1. Introduction
Natural recruitment to adulthood is essential for the sustainability of fish populations. In north-temperate lakes, littoral zone habitats are critical in early life history stages of fishes by providing suitable conditions for egg incubation, hatching, refuge, and foraging that insure survivorship [
1,
2,
3,
4,
5]. Successful recruitment of young fishes through ontogeny and into adulthood are due to a series of intricately timed predator-prey interactions that often rely on suitable littoral zone habitat [
2,
4,
6,
7,
8]. These critical habitats are especially sensitive to degradation from extrinsic, anthropogenic factors such as removal/manipulation of aquatic vegetation and coarse woody habitat [
9,
10,
11,
12,
13], and chemical pollution [
14]. Often, lakeshore residential development can directly influence littoral zone habitat and fish communities [
3,
15,
16,
17,
18,
19,
20,
21].
Previously extraneous chemicals continually become present in surface and groundwater through a variety of point and non-point sources, including industrial waste, application and leaching from agricultural practices, leaching from landfills, waste storage tank leakage, and municipal sewer and septic tank leakage [
22]. Many studies of chemical influences on fishes have been focused on increased exposure to steroid hormones, as these are commonly used chemicals that frequently leach into waterways (i.e., wastewater treatment effluents, combined sewer overflow events, confined animal feeding operations). Chemicals have the potential to cause significant negative influences on fish populations and their abilities to naturally reproduce through direct influences on young fishes [
23]. Exposures to steroid hormones have been known to cause changes in sex and influence early life stages in fishes [
24,
25,
26,
27,
28,
29]. With evidence of direct negative influences of extraneous chemicals, preventing and limiting them in waterbodies is important for maintaining sustainable fish populations. Identifying and limiting potential chemical pollution sources through protective riparian buffers, shoreline development regulations, and regular inspection and regulation of surrounding sewage waste systems are common management practices to ensure reduced exposure in aquatic ecosystems [
30].
Direct application of aquatic herbicides into lakes bypasses precautionary measures in place to conserve shoreline and lake health but is frequently conducted to control and eradicate aquatic macrophytes such as invasive Eurasian watermilfoil (
Myriophyllum spicatum) (EWM). Invasive EWM is widespread in north-temperate lakes and is currently documented in 908 Wisconsin waterbodies [
31]. Eurasian watermilfoil is especially concerning to lake managers and surrounding property owners as it can crowd out and hybridize with native submersed aquatic vegetation [
32], spread rapidly [
33], and create dense mats that can become a nuisance to lake recreators. Mitigation of EWM through manual harvest and chemical treatments of aquatic herbicides are commonly used to reduce overabundant populations and associated negative influences. Chemical treatments typically use the aquatic herbicide 2,4-Dichlorophenoxyacetic acid (2,4-D) in a granular or liquid form and applied in spot and whole-lake treatments. Low environmental concentrations of 2,4-D have previously been thought to have minor influences on native aquatic fauna; however, laboratory studies have indicated that even low concentrations can have significant influences on development and survivorship of fathead minnow (
Pimephales promelas), a common prey fish species found in north-temperate lakes [
34,
35,
36]. In addition, Dehnert et al. (2021) [
37] concluded in a laboratory setting that environmental concentrations of 2,4-D had a negative influence on embryonic and larval survivorship of six different fish species native to north-temperate lakes: northern pike (
Esox lucius), walleye (
Sander vitreus), largemouth bass (
Micropterus salmoides), white sucker (
Catostomus commersonii), yellow perch (
Perca flavescens), and white crappie (
Pomoxis annularis). With 2,4-D applications regularly occurring in north-temperate lakes to mitigate negative influences of EWM, this raises a concern for aquatic ecosystems and fish populations.
There are still many unknowns associated with chemical herbicide treatments that warrant further investigation. For example, variable delivery methods, chemical fillers that are commonly used in conjunction with 2,4-D, timing of treatments as they relate to north-temperate lake fish life histories, degradation rates of chemicals in natural aquatic ecosystems, the extent of the spread of chemicals during treatment, and any potential legacy effects of chemicals within sediment. These variables associated with chemical treatments have the potential to negatively influence littoral habitats, reproduction, and food web interactions essential to the sustainability of fish populations. Thus, there is a critical need to better understand the potential influences of these aquatic herbicide treatments on fish communities in natural ecosystems.
We observed natural recruitment failures in several fish species during 2012 fisheries surveys on Lake Ellwood, Florence County, Wisconsin coincident with long-term chemical treatments of EWM. Therefore, we conducted a whole-lake experiment testing for fish community and aquatic ecosystem responses to the cessation of long-term EWM chemical treatments on Lake Ellwood. Fish community and aquatic ecosystem responses on Lake Ellwood were compared to two nearby reference systems with no history of EWM chemical treatment. Our primary objective was to test for Lake Ellwood fish community responses to the cessation of long term EWM chemical treatment (2003–2012) during 2013–2019 and to explore plausible mechanisms for the observed recruitment failures of several fish species. To encompass a whole-lake ecosystem approach, we also recorded limnological conditions, zooplankton density, and submersed aquatic vegetation coverage. We hypothesized that the long-term 2,4-D treatments on Lake Ellwood had: (1) direct, lethal effects on fish recruitment; (2) negative effects on fish recruitment due to sublethal effects from zooplankton community change; and (3) caused large-scale declines in aquatic macrophyte abundance negatively influencing juvenile fish survivorship and foraging opportunities. Results from our study provide insight into how recruitment failures in several Lake Ellwood fish species occurred and provide valuable knowledge on the responses of a fish and zooplankton community following cessation of long-term aquatic herbicide treatments. Ultimately, our findings may inform lake manager and lake property owner decisions regarding best practices for invasive aquatic macrophyte control in north-temperate lakes.
2. Materials and Methods
2.1. Study System and Reference Lakes
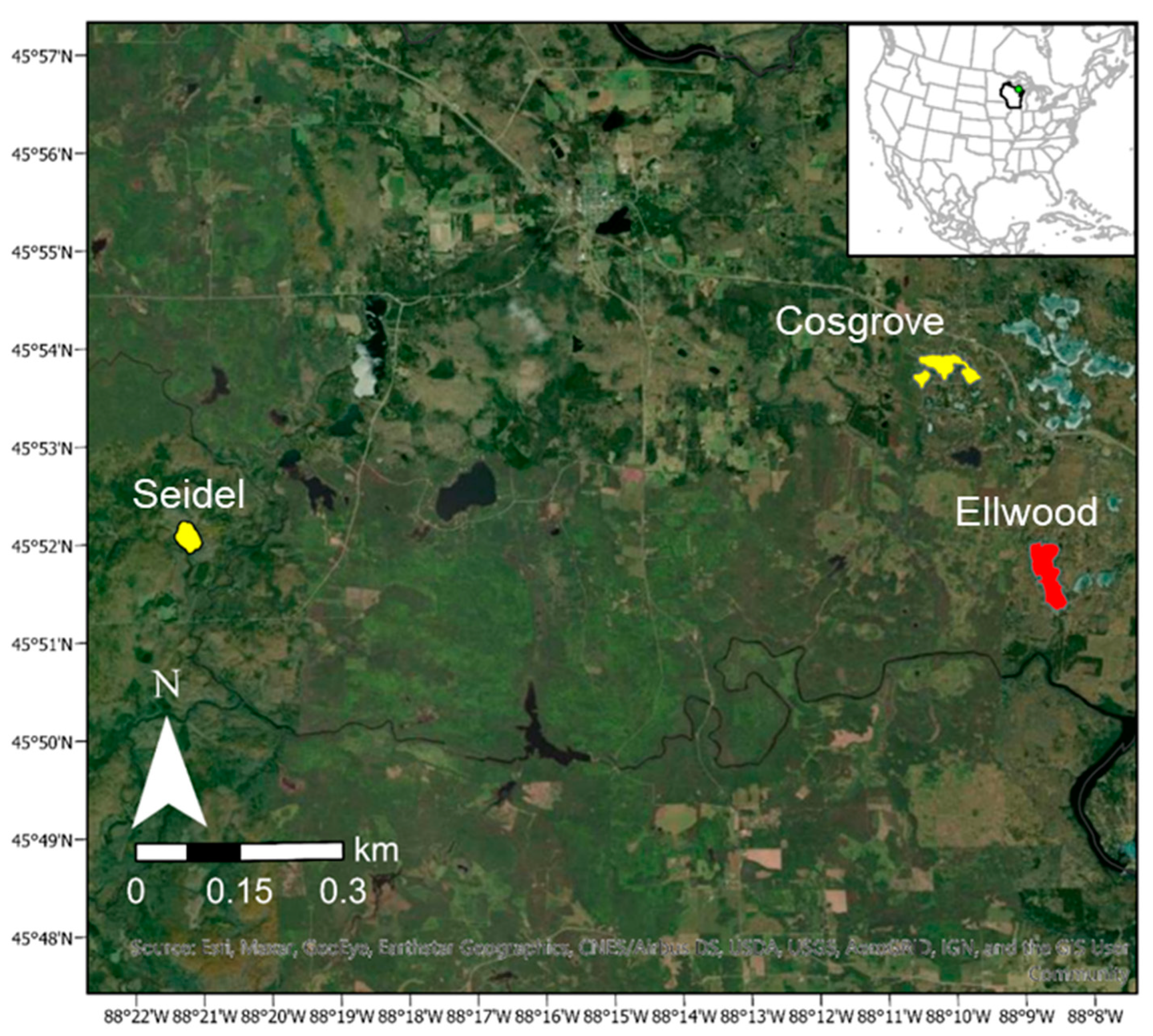
Lake Ellwood is a 54-ha mesotrophic, seepage lake located in Florence County, Wisconsin (N 45.86155, W 88.14315) (
Figure 1).
