1. Introduction
The common carp (
Cyprinus carpio) has a farming history of more than 8000 years in some parts of China [
1]. Nowadays, the common carp is farmed globally with a production higher than 4.41 million tons in 2019 [
2]. The Yellow River carp is a subspecies of the common carp that has a large yield in northern China. The rapid development of aquaculture has led researchers to focus their work on common carp genetics and breeding [
3,
4,
5,
6].
Among the methods used in breeding research, such as selective breeding and cross-breeding, quantitative trait locus (QTL) mapping is important, as it helps screen trait-related markers and assists breeding; studies on growth, feed conversion rate, disease resistance, sex, and muscle quality have been carried out using QTL [
7]. Completion of the mapping of the carp genome resulted in the determination of the QTL location with increased accuracy [
8,
9]. Growth is one of the most important breeding traits. In research on growth-related QTL mapping, Laghari et al. [
10] analyzed three traits of common carp F1 families 300 days post hatching, detecting four major-effect QTLs that explained more than 20% of the phenotypic variance. Peng et al. [
11] mapped the body weight (BW), body length (BL), and carcass weight of Yellow River carp (
C. c. haematopterus) 18 months after hatching (MAH) and obtained a total of 22 QTLs and several growth-related candidate genes. Feng et al. [
12] identified 21 QTLs related to BW, total length, BL, body height (BH), or head length in Yangtze River common carp at 9 MAH using microsatellite and single nucleotide polymorphism (SNP) markers. By injecting passive integrated transponder (PIT) markers into common carp at 3 MAH and measuring their growth traits at 3 and 8 MAH, Su et al. [
4] not only located 18 growth-related QTLs but also found a single shared QTL that partially controlled the BL, body depth, and body width. Wang et al. [
13] detected a total of 14 major-effect QTLs and 17 candidate genes associated with BL, body thickness (BT), BH, or BW in three growth stages. However, there are few reports on cold-tolerant growth.
While studying the cold tolerance of common carp, Sun et al. [
14] tagged 100 common carp before overwintering and identified living and dead individuals after overwintering. In that study, a genetic linkage map was constructed, and four putative markers associated with cold tolerance were identified, but only one marker could be mapped on a linkage group (LG). Liang et al. [
15] detected a large number of cold-response genes in different tissues at different temperatures by transcriptomic analysis. Sun et al. [
16] researched the effect of high and low temperatures on the common carp liver transcriptome. The results showed that the change in temperature had a great influence on the energy metabolism and metabolic process of cell membranes. Ge et al. [
17] revealed that FoxO-related signal pathways play an important role in the formation and acclimation of the cold tolerance of Songpu mirror carp. Long et al. [
18] analyzed the low-temperature-acclimation transcriptome of three common carp strains and found that low-temperature acclimation was consistent with the upregulation of genes related to cholesterol biosynthesis, and the content of total cholesterol in the brain increased significantly under cold stress.
Although many studies have been conducted on growth-related QTL mapping and cold tolerance, studies on growth-related QTL during the overwintering period have not yet been reported. However, during winter, the growth of Yellow River carp is almost stagnant. Prolonging the growth period of the Yellow River carp into the winter would play an important role in promoting the development of its farming. It is possible to screen QTL and candidate genes related to overwintering growth by using growth-related QTL mapping of overwintering. The QTL and candidate gene may also be related to cold tolerance. The aim of this study is to provide a reference for the growth and cold-tolerant breeding of Yellow River carp. To this end, we used high-density genetic maps of Yellow River carp [
13] constructed by our group to locate growth-related QTL and to screen major candidate genes according to the BL, BH, BT, and BW of Yellow River carp during overwintering.
