Abstract
In tropical regions, temperature is the fundamental environmental factor controlling the reproduction-related physiological activities of fish. Tropical fish are particularly sensitive to climate change since they develop in a relatively stable thermal environment. A review was done to assess the potential effect of temperature rise on reproduction and population structure in the commercially important hermaphrodite grouper and wrasse species, and in gonochoric snapper species in the southern Gulf of Mexico. Temperature increase can disturb the aromatase synthesis and/or activity, which can affect the reproductive cycle and sexual differentiation in all studied species and the sexual inversion process in sequential hermaphrodites. Moreover, a mistiming or discontinuity in spawning seasonality could occur, with an alteration in the sex ratio in favor of males and a consequent reduction in populations’ fecundity. Furthermore, if the level of fishing exploitation enhances species’ sensitivity to environmental changes, then the stock of red grouper Epinephelus morio would be more affected by temperature increases than other species because it is the only fish population in the Campeche Bank currently assessed as overexploited.
1. Introduction
Among many effects of climate change, the rise of atmospheric greenhouse gases (particularly carbon dioxide) has led to an increase in average sea surface temperatures (SST). Fish are ectotherms and therefore extremely sensitive to changes in environmental temperature. Tropical marine fishes living in a relatively thermal stable environment and close to upper thermal limits are especially vulnerable to increases in SST [1,2]. Rising temperatures in the ocean can affect all the successive lifecycle phases of fish (embryo, larva, juvenile, and adult), as well as impact all levels of their biological and ecological organization (individual, population, community, and ecosystem) [3,4,5]. Fish biological functions, such as reproduction and growth, generally respond positively to slight increases in environmental temperature but can also degrade when temperatures exceed a species’ thermal optimum. The aerobic thermal windows at which animals can function best, wideness during the individual life cycle, and body size. Nevertheless, adult spawners present a narrower thermal window making them more sensitive to warming because a minimal temperature rise can surpass the individuals’ upper critical temperature-limit necessary to reproduce [2,6].
Phenological observations generate indicators considered fundamental in demonstrating changes in the lifecycles of living beings [7]. First coined and used by the Belgian botanist Charles Morrel in a 1953 publication [7,8], the term phenology can be defined as, the study of the timing of recurring biological events, the causes of their timing with regard to biotic and abiotic forces, and the interrelation among phases of the same or different species [8]. The biological rhythms of living beings involve the synchronization of an organism’s numerous physiological functions with periodic variations of the surrounding environment. For example, reproduction in teleosts is mainly controlled by two environmental factors: the photoperiod and temperature [9,10]. The influence of these factors on the reproductive cycles of fish varies by species and geographical distribution areas [11]. In cold, temperate, and subtropical regions, photoperiod apparently plays a greater role than in tropical regions where temperature and a combination of other stimuli such as rainfall, tides, or lunar cycles would be the dominant factors [11,12]. Nevertheless, sea temperature may not only affect the different phases of reproductive activity in teleosts (i.e., gametogenesis, the timing of spawning, gonad regression, and egg quality) but on gonad differentiation and sexual inversion of sequentially hermaphroditic species [13,14,15].
The most accurate data available on the mechanisms involved in fish reproductive process disturbances in response to temperature are generally for temperate regions species, such as salmon, obtained through laboratory experiments in a controlled environment [15]. For tropical fish, the possible effects of climate change on different aspects of their biology and at the population level have not been evaluated yet. Any impact climate change could have on reproduction in commercially important species would be in addition to the effects of commercial fishing pressure on their population dynamics. In conjunction, these two impacts could provoke strong repercussions in the socioeconomics of human populations that depend on these species [4,14].
In the southern Gulf of Mexico, the grouper (Epinephelidae) and snapper (Lutjanidae) complex is a main component of the finfish fishery based in the state of Yucatan, Mexico. In 2014, these species represented 56% of finfish live weight catch landed in the state (15,555 metric tons): 39% (6092 metric tons) was grouper and 17% (2544 metric tons) was snapper (22% of the latter was red snapper Lutjanus campechanus) [16]. The grouper–snapper fishery on the Yucatan Peninsula continental shelf (i.e., Campeche Bank) is in decline [17], and the red grouper Epinephelus morio stock is overexploited [18]. The 69% decline in grouper catches since 1972 (19,886 metric tons) has driven an intensification of fishing efforts towards other potentially profitable species such as hogfish Lachnolaimus maximus [16,17]. Moreover, there is evidence of how climate change environmental variables may affect the productivity of various fishery stocks on the Campeche Bank, particularly on red grouper’s stock abundance, in which an increase in SST might have an effect on the species’ reproductive process and recruitment success [19].
By reviewing the literature available on the effects of climate change on fish species reproduction, the present study objective was to evaluate its possible consequences on reproduction and population structure in a case study of ten commercially important hermaphroditic and gonochoric tropical fishes. Therefore, we analyze the possible repercussions of temperature rise on the reproductive cycle, sex determination, and sex change of grouper, snapper, and hogfish species, which populations on the Campeche Bank face heavy fishing pressure and potential climate change effects.