Lake Ellwood has a mean depth of 4.6 m and maximum depth of 7.6 m with bottom substrate of 85% sand, 10% gravel, 3% rock, and 2% muck. Lake Ellwood supports a fish community with historic naturally reproducing populations of northern pike, largemouth bass, smallmouth bass (
Micropterus dolomieu), bluegill (
Lepomis macrochirus), yellow perch, black crappie (
Pomoxis nigromaculatus), rock bass (
Ambloplites rupestris), pumpkinseed (
Lepomis gibbosus), white sucker, bluntnose minnow (
Pimephales notatus), Iowa darter (
Etheostoma exile), and a stocked population of walleye
(Sander vitreus). Eurasian watermilfoil was discovered in the lake in 2002 and the lake association hired private consultants to treat the non-desirable mats that had begun to form. The Wisconsin Department of Natural Resources (WDNR) issued aquatic plant management permits approving chemical treatments during 2003–2012 (
Table 1).
A fisheries survey was conducted on Lake Ellwood in 2012 by WDNR fisheries management staff. Despite substantially increased sampling effort, no age-0 northern pike, largemouth bass, smallmouth bass, black crappie, and pumpkinseed were encountered and low numbers of age-0 bluegill (1.2 fish/km of shoreline) and yellow perch (2.9 fish/km of shoreline) were observed in electrofishing surveys. Past fisheries surveys indicated that these species-maintained populations and naturally reproduced in Lake Ellwood with abundances comparable to other northern Wisconsin lakes. Permitting of EWM chemical treatments on Lake Ellwood were denied by the WDNR in 2013 due to fish recruitment concerns (G. Matzke, Wisconsin Department of Natural Resources, personal communication and unpublished data).
Two nearby lakes with relatively similar lake characteristics and no history of herbicide chemical treatment were chosen as references systems to compare with responses observed in the fish and zooplankton communities, submersed vegetation coverage, and limnological conditions in Lake Ellwood after the cessation of chemical treatment. Cosgrove Lake is a 31-ha mesotrophic, seepage lake with no history of EWM (
Figure 1). Cosgrove Lake has a mean depth of 1.8 m and a maximum depth of 7.9 m with bottom substrate consisting of 75% sand, 5% gravel, 0% rock, and 20% muck. The fish community consists of northern pike, walleye, largemouth bass, smallmouth bass, bluegill, rock bass, black crappie, yellow perch, white sucker, and bluntnose minnow. Seidel Lake is a 21-ha mesotrophic, drainage lake with a mean depth of 4.6 m and maximum depth of 13.4 m with EWM present, but no history of chemical treatment (
Figure 1). The fish community in Seidel Lake comprises largemouth bass, bluegill, pumpkinseed, black crappie, rock bass, Iowa darter, golden shiner (
Notemigonus crysoleucas), common shiner (
Luxilis cornutus), black bullhead (
Ameiurus melas), yellow bullhead (
Ameiurus natalis), warmouth (
Lepomis gulosus), central mudminnow (
Umbra limi), and tadpole madtom (
Noturus gyrinus). Because of slight differences in the fish communities among our study and reference lakes, and the fact that abiotic and biotic conditions among lakes and fish populations may differentially influence species-specific growth, we only used spring fyke netting (fish/net night) and electrofishing surveys (fish/km) and age-0 fall recruitment electrofishing surveys (fish/km) to test for a post-chemical treatment effect on fish between Lake Ellwood and the reference lakes. In this manner, a post-chemical treatment effect on Lake Ellwood would result in a change in these metrics, while these metrics would stay the same or change in the opposite direction on the reference lakes.
2.2. Limnological and Submersed Aquatic Vegetation Coverage Responses
To test whether the fish recruitment failures were in response to water quality conditions, limnological characteristics (Secchi depth, temperature-dissolved oxygen profiles, and chlorophyll-a concentration) were collected monthly (June–September) during 2014–2019 at the deepest portions of Lake Ellwood, Cosgrove Lake, and Seidel Lake. Mean values were calculated for each lake and trends over the study period were visually assessed for extreme events or trends.
To test the hypothesis that large-scale declines in aquatic macrophyte abundance due to herbicide treatment negatively influenced juvenile fish survivorship and foraging opportunities, vegetation sonar surveys were conducted to estimate submersed vegetation coverage (percent plant biovolume (BvP) and percent area covered (PAC)) on Lake Ellwood and the reference systems. Sonar surveys were conducted in August on Lake Ellwood during 2013–2019, and Cosgrove and Seidel lakes during 2014–2019 using a Lowrance Hook² sonar unit paired with
BioBase vegetation mapping software. Annual PAC and BvP values were log
10 + 2 transformed to satisfy assumptions of normality and simple linear regression was used to test for temporal trends over the study period. We used the null hypothesis of no change in PAC or BvP over time (α = 0.05). Kolmogorov–Smirnov tests were used to test for differences in submersed aquatic vegetation values between Lake Ellwood and the reference lakes (α = 0.05). All statistical tests were conducted using the open-source software R (v 4.1.0) [
38].
2.3. Zooplankton Responses
During 2014–2019, three entire water column zooplankton tows were conducted monthly (June–September) using an 80 μm mesh, 12.7 cm aperture diameter Wisconsin plankton net in the deepest portion of Lake Ellwood, Cosgrove Lake, and Seidel Lake. Zooplankton samples were preserved in ethanol, enumerated under a microscope, and identified to the lowest taxonomic level possible. Mean annual zooplankton densities (ind. L−1) were then calculated for zooplankton taxonomic groups found within each lake. Mean zooplankton densities were log10 + 2 transformed to satisfy assumptions of normality and simple linear regression was used to test for temporal trends over the study period (α = 0.05). Kolmogorov–Smirnov tests were used to test for differences among zooplankton densities among Lake Ellwood and the reference lakes (α = 0.05).
2.4. Fish Community Responses
Comprehensive fishery surveys with spring, summer, and fall sampling efforts were conducted during 2012–2019 to test for fish community responses to the cessation of EWM treatments on Lake Ellwood. Similar data were also collected from Cosgrove Lake (2013–2019) and Seidel Lake (2014–2019) to allow for comparison. Spring adult mark-recapture population estimates were conducted for northern pike, largemouth bass, and smallmouth bass on Lake Ellwood in 2012, 2015, and 2019. Immediately after ice out in each year, northern pike were captured in fyke nets and marked and primarily recaptured with fyke nets and some supplementary AC boom electrofishing. Northern pike that expressed gametes or were of unknown sex ≥ 305 mm in length were considered adults. Largemouth bass and smallmouth bass were marked and recaptured with AC boom electrofishing in late-May/early June. Largemouth and smallmouth bass that expressed gametes or were of unknown sex > 203 mm in length were considered adults. Mark-recapture adult population abundance was estimated using the Chapman-modified Petersen method [
39]. Annual fall boom AC electrofishing runs (2.41–4.43 km in length) in historical sampling locations were conducted on Lake Ellwood during 2012–2019 to index relative abundance (fish/km of shoreline, catch-per-unit-effort, CPE) of age-0, age-1, and adult fishes. Fall electrofishing surveys primarily focused on juvenile fishes to index annual recruitment; however, all fishes encountered were collected including adults. Similar annual surveys were conducted on Cosgrove Lake (1.61–2.51 km) during 2013–2019 and Seidel Lake (0.84–0.87 km) during 2014–2019. In addition to fall electrofishing, mini-fyke net surveys were conducted during early fall to capture fishes less susceptible to electrofishing and to evaluate annual recruitment (fish/net night, relative abundance). These surveys were conducted on Lake Ellwood during 2013–2019 (16–20 net nights annually), in Cosgrove Lake during 2013–2019 (12–16 net nights annually), and in Seidel Lake during 2014–2019 (11–12 net nights annually). To test for temporal trends in relative abundance after the chemical treatment cessation, annual age-specific CPE of fish captured during fall electrofishing surveys was calculated. Natural recruitment was indexed using CPE of age-0 fish, relative juvenile abundance was indexed using CPE of age-1 fish, and adult fish relative abundance was indexed using CPE of age-2+ fish. This was conducted for gamefish and panfish species with sufficient sample sizes, which included northern pike, largemouth bass, smallmouth bass, bluegill, black crappie, pumpkinseed, yellow perch, and rock bass. Total CPE of all age classes present were calculated for non-gamefish species with sufficient sample sizes, which included white sucker and bluntnose minnow. Species- and age-specific CPE values were log
10 + 2 transformed to satisfy assumptions of normality and simple linear regression was used to test for temporal trends over the study period (α = 0.05). The same analyses were conducted for Cosgrove Lake during 2013–2019 and Seidel Lake during 2014–2019. Kolmogorov–Smirnov tests were used to test for fish CPE differences among Lake Ellwood and the reference lakes (α = 0.05).