4. Discussion
Our study on the growth-related QTLs of Yellow River carp during overwintering showed that the family grew slowly during the overwintering period. There were significant differences in the added value of BW among individuals, and the increase in BW resulted mainly from the increase in BL. At present, there are some reports about the growth-related QTL of fish at a certain growth stage. For example, Sauvage et al. [
31] tagged brook charr (
Salvelinus fontinalis) with PIT at the age of 5 months, collected the growth data of four different growth periods (May 2009, July 2009, August 2009, and November 2009), and mapped the growth rate-related QTLs in different growth periods. Zhang et al. [
32] measured the growth rate of common carp at three time points (3, 4, and 5 MAH) and identified 15 genomic regions associated with growth rate. Wang et al. [
13] carried out a QTL mapping study on four growth traits of Yellow River carp at the age of 13, 17, and 13–17 months. However, there is no research on the QTL location related to the growth of fish during overwintering. This may be due to the slow growth of carp during overwintering; some scholars think that the research is of little significance. Although carp grow slowly during overwintering, there are great differences among individuals. Thus, we can screen the major-effect QTL during overwintering by detecting the QTL related to growth traits. These QTL may also be related to the cold tolerance of carp.
At present, the research on the cold tolerance of fish is mainly focused on the transcriptomic analysis of the common carp [
18,
20], Nile tilapia (
Oreochromis niloticus) [
33], yellow drum (
Nibea albiflora) [
34],
Onychostoma macrolepis [
35], etc. However, there are few studies on cold-tolerance-related QTL. At present, there is only one report on the location of cold-tolerance-related QTL in common carp [
14]. In this study, the dead and surviving fish were classified into two groups after overwintering. Four RAPD markers that may be associated with cold tolerance were screened, but only one was located in the linkage group. In this study, 300 fish were initially marked, but only 198 fish were used because the samples were collected at the age of 17 MAH for QTL mapping. There were still 207 fish at the age of 17 MAH, but the growth data of nine fish were obviously wrong during the overwintering period, so these growth data were excluded. In future research, we should develop devices that can automatically and accurately obtain the phenotypic traits of fish and so improve the accuracy of the results [
36].
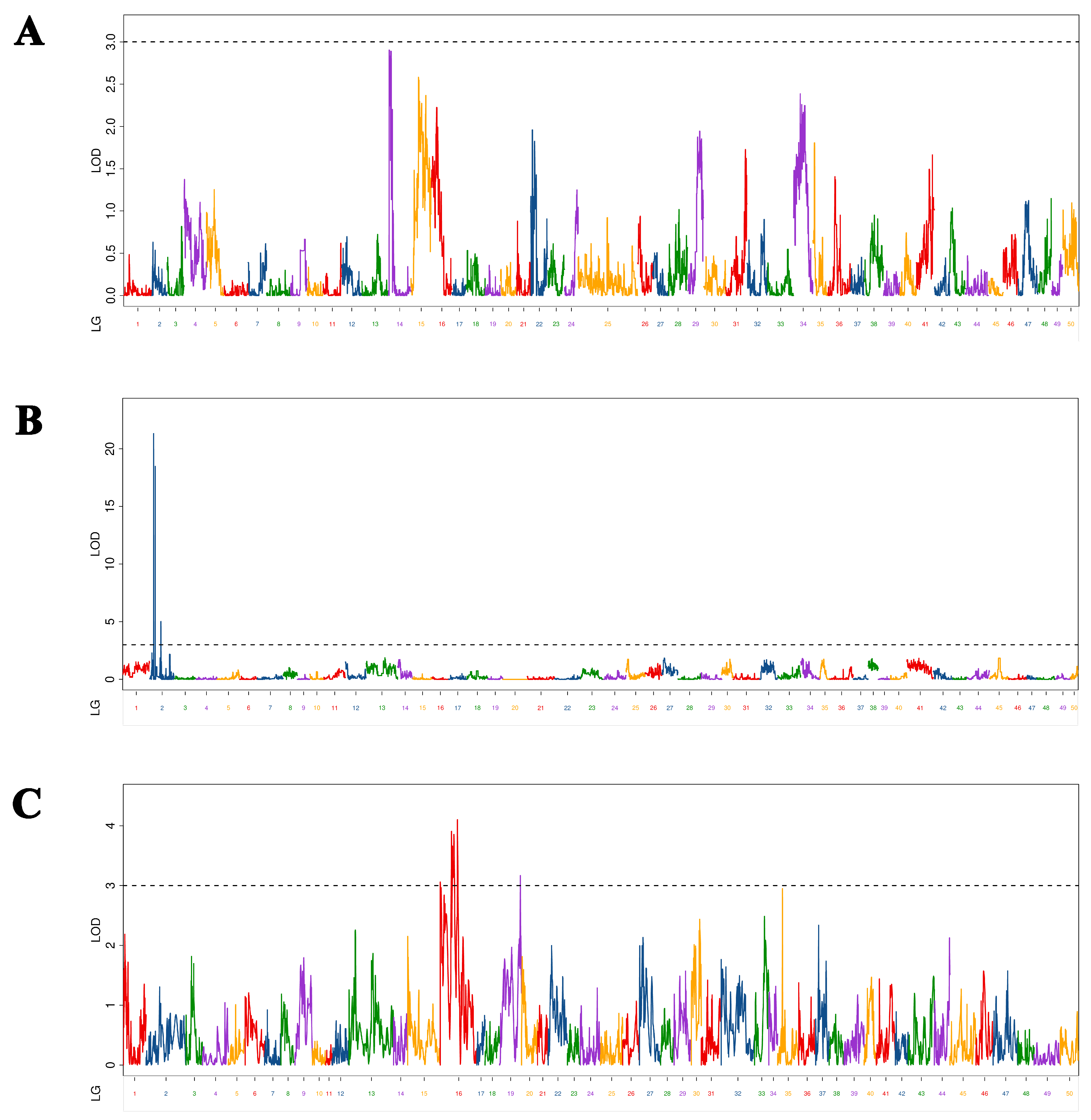
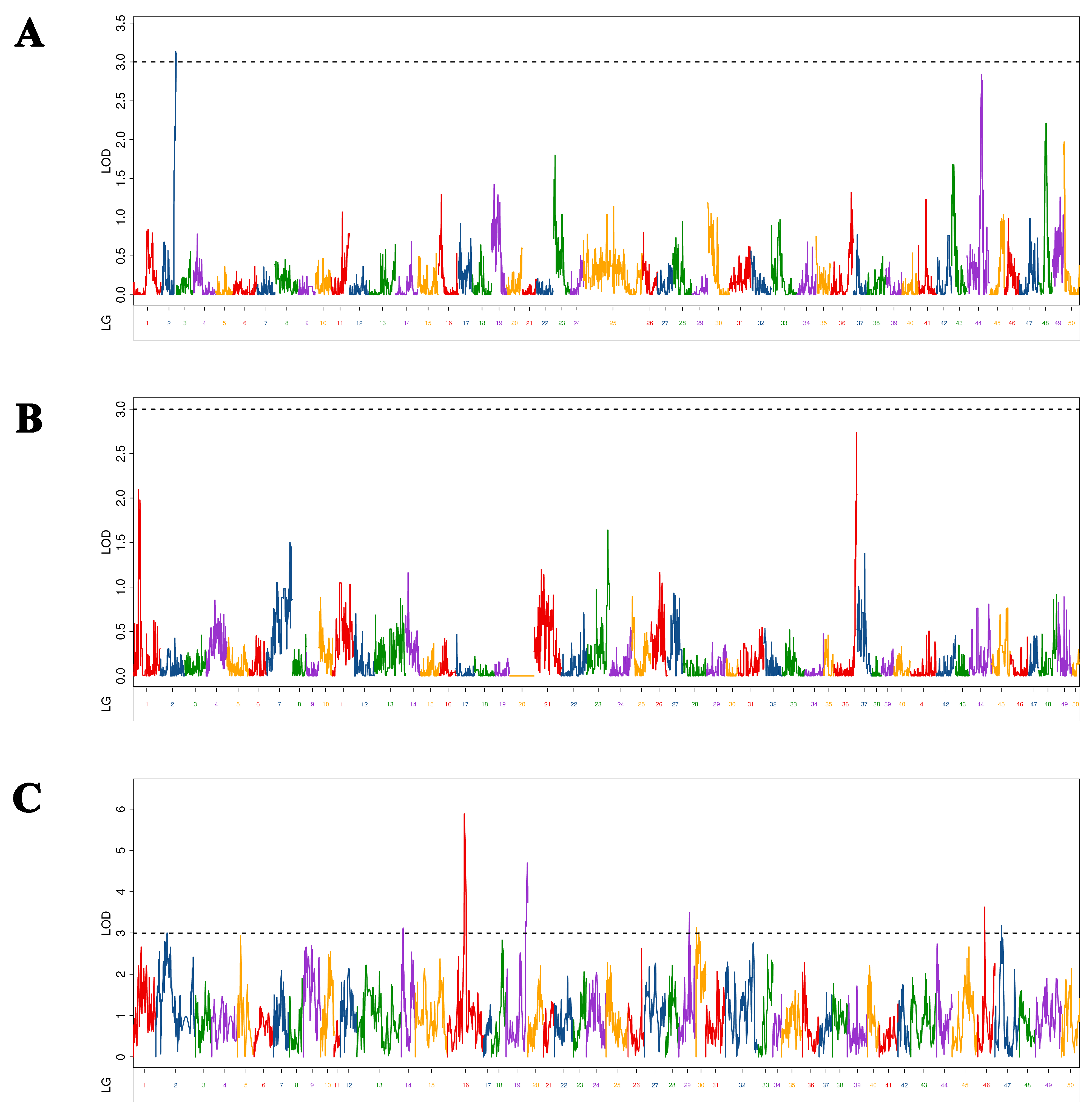
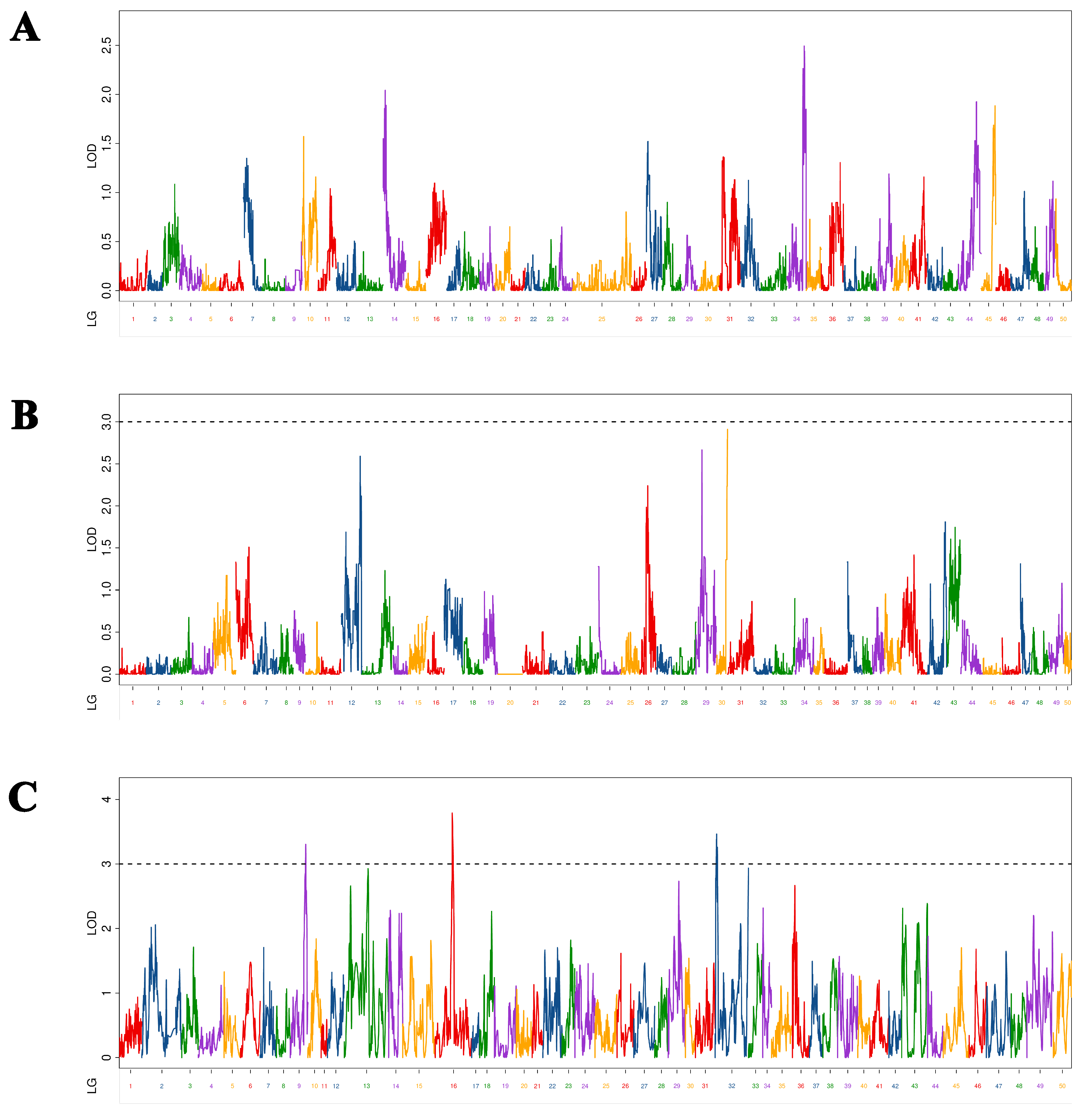
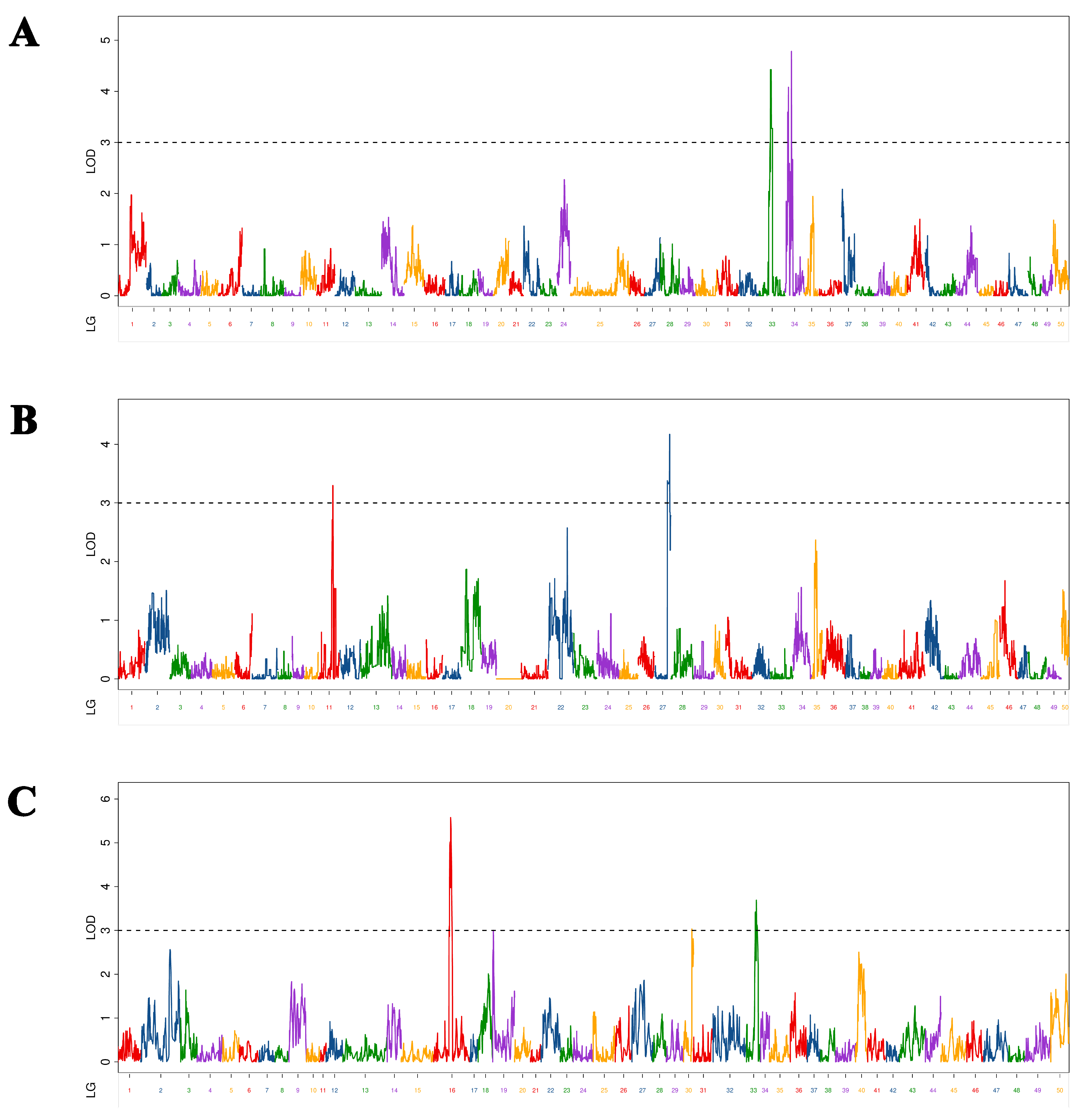
