [Google Scholar] [CrossRef]
- McLeod, I.M.; Rummer, J.L.; Clark, T.D.; Jones, G.P.; McCormick, M.I.; Wenger, A.S.; Munday, P.L. Climate Change and the Performance of Larval Coral Reef Fishes: The Interaction between Temperature and Food Availability. Conserv. Physiol. 2013, 1, cot024. [Google Scholar] [CrossRef] [Green Version]
- Killen, S.S.; Marras, S.; Ryan, M.R.; Domenici, P.; Mckenzie, D.J. A Relationship between Metabolic Rate and Risk-Taking Behaviour Is Revealed during Hypoxia in Juvenile European Sea Bass. Funct. Ecol. 2012, 26, 134–143. [Google Scholar] [CrossRef]
- Donelson, J.M.; Wong, M.; Booth, D.J.; Munday, P.L. Transgenerational Plasticity of Reproduction Depends on Rate of Warming across Generations. Evol. Appl. 2016, 9, 1072–1081. [Google Scholar] [CrossRef]
- Tenugu, S.; Pranoty, A.; Mamta, S.-K.; Senthilkumaran, B. Development and Organisation of Gonadal Steroidogenesis in Bony Fishes—A Review. Aquac. Fish. 2021, 6, 223–246. [Google Scholar] [CrossRef]
- Idler, D.R.; Bitners, I.I.; Schmidt, P.J. 11-Ketotestosterone: And androgen for Sockeye Salmon Can. J. Biochem. Physioogyl. 2011, 39, 1737–1742. [Google Scholar] [CrossRef]
- Ueda, H.; Kambegawa, A.; Nagahama, Y. Involvement of Gonadotrophin and Steroid Hormones in Spermiation in the Amago Salmon, Oncorhynchus Rhodurus, and Goldfish, Carassius Auratus. Gen. Comp. Endocrinol. 1985, 59, 24–30. [Google Scholar] [CrossRef]
- Yoneda, M.; Wright, P.J. Effect of Temperature and Food Availability on Reproductive Investment of First-Time Spawning Male Atlantic Cod, Gadus Morhua. ICES J. Mar. Sci. 2005, 62, 1387–1393. [Google Scholar] [CrossRef]
- Mayer, I.; Borg, B.; Schulz, R. Seasonal Changes in and Effect of Castration/Androgen Replacement on the Plasma Levels of Five Androgens in the Male Three-Spined Stickleback, Gasterosteus Aculeatus L. Gen. Comp. Endocrinol. 1990, 79, 23–30. [Google Scholar] [CrossRef]
- Rideout, R.M.; Rose, G.A.; Burton, M.P.M. Skipped Spawning in Female Iteroparous Fishes. Fish Fish. 2005, 6, 50–72. [Google Scholar] [CrossRef]
- Rideout, R.M.; Tomkiewicz, J. Skipped Spawning in Fishes: More Common than You Might Think. Chang. Publ. Wiley 2011, 3, 176–189. [Google Scholar] [CrossRef] [Green Version]
- Burton, M.P.M.; Penney, R.M.; Biddiscombe, S. Time Course of Gametogenesis in Northwest Atlantic Cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 1997, 54, 122–131. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, B.; Hansen, L.P. Energetic Cost of Spawning in Male and Female Atlantic Salmon (Salmo salar L.). J. Fish Biol. 1991, 39, 739–744. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty Acid Requirements in Ontogeny of Marine and Freshwater Fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Parma, L.; Bonaldo, A.; Pirini, M.; Viroli, C.; Parmeggiani, A.; Bonvini, E.; Gatta, P.P. Fatty Acid Composition of Eggs and Its Relationships to Egg and Larval Viability from Domesticated Common Sole (Solea Solea) Breeders. Reprod. Domest. Anim. 2015, 50, 186–194. [Google Scholar] [CrossRef]
- Vasconi, M.; Aidos, L.; Di Giancamillo, A.; Bellagamba, F.; Domeneghini, C.; Moretti, V.M. Effect of Temperature on Fatty Acid Composition and Development of Unfed Siberian Sturgeon (A. Baerii) Larvae. J. Appl. Ichthyol. 2019, 35, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.R.; Baptista, M.; Rosa, I.C.; Dionísio, G.; Gomes-Pereira, J.; Paula, J.R.; Figueiredo, C.; Bandarra, N.; Calado, R.; Rosa, R. “Gone with the Wind”: Fatty Acid Biomarkers and Chemotaxonomy of Stranded Pleustonic Hydrozoans (Velella Velella and Physalia Physalis). Biochem. Syst. Ecol. 2016, 66, 297–306. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Perez, K.O. Metabolic Programming Mediated by an Essential Fatty Acid Alters Body Composition and Survival Skills of a Marine Fish. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151414. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Fuiman, L.A. Maternal Diet Affects Utilization of Endogenous Lipids by Red Drum Sciaenops Ocellatus Embryos and Early Larvae. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 256, 110639. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. Effect of Broodstock Nutrition on Reproductive Performance of Fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Faleiro, F.; Diniz, M.; Machado, J.; Pousão-Ferreira, P.; Peck, M.A.; Pörtner, H.O.; Rosa, R. Oxidative Stress and Digestive Enzyme Activity of Flatfish Larvae in a Changing Ocean. PLoS ONE 2015, 10, e0134082. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).