Spring fyke netting surveys were conducted during 2012, 2015, and 2019 on Lake Ellwood and during 2015 and 2019 on Cosgrove and Seidel lakes to collect additional fish information and hard structures for fish age estimation. Spring fyke netting surveys on Lake Ellwood consisted of 28 net nights in 2012, 20 net nights in 2015, and 32 net nights in 2019. Spring fyke netting surveys on Cosgrove Lake and Seidel Lake consisted of 16 and 32 net nights and 12 and 20 net nights, respectively. Summer fyke netting surveys were also conducted on Lake Ellwood during 2012, 2015, and 2019 and consisted of 15–16 net nights each. Similarly, summer fyke netting surveys were conducted on Cosgrove (13 and 15 net nights) and Seidel lakes (10 and 12 net nights). Entire shoreline spring-early summer boom AC electrofishing runs were conducted on Lake Ellwood during 2012 (29.72 km), 2015 (27.15 km), and 2019 (37.55 km) to collect additional fish information and aging structures. Similar electrofishing runs were conducted on Cosgrove Lake during 2015 (24.07 km) and 2019 (24.77 km) and Seidel Lake during 2015 (21.71 km) and 2019 (16.98 km).
Fish age information was obtained non-lethally from collected hard structures (dorsal fin spines, anal fin spines, anal fin rays, and scales) during spring and early summer fish surveys. Aging structures were collected from five fish per 13 mm length bin for each species and used to develop an age-length key for age extrapolation. To improve species-specific age estimation accuracy and precision, various hard structures were used depending on fish length [
40]. For northern pike, anal fin rays were used for fish ≥ 457.2 mm and scales were used for fish ≤ 457.2 mm. For largemouth and smallmouth bass, dorsal fin spines were used for fish ≥ 203.2 mm and scales were used for fish ≤ 203.2 mm. For yellow perch, anal fin rays were collected for fish ≥ 152.4 mm and scales were used for fish ≤ 152.4 mm. For black crappie, anal spines were used for fish ≥ 165.1 mm and scales were used for fish ≤ 165.1 mm. For bluegill and rock bass, anal spines were used for fish ≥ 127 mm, and scales were used for fish ≤ 127 mm. Hard structures were processed (dried, spines and rays cut and polished) and analyzed under a microscope. Age estimation was conducting by counting annuli on processed hard structures. Age estimation was conducted by a consistent, experienced reader during all three years of age estimation. To test for potential influences on fish growth rates during the chemical treatment period, mean length-at-age estimates were calculated for northern pike, largemouth bass, smallmouth bass, and bluegill on Lake Ellwood using age estimates from structures collected during spring-early summer fyke netting and electrofishing in 2012, 2015, and 2019. Mean length-at-age estimates were calculated for Lake Ellwood during the chemical treatment (2003–2012) and post-chemical treatment (2013–2019) periods. Treatment and post-treatment mean length-at-age were log
10 transformed and compared using Welch’s t-tests in program R (α = 0.05) [
38]. Length and age structure were compared using length frequency histograms and density ridge plots with the “ggridges” package in program R [
38] for Lake Ellwood in 2012, 2015, and 2019, and Cosgrove and Seidel lakes in 2015 and 2019.
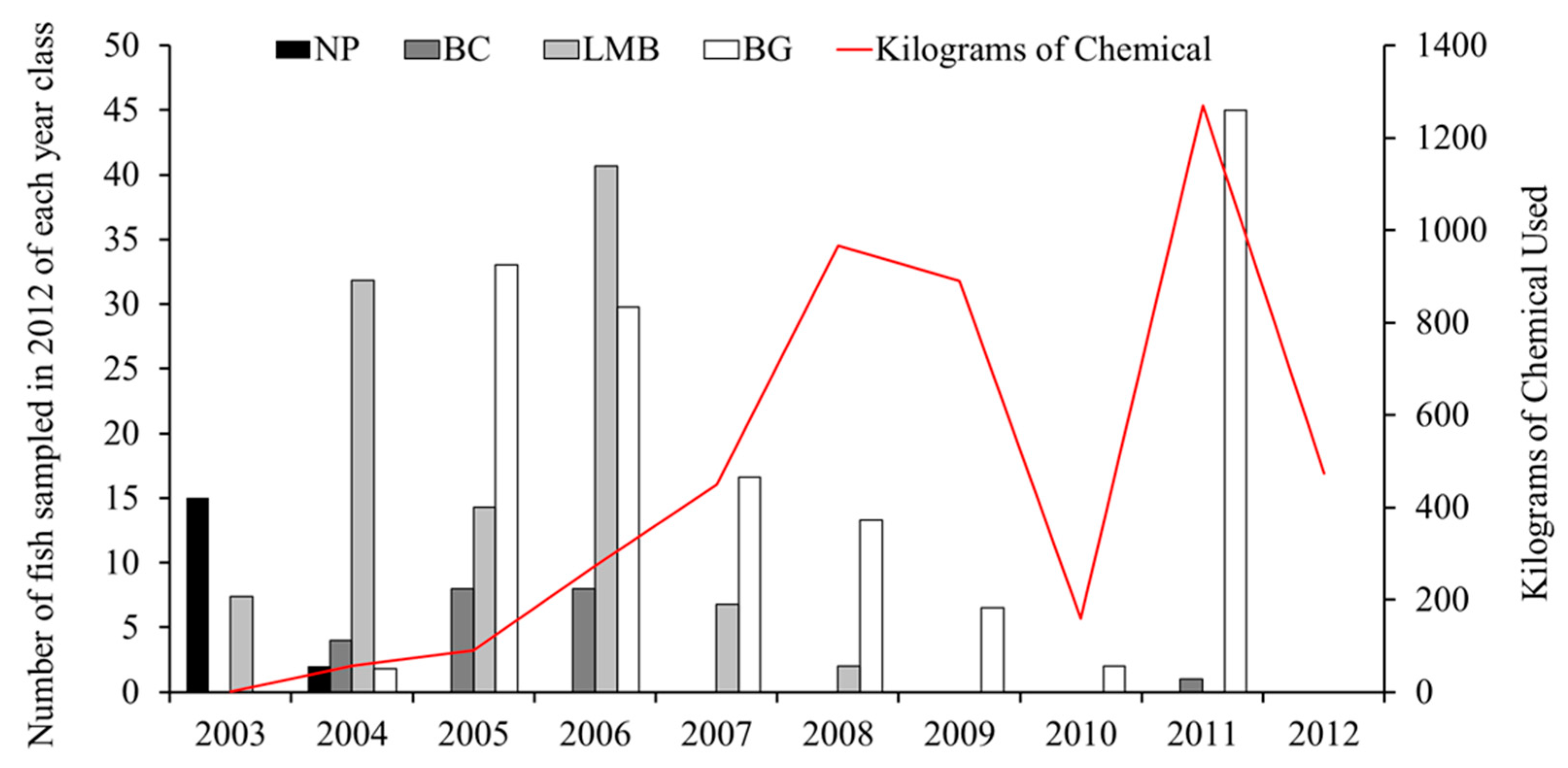
Due to a lack of pre-treatment data, we back-calculated year class strength from age estimates calculated during spring and summer sampling efforts in 2012. Year class strength (No. of sampled fish present in 2012 per each year of treatment) was estimated for northern pike, largemouth bass, smallmouth bass, black crappie, and bluegill. Year class strength trends were plotted and overlayed with amounts of chemical used in treatments and assessed for failed year classes.
3. Results
3.1. Limnological and Submersed Aquatic Vegetation Coverage Responses
Limnological conditions did not change in Lake Ellwood following the cessation of EWM chemical treatments and in the reference lakes during 2013–2019 (
Table 2).