In this study, as different maps (maternal, paternal, and HH) are constructed with different markers, the QTL mapping results of the same trait on these maps are very different. Twenty-nine QTLs related to overwintering growth were located and distributed on 12 chromosomes. The characteristics of a large number and dispersion of QTL were similar to those mentioned by other studies on common carp growth-related QTL [
10,
11,
12,
37]. The largest number of growth-related QTLs in common carp was 165, distributed in 30 linkage groups [
38], indicating that common carp growth-related traits were determined by multiple genes. The results of the present study show that these growth-related, complex traits are controlled by a large number of genes. It is generally assumed that the higher the LOD value, the higher the confidence, and the higher the PVE, the greater the role of QTL. In this study, there were only two BL-related QTLs that had LOD > 10 and PVE > 20%, which were values similar to those obtained by a study on the Yangtze River common carp growth-related QTL [
12]. In that study, the authors found that one BW-related QTL had LOD > 10 and PVE > 20%. However, although several QTLs have been found in studies of Yellow River carp [
11] and common carp [
10] with PVE >20%, none had LOD >10%. Therefore, assumptions regarding whether there was a QTL that had a significant effect on different growth traits of common carp at different growth stages were quite different. However, these QTLs with significant effects are the ones we should focus on, as they can be used in molecular-assisted breeding, similar to the two QTLs that had significant effects and were screened in this study. The molecular markers and genes will help increase overwintering growth and prolong the growth period. In this study, C-QTL contained seven QTLs associated with four growth traits and had obvious pleiotropy. In previous studies, certain QTLs appeared pleiotropic in different periods [
13] or different families [
38]. The consensus QTLs may be related to housekeeping genes if their PVE is very low. In this study, the PVE of C-QTL was low, which may have little effect on overwintering growth. Although many markers were located in genes, the major QTLs were located in intergenic regions.
Among the three overwintering growth-related genes screened in this study,
fnd3b is a protooncogene involved in the occurrence and development of cancer [
39,
40]. The protein Fnd3b expressed in this gene is a positive regulator of adipogenesis, related to adipocyte differentiation, and is an RNA-binding protein [
41,
42]. Other studies have shown that Fnd3b affects human craniofacial development [
43] and is related to the domestication of chickens [
44]. Ghsr, the receptor of growth hormone secretory hormone (Ghrelin) and growth hormone-releasing peptide, is a G protein coupling protein [
45]. The Ghsr/Ghrelin system is an important pathway for the regulation of growth hormone secretion [
46,
47]. Ghsr has been found to play an important role in energy balance and metabolism as well as digestion and GH secretion in fish [
48]. However, in the study of fish QTL, no QTL related to Ghsr was found. Pld1 is an enzyme widely distributed in bacteria, fungi, animals, and plants [
49]. It plays a key role in signal transduction, membrane transport, and cytoskeletal regulation [
50,
51,
52]. Lipid catabolism plays an important role in enhancing the cold tolerance of fish [
53]. Zheng et al. [
54] studied the QTL related to muscle fat content and abdominal fat in common carp and located 18 genome-wide QTLs with suggestive significance. This result was different from the genes found in this study, which might be due to the different traits examined in the two studies. These three genes could be used as important candidates to study the growth and metabolism of Yellow River carp during overwintering.
In conclusion, this is the first study on the growth-related QTL mapping in overwintering of Yellow River carp. In this study, 29 QTLs, including two major-effect QLTs and one C-QTL related to overwintering growth traits, were detected. Additionally, we screened three genes related to overwintering growth (fnd3b, ghsr, and pld1), which were related to fat formation, growth, membrane transport, etc. The results of this study provide information for the breeding of Yellow River carp to improve growth and cold tolerance during overwintering.