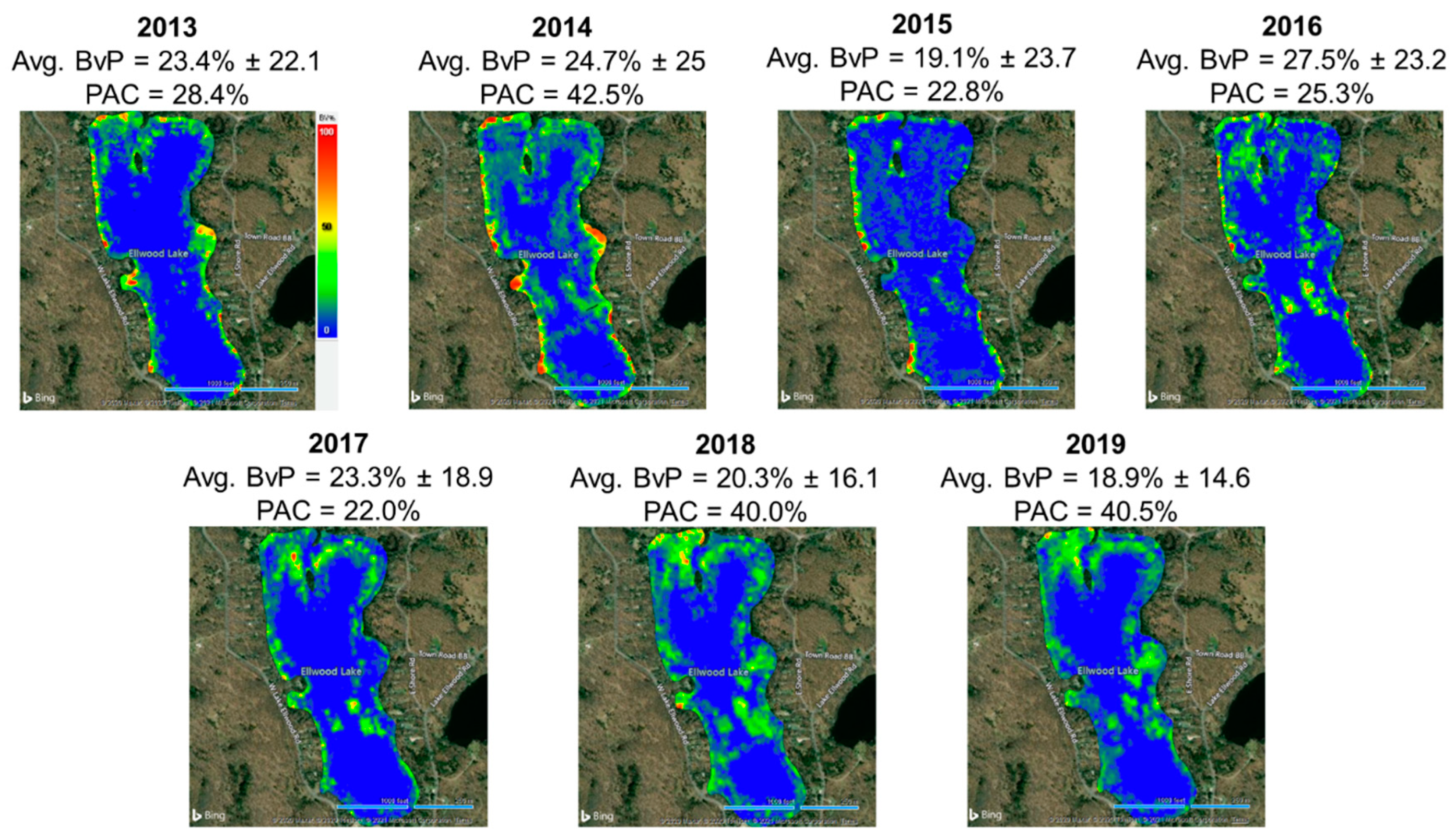
Annual submersed aquatic vegetation PAC and BvP did not change on Lake Ellwood following chemical treatment cessation (2013–2019) (both
p > 0.05) (
Figure 2).
Percent area covered values estimated from surveys in Lake Ellwood indicated initial increases during 2013–2014 (PAC = 28.4–42.5); however, similar values were estimated in 2018 (PAC = 40.0) and 2019 (PAC = 40.5) (
Figure 2). Percent plant biovolume values remained relatively stable in Lake Ellwood during 2013–2019 (range = 18.9–27.5) (
Figure 2). Variation in BvP and PAC within the reference systems showed wider ranges and exhibited larger PAC and BvP value increases and decreases during 2014–2019 in Cosgrove Lake (PAC range = 7.0–56.0, BvP range = 12.6–31.8) and Seidel Lake (PAC range = 17.9–33.9, BvP range = 33.1–62.4). Kolmogorov–Smirnov tests showed no differences in Lake Ellwood submersed aquatic vegetation PAC and BvP when compared to the reference systems (
p > 0.05).
3.2. Zooplankton Responses
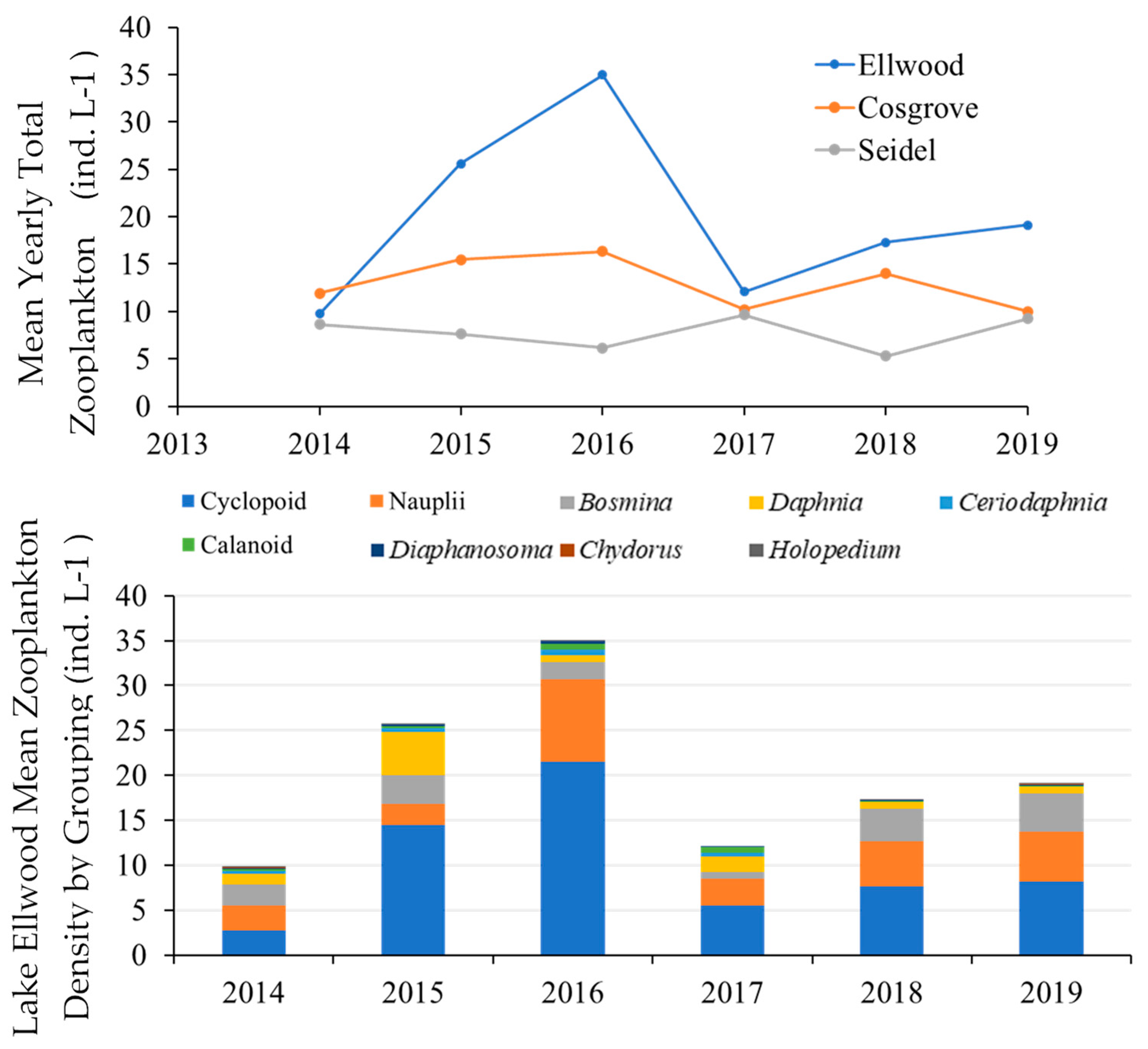
Total zooplankton density in Lake Ellwood increased from 2014–2016 and stabilized thereafter (
Figure 3).
Annual mean densities of total zooplankton increased from about 9.8 ind. L−1 in 2014 to 25.6 ind. L−1 in 2015 to 35 ind. L−1 in 2016 and ranged from about 12.1–19.1 ind. L−1 during 2017–2019. The observed increase in total zooplankton density was predominantly due to high densities of cyclopoid copepods (22.8–46.7 ind. L−1) collected in sampling efforts during 2015 and 2016. Nauplii also showed high densities (34.3–35.3 ind. L−1) in August, 2016 that contributed to the relatively high mean total zooplankton density in 2016. Total zooplankton density did not differ during 2014–2019 within Lake Ellwood for any zooplankton taxonomic grouping (all p > 0.05). Kolmogorov–Smirnov tests indicated significantly different total zooplankton densities among Lake Ellwood and the reference systems for calanoid copepods (Ellwood-Cosgrove, p = 0.002, Ellwood-Seidel, p < 0.001), cyclopoid copepods (Ellwood-Cosgrove, p = 0.02, Ellwood-Seidel, p < 0.001), Daphnia (Ellwood-Cosgrove, p = 0.02, Ellwood-Seidel, p = 0.02), and Bosmina (Ellwood-Cosgrove, p = 0.001, Ellwood-Seidel, p = 0.002).
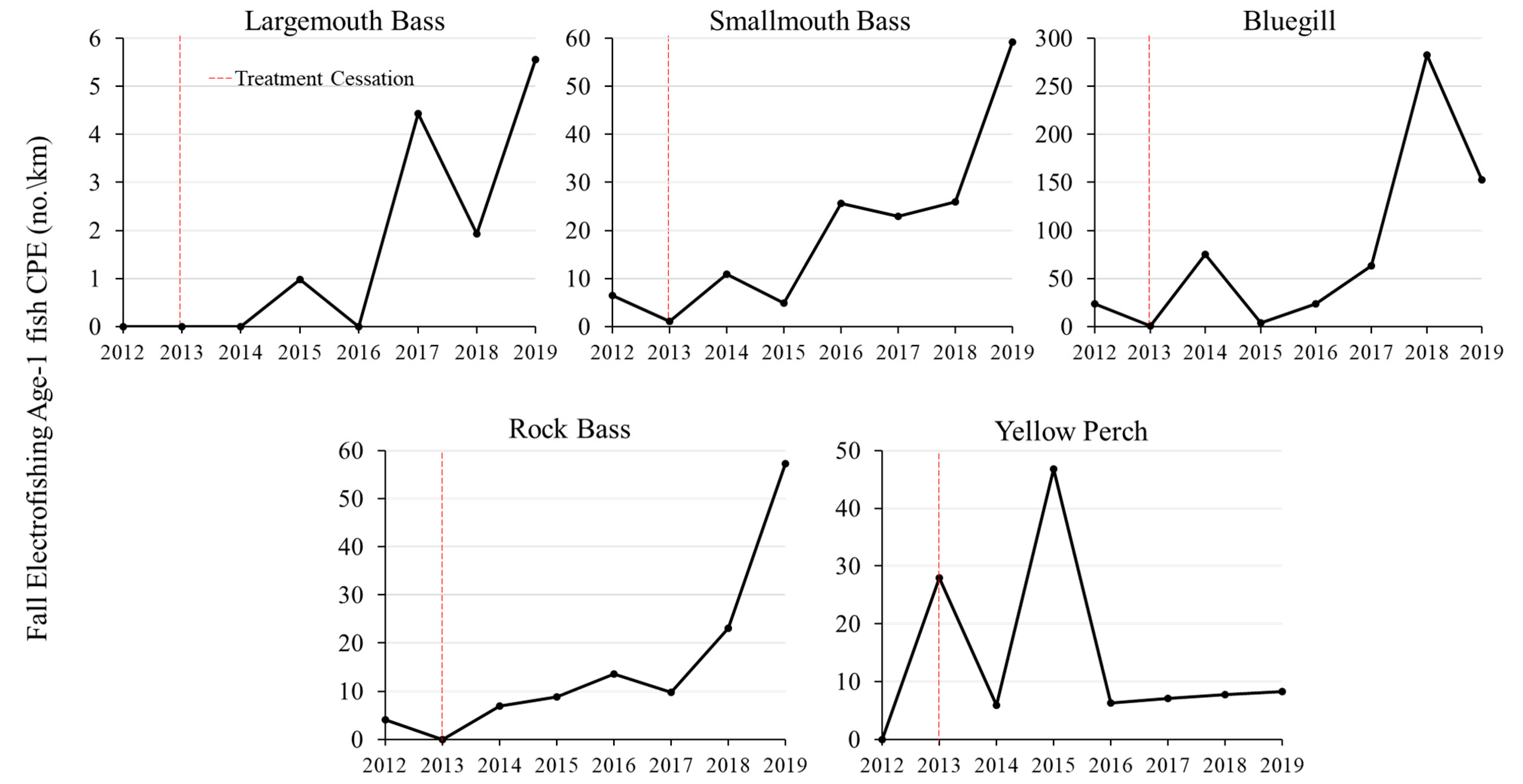
3.3. Juvenile fish Relative Abundance Responses
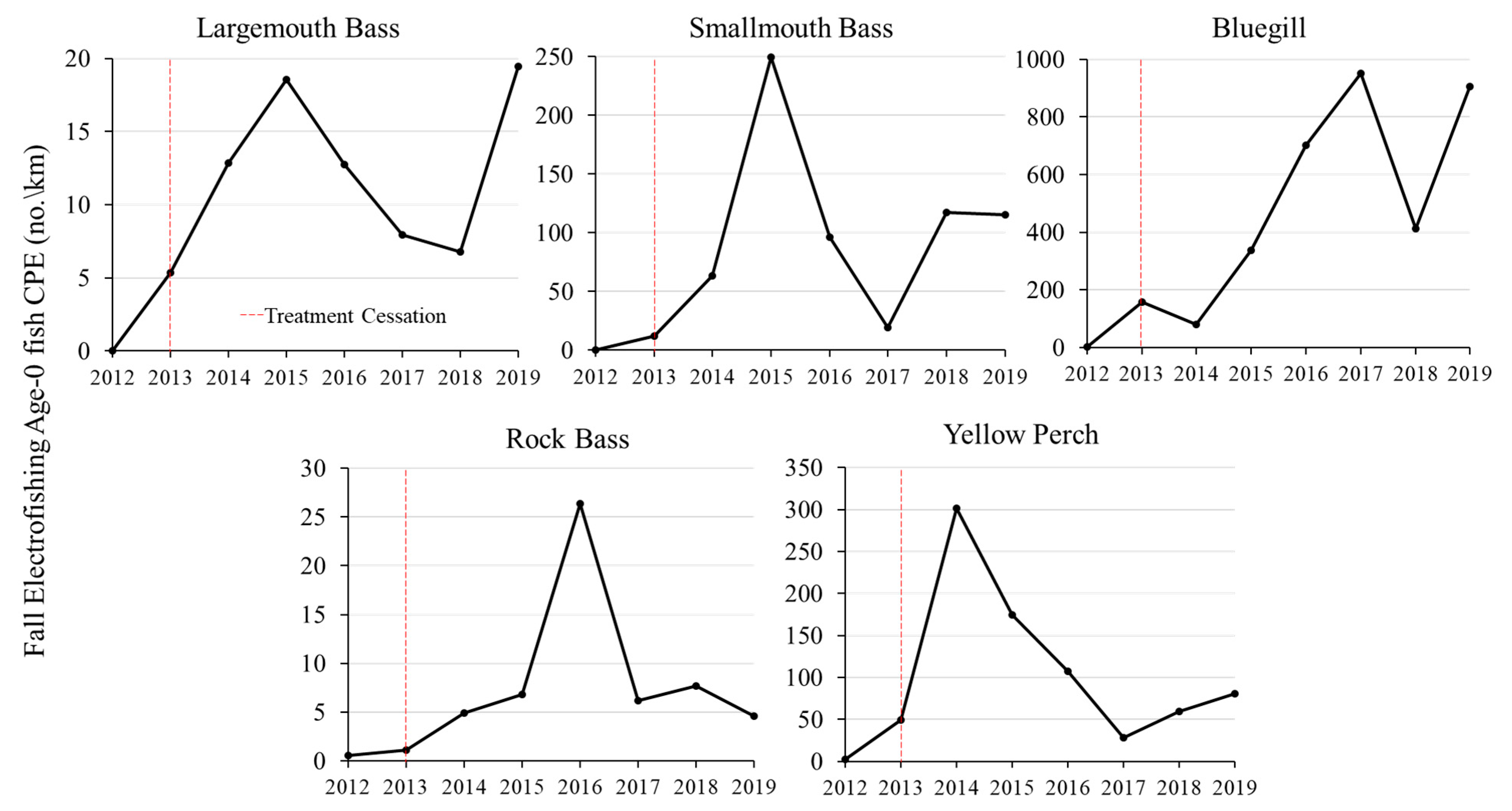
Age-0 relative abundance for several fish species increased following the cessation of chemical treatments on Lake Ellwood. Because fall mini-fyke net surveys for age-0 CPE showed similar trends to fall electrofishing with lower sample size, we used fall electrofishing age-0 CPE (fish/km of shoreline) as the primary metric of relative abundance of age-0 fish. Electrofishing CPE of age-0 fish in Lake Ellwood immediately increased between 2012 and 2013 for largemouth bass (0 fish/km to 5.4 fish/km), smallmouth bass (0 fish/km to 11.8 fish/km), bluegill (1.2 fish/km to 156.6 fish/km), yellow perch (2.9 fish/km to 49.4 fish/km), and rock bass (0.6 fish/km to 1.1 fish/km) following the cessation of chemical treatments (
Figure 4).
Relative abundance of age-0 fish steadily increased to peak values in 2015 and 2016 and then stabilized thereafter for largemouth bass, smallmouth bass, yellow perch, and rock bass. Age-0 bluegill CPE significantly increased throughout the study period (f = 11.2, df = 1.6, p = 0.02, r2 = 0.65) with a peak increase to 952.6 fish/km in 2017. Age-0 black crappie and pumpkinseed, which were present in Lake Ellwood prior to the chemical treatments (i.e., observed in 2002) were not observed post-treatment.
Cosgrove Lake (reference) age-0 largemouth bass (mean 15.9 ± 4.8 SE fish/km), age-0 smallmouth bass (3.0 ± 0.7 fish/km), age-0 yellow perch (15.5 ± 8.6 fish/km), age-0 bluegill (74.8 ± 34.9 fish/km), and age-0 black crappie (1.7 ± 1.1 fish/km) relative abundance did not change during 2013–2019 (all p > 0.05). Seidel Lake (reference) age-0 largemouth bass relative abundance significantly increased during 2014–2019 (mean 25.4 ± 10.0 SE fish/km; f = 19.7, df = 1.4, p = 0.01, r2 = 0.79), whereas age-0 yellow perch (51.4 ± 30.5 fish/km), age-0 bluegill (51.0 ± 11.5 fish/km), and age-0 black crappie (14.8 ± 4.2 fish/km) relative abundance did not change during the study period (all p > 0.05). The changes observed in age-0 fish recruitment in Lake Ellwood, and the relative stability of age-0 recruitment in the reference lakes, suggested a post-chemical treatment effect.
Age-1 relative abundance of several fish species increased post-chemical treatment in Lake Ellwood (
Figure 5).
Largemouth bass age-1 CPE significantly increased in Lake Ellwood during 2013–2019 (0 fish/km in 2013, 1.0 fish/km in 2015, 5.6 fish in 2019) (f = 13.6, df = 1.6, p = 0.01, r2 = 0.69). Smallmouth bass and rock bass age-1 CPUE also significantly increased during 2013 –2019 (smallmouth bass; 1.1 fish/km in 2013, 25.6 fish/km in 2016, and 59.2 fish/km in 2019) (f = 16.3, df = 1.6, p = 0.01, r2 = 0.73) (rock bass, 0 fish/km in 2013, 13.6 fish/km in 2016, 57.3 fish/km in 2019) (f = 18.1, df = 1.6, p = 0.01, r2 = 0.75). Bluegill age-1 CPE increased during 2013–2019, but did not statistically change over time. Age-1 CPE of yellow perch peaked in 2013 (27.9 fish/km) and 2015 (46.8 fish/km); however, was lower in 2014 (5.9 fish/km) and during 2016–2019 (6.4–8.3 fish/km). No age-1 black crappie or pumpkinseed were collected during the study period.
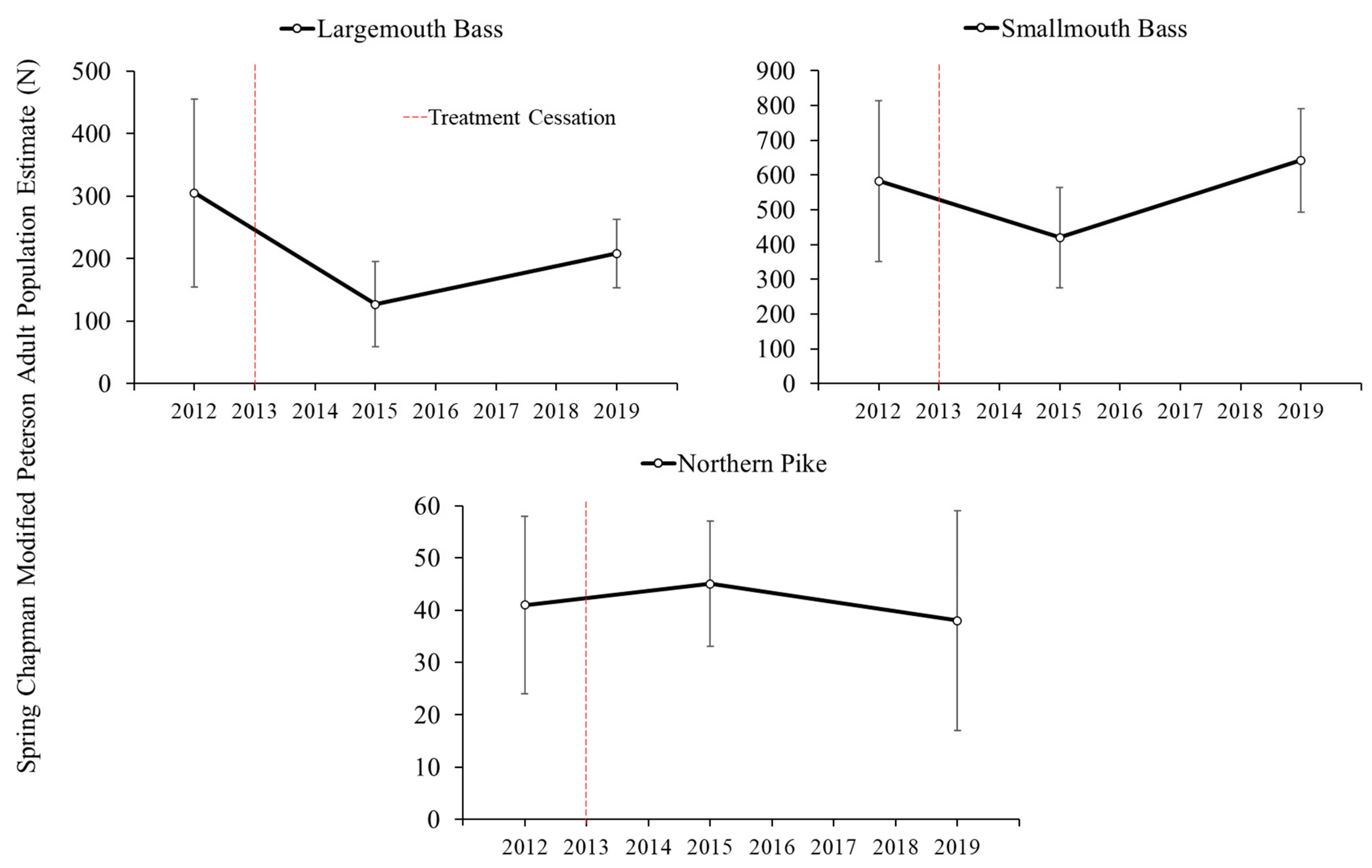
3.4. Adult Fish Abundance, Relative Abundance, Size Structure, and Growth Responses
Adult fish responses to the cessation of chemical treatments on Lake Ellwood were variable. Adult population estimates of northern pike remained relatively stable from 2012 (41 ± 17 fish) to 2015 (45 ± 12 fish) and 2019 (38 ± 21 fish) on Lake Ellwood (
Figure 6).
Largemouth bass and smallmouth bass adult population estimates on Lake Ellwood decreased in 2015 relative to 2012 and increased from 2015 to 2019 (
Figure 6). Adult largemouth bass abundance in 2012, 2015, and 2019 on Lake Ellwood was 305 ± 150 fish, 127 ± 68 fish, and 208 ± 55 fish, respectively (
Figure 6). Adult smallmouth bass abundance on Lake Ellwood was 583 ± 231 fish in 2012, 420 ± 144 fish in 2015, and 642 ± 148 fish in 2019 (
Figure 6). Adult (age-2+) largemouth bass CPE ranged from 2.3–4.3 fish/km during 2012–2015, zero were collected in 2016, and ranged from 0.9–4.6 fish/km during 2017–2019. Adult smallmouth bass CPE was relatively high during 2013–2015 (14.8–19.5 fish/km), decreased in 2016 to 3.7 fish/km, then stabilized during 2017–2019 (9.3–11.6 fish/km). Adult northern pike CPE decreased from 2013–2016 (2.1–0.9 fish/km) and no adult northern pike were captured during fall electrofishing surveys during 2017–2019 resulting in a significant decline throughout the study period (
f = 7.6,
df = 1.6,
p = 0.03,
r2 = 0.56). Adult bluegill CPE decreased from 12.9 fish/km in 2013 to 0.9 fish/km in 2016, then increased to 76.0 fish/km in 2019. Adult yellow perch CPE remained low during 2013–2019 (3.2 fish/km in 2013, 0 fish/km in 2014, ranged from 0.9–2.9 fish/km during 2015–2019). Adult rock bass CPE remained stable during 2013–2018 (6.5–13.9 fish/km) and increased in 2019 (22.2 fish/km). No adult black crappie or pumpkinseed were captured during fall relative abundance surveys. Bluntnose minnow CPE significantly increased during 2012–2019 (
f = 7,
df = 1.6,
p = 0.04,
r2 = 0.54). Bluntnose minnow CPE was 0.6 fish/km in 2012, 156.4 per/km in 2016, and then stabilized thereafter at 44.2–110.1 fish/km during 2017–2019. White sucker CPE significantly decreased during 2012–2019 (
f = 8,
df = 1.6,
p = 0.03,
r2 = 0.58). White sucker CPE was stable during 2012–2016 (2.7–7.8 fish/km), but then decreased to 0.9 fish/km in 2017, 0 fish/km in 2018, and 0.9 fish/km in 2019.
Cosgrove Lake (reference) largemouth bass CPE was 44.7 fish/km and 54.9 fish/km in 2015 and 2019, respectively. Cosgrove lake smallmouth bass CPE was 0.8 fish/km in 2015 and 2.3 fish/km in 2019. Relative abundance of largemouth bass in Seidel Lake (reference) was 1.9 fish/km in 2015 and 4.6 fish/km in 2019. Relative abundances of yellow perch, bluegill, rock bass, and black crappie in Cosgrove Lake in 2015 and 2019 were 1.9 and 0.9 fish/net night, 10.7 and 9.3 fish/net night, 5.0 and 6.73 fish/net night, and 0.2 and 16.5 fish/net night, respectively. In Seidel Lake, CPE of bluegill was 24.1 and 2.9 fish/net night in 2015 and 2019. Seidel Lake black crappie CPE was 5.0 fish/net night in 2015 and 4.4 fish/net night in 2019. Again, changes observed in fish CPE in Lake Ellwood, and the relative stability of fish CPE in the reference lakes, suggested a post-chemical treatment effect.
Back calculation of year-class strength from age estimates of fish collected during 2012 spring and summer surveys showed evidence for multiple failed year-classes of northern pike (2005–2012), largemouth bass (2009–2012), black crappie (2007–2010, 2012), and bluegill (2012) during the chemical treatment period (
Figure 7).
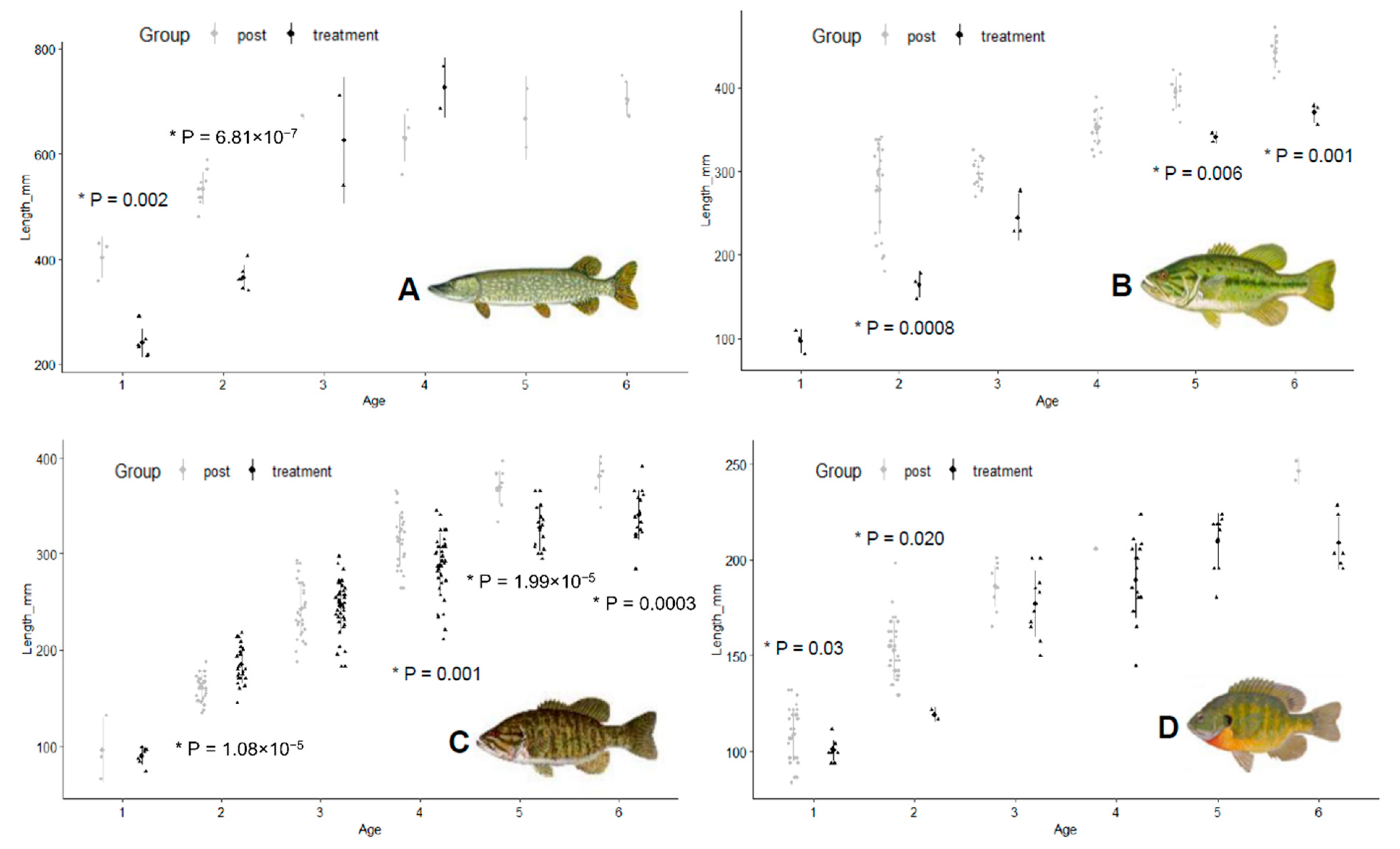
Welch’s
t-tests between treatment and post- treatment mean length-at-age estimates showed significantly smaller lengths for several fish species (
Figure 8).
Age-1 (
t = 7,
df = 4,
p = 0.002) and age-2 (
t = 12.2,
df = 9,
p < 0.001) northern pike showed significantly lower mean lengths during chemical treatment (
Figure 8). Largemouth bass showed significantly lower mean length-at-age during chemical treatments for age-2 (
t = 7.3,
df = 5,
p < 0.001), age-5 (
t = 7,
df = 3,
p = 0.006), and age-6 fish (
t = 7,
df = 4,
p = 0.001) (
Figure 8). Smallmouth bass showed significantly lower mean length-at-age estimates during chemical treatments for age-4, (
t = 3.5,
df = 64,
p = 0.001), age-5 (
t = 5.3,
df = 24,
p < 0.001), and age-6 (
t = 4.4,
df = 18,
p < 0.001) (
Figure 8). Length-at-age mean estimates for bluegill were significantly lower for age-1 (
t = 2.5,
df = 33,
p = 0.02) and age-2 fish (
t = 8.9,
df = 3,
p = 0.003) (
Figure 8). In contrast, age-2 smallmouth bass showed significantly higher mean length-at-age during the chemical treatment period compared to post-treatment (
t = 5,
df = 43,
p < 0.001) like age-2 rock bass (
t = 3,
df = 10,
p = 0.01).
4. Discussion
Our results provide multiple lines of evidence to suggest that ten years of EWM chemical treatment on Lake Ellwood contributed to failed natural recruitment in several north-temperate native fish species. The cessation of chemical treatments in 2012 resulted in a fish and zooplankton community that appeared to rebound rapidly for most species in the years immediately after treatments were ceased and then stabilized to abundances that were like our reference lakes and other northern Wisconsin lakes by 2019. Comparison of Lake Ellwood fish and zooplankton community responses to the two references systems suggested a cessation of chemical treatment effect. Our hypotheses of a lethal effect of the chemical treatment and sublethal effects due to changes in the zooplankton community on fish recruitment were supported [
37], whereas our hypothesis of submersed aquatic vegetation loss leading to a reduced refuge or greater mortality effect on recruitment was not.
The recovery in fish recruitment observed in Lake Ellwood did not correspond with an increase in submersed aquatic vegetation abundance. This suggests that the loss of vegetation from chemical treatment did not cause failed recruitment through factors such as a loss of refuge habitat and associated increased predation risk [
12,
41]. Therefore, natural recruitment declines appeared to be driven by direct lethal effects of chemicals (2,4-D) on fish or sublethal mechanisms from high mortality rates during ontogeny related to changing zooplankton community dynamics. The highest BvP and PAC of submersed vegetation on Lake Ellwood occurred during 2014–2016; however, BvP and PAC were lower in subsequent years with similar increases observed during 2018 and 2019. Given our observations, a major change in the aquatic macrophyte community was not evident and coincident with the fish and zooplankton community responses observed in Lake Ellwood during 2013–2019. Wider ranges and fluctuations in BvP and PAC of submersed aquatic vegetation were observed within our reference systems, whereas similar increases and changes in the fish and zooplankton community did not occur compared to Lake Ellwood. These fluctuations of submersed aquatic vegetation in the reference systems were more likely a result of natural inter-annual variability. Hand pulling and Diver-Assisted-Suction-Harvest (DASH) of EWM did occur after chemical treatments in Lake Ellwood and could have also played a role in the submersed aquatic vegetation responses. Although submersed aquatic vegetation surveys using sonar proved to be a convenient and viable way of testing for changes during our study, future research on EWM chemical treatments should include point-intercept sampling techniques to provide additional fine-scale submersed aquatic vegetation data on lake treatment areas.
2,4-D has been shown to have direct lethal effects on certain fishes in laboratory settings [
34,
35,
36,
37]. Our results within a natural system support previous laboratory findings suggesting that EWM chemical treatments using 2,4-D induced lethal effects on eggs, larval fishes, and age-0 recruitment even at low concentrations, especially if applications take place over an extended period. We observed a positive response to the cessation of chemical treatments in the rebounding populations of largemouth bass, smallmouth bass, bluegill, and yellow perch in our fish community analysis. Although adult northern pike abundance declined over time, the initial decrease in abundance of adult largemouth and smallmouth bass after the cessation of chemical treatments was followed by an increase as new juveniles recruited to adulthood. However, black crappie (historically present in Lake Ellwood) were not observed in our fish surveys post-chemical treatment. This is especially concerning as black crappie are an important native fish species in many north-temperate lakes and highly sought after by anglers in the region [
42,
43]. Further, black crappie abundances have increased in Wisconsin statewide over time [
44]. Black crappie populations are known to have variable natural recruitment [
45] and could be negatively influenced if year classes are repeatedly suppressed. In addition, white sucker relative abundance significantly decreased in combination with an aging adult population that had noticeably less healthy body condition in 2019 surveys compared to previous surveys (G. Matzke, Wisconsin Department of Natural Resources, personal communication). White sucker predominantly feed on zoobenthos and zooplankton [
46] and may have been negatively influenced due to zooplankton community or zoobenthos (although not studied here) changes associated with the EWM chemical treatments on Lake Ellwood. White sucker population dynamics may also be influencing the decreasing abundance and length of adult northern pike present in Lake Ellwood (G. Matzke, Wisconsin Department of Natural Resources, personal communication), as white sucker can be a common forage item found in northern pike diets [
47]. The combination of laboratory and whole-lake evidence that 2,4-D can cause lethal effects on fish species native to north-temperate lakes [
37,
48] and the continued application of these chemicals to waterbodies to reduce invasive macrophytes such as EWM is concerning. Increased whole-lake aquatic ecosystem monitoring before and after submersed aquatic vegetation chemical treatments may be necessary to ensure fish populations are not being negatively influenced.
Zooplankton are a vital link between primary producers [
49] and consumers to ensure that energy and nutrient transfer occurs in food webs of north-temperate lakes [
50,
51]. Zooplankton are a critical component of larval fish diets during ontogeny. Therefore, high mortality may result from changes in zooplankton community composition or overall reductions in zooplankton densities [
52]. Due to failed year classes of fish species observed in Lake Ellwood, we would have expected zooplankton densities to have been consistently high in the absence of few or no age-0 fish [
50,
51] and lower during periods of greater natural recruitment such as those observed following the cessation of chemical treatments on Lake Ellwood. Interestingly, total zooplankton densities increased immediately following the cessation of chemical treatments on Lake Ellwood. Total zooplankton density increases coincided with age-0 fish recruitment in Lake Ellwood, suggesting that a chemical treatment effect occurred and that the zooplankton response may have been even greater in the absence of fish predation. Similarly, total zooplankton density increases in Lake Ellwood in comparison to the relative stability of densities observed in the reference systems suggest a chemical treatment effect. Despite increasing trends in most zooplankton species in Lake Ellwood post-chemical treatment, copepods (cyclopoid copepods, nauplii) exhibited the strongest increases and greatest influence on total zooplankton density. Copepods are known to be a prominent source of meiofauna found within sediments in marine environments [
49], and this may suggest that interactions with 2,4-D within sediments or potential legacy effects in Lake Ellwood may have been inducing lethal effects suppressing abundances. 2,4-D has been shown to decrease total abundance and taxon richness of some zooplankton species. Mixtures with additional commercial herbicides have been shown to specifically have significant negative influences on copepod abundances [
53]. The chemical treatments on Lake Ellwood could have led to low availability for a previously common zooplanktonic prey for age-0 fishes and contributed to the recruitment failures we observed.
Several limitations existed within our study, and additional hypotheses and questions gleaned from our results require additional research. First, whole-lake experiments typically use a deliberate manipulation to test for a treatment effect [
54,
55]. Ideally, hypothesis testing would have involved the treatment effect of long-term chemical application versus a treatment of chemical cessation. In this case, pre-treatment aquatic ecosystem monitoring would have been preceded by during- or post-treatment monitoring. Unfortunately, consistent pre- and during-chemical treatment data was limited (but see 2012 population estimates); however, we reconciled this for fish recruitment through the back calculation of year class strength provided by aging structures. The lack of pre-and during-treatment data limited traditional statistical methods of Before-After-Control-Impact study designs [
56,
57,
58], and further highlights the need for monitoring and documentation in systems during EWM mitigation efforts. Such efforts may require the need for cross agency/consultant cooperation to achieve these logistically intensive monitoring efforts. Additional metrics that would have been beneficial to collect within our study, and we suggest for future research efforts, would be measurements of 2,4-D concentrations within the sediment during and after chemical treatment to test for legacy effects on sediment concentration and seed bank viability. Additionally, benthic macroinvertebrate monitoring and increased data collection (e.g., length/weight measurements, age-specific CPE) of non- gamefish species for forage consequences on adult gamefish would be beneficial due to the plausible connection observed with low body condition and abundances observed in white sucker and decreasing abundances of northern pike. It is also possible that intra- and inter-specific interactions within and between the fish community and inter-annual variability may have influenced our findings and conclusions. Nevertheless, we reasoned that intra- and inter-specific interactions in the fish community were not plausible mechanisms leading to bluegill and largemouth bass recruitment declines because they are generalist species [
59], not species of conservation concern in Wisconsin or elsewhere in their native range [
14,
44], and often invasive species in their non-native range [
60]. Furthermore, fusiform and soft-bodied fish prey are generally selected for by piscivorous fishes over deep-bodied and spiny-rayed fish prey [
55,
61,
62,
63]. We also reasoned that inter-annual variability in bluegill and largemouth bass natural recruitment was not likely a major driver of our observations given the length of our time series, the longevity of these species [
59], and the fact that nest-guarding species have generally shown more consistent natural recruitment versus that of broadcast spawners such as walleye [
44,
64,
65,
66].
Despite our study limitations, we present multiple lines of evidence to suggest that long-term chemical treatments with 2,4-D in north-temperate lakes have the potential to cause fish recruitment declines. Potential direct lethal mechanisms for failed recruitment supported from our results in Lake Ellwood include influences on adult reproductive capabilities, egg quality degradation, larval development, and hatching influences as supported by laboratory studies [
37]. A potential sublethal effect supported by our results is the lack of suitable forage availability (decreased total zooplankton abundance and community changes) during ontogeny. Although our results do not support the loss of refuge as a mechanism for fish recruitment declines from changes in SAV, this factor is another potential outcome of chemical treatments that could influence fish populations and should be considered when planning invasive submersed aquatic vegetation control efforts. Submersed aquatic vegetation chemical management interventions should be designed as deliberate learning experiments [
67,
68]. Many current invasive macrophyte chemical treatments lack sufficient preliminary, during, and post-treatment monitoring of fish, zooplankton, and submersed aquatic vegetation communities to achieve a thorough understanding of potential aquatic ecosystem influences.
Long-term submersed aquatic vegetation chemical treatments have the potential to cause failed year classes in fishes due to direct lethal effects and changes to aquatic ecosystems. In the absence of an intervention (e.g., fish stocking), naturally reproducing fish populations may be negatively influenced or extirpated from lakes if 2,4-D treatments are persistent or legacy effects occur beyond species-specific fish longevities. Our results suggest that if chemical treatments are a warranted management decision for invasive submersed aquatic vegetation control in a waterbody, fish community composition needs to be acknowledged to account for species with observed higher laboratory and north-temperate lake vulnerabilities ([
37], species negatively affected here), 2,4-D alternatives should be considered, management plans limited in spatial scope and frequency within lakes, and sufficient preliminary, during, and post-treatment data obtained to prevent long-term recruitment declines in certain fish species in north-temperate lakes.
Overall, our results showed no post-chemical cessation changes in the limnology or submersed aquatic vegetation community, an increase and then stabilization in total zooplankton density, and an increase in fish recruitment for several fish species following years of failed recruitment during the 2,4-D chemical treatment period on Lake Ellwood. The relative lack of change in these variables in our reference lakes suggest a post-chemical treatment effect on Lake Ellwood. Our study highlights a critical need to further investigate potential mechanisms for failed fish natural recruitment in lake ecosystems with on-going or historic aquatic herbicide treatments through additional research or other whole-lake studies. For example, the timing of chemical treatments in relation to fish life histories (particularly spawning), influences of the frequency and duration of treatments, the concentration and mixture of chemicals (2,4-D and other aquatic herbicides) used in treatments, and the longevity, spread, and degradation of these chemicals in waterbodies during and after treatments.