2. Regulation of Fish Reproduction
Seasonal changes in proximal environmental factors modulate the neuroendocrine and endocrine activity of the hypothalamic–pituitary–gonadal axis (HPG), which regulates reproductive function in fish [9,10]. The gonadotropin-releasing hormone (GnRH) produced by the hypothalamus stimulates the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the pituitary, which in turn induce the production of steroid sex hormones such as estradiol-17β (E2) and 17,20β-dihydroxy-4-pregnen-3-one (17,20βP) in the ovaries, and testosterone (T) and 11-ketotestosterone (11KT) in the testicles. One notable aspect of teleost reproduction is the fundamental role played in steroidogenesis by certain enzyme complexes, such as aromatase (P450aro) in females and 11β-hydroxylase (11βH) in males. In the ovaries, aromatase causes the transformation of T (produced as a precursor) into E2, while in the testicles 11βH mediates conversion of T to 11KT [9,10,20]. The sexual hormones produced by the gonads stimulate gametogenesis in both sexes; vitellogenesis and oocyte maturation in females and spermiation in males. These hormones also control the development of secondary sexual characteristics and reproductive behavior [9,10], as well as modulate the physiological processes of gonad differentiation and sex change in sequential hermaphroditic species [21,22,23].
3. Influence of Temperature in Fish Reproduction
Very little is known of the physiological role of temperature on the modulation of fish reproductive activity, especially in tropical fish. Fluctuations in temperature most probably cause variations in the expression of genes controlling the synthesis of reproductive hormones and their associated enzymes at the level of certain crucial links in HPG axis functioning [12]. Temperature acts directly at the cellular level on metabolic activities affecting the synthesis, structure, and activity of the neurohormones, hormones, and enzymatic complexes involved in steroidogenesis since these are thermosensitive and thermolabile molecules [9]. Temperatures above organisms’ thermal physiological tolerance range can cause negative effects on their reproductive processes by (1) inhibiting the expression of the genes that control the synthesis of reproductive hormones and associated enzymes; (2) altering the activity levels of hormones in the bloodstream and enzymes in the gonads; and (3) modifying the specific affinity of reproductive hormone receptor cells [15,24]. Consequently, a higher-than-optimal temperature can affect oocyte development and maturation; the timing of ovulation and spawning; and egg quality and reproductive physiology in females [25].
4. Effects of Rising Temperature in Fish Reproduction
4.1. Effect on Reproductive Cycle
Even relatively slight increases in temperature can provoke endocrinal changes in the HPG axis of fish, particularly in tropical fish [12]. In these species, temperatures above 30 °C can begin to disturb the endocrine activities involved in reproduction [15,26]. An increased temperature generally leads to mistiming in fish reproductive cycles. Experimental studies on different species suggest that this effect is more notable in females than males [24]. This mistiming in a female’s reproductive cycle results from the decrease or inhibition of aromatase and E2 synthesis and activity when environmental temperature increases above a species’ physiological thermal tolerance range. How off the timing will depend on the season during which the temperature increase occurs in relation to the season during that a species reproduces. Temperature effects will be strongly dependent not only on the overall temperature but on the annual pattern of thermal change as well. Therefore, in temperate regions, high temperatures can advance or truncate the reproduction season in spring or summer spawning species and delay the season in fall spawning ones [15,24]. In equatorial regions, fish species commonly reproduce during a large portion of the year and even year-round. Temperature increases in these regions can advance the beginning of the reproduction season in species, which start reproducing after crossing a thermal threshold. The reproductive season can overextend as long as the upper limit of the reproduction-compatible temperature range is not exceeded. If the maximum temperature exceeds a tropical species’ physiological tolerance range during reproduction season, ovulation and spawning can be eventually temporary interrupted [3,24]. Indeed, extreme temperatures alter gonad development and block gamete emissions by inhibiting the genes that code steroidogenic enzymes in gonads of both sexes [12,15,24]. In consequence, mistiming in fish reproductive cycles can easily cause negative changes in gamete quality and on larvae development due to a mismatch between the timing of hatching and favorable environmental conditions for larval survival [5,15,24].
4.2. Effect on Sexual Determination
Sex determination in many gonochoristic fishes is controlled by genetic factors (genotypic sex determination-GSD) or environmental factors (environmental sex determination-ESD). With GSD, sex is determined at conception by genes generally located in the sexual chromosomes. With ESD, sex is determined after fertilization by environmental factors, primarily temperature during a critical period of cell sensitivity (embryonic or larval stage). This is referred to as temperature sex determination (TSD) [27]. However, sex determination in some GSD species can be influenced during embryo and larva development by environmental effects of exogenous factors (GSD + EE) and especially by temperature effects (GSD + TE) [28,29]. Therefore, in species with GSD + TE, the temperature can eventually orient final gonad differentiation (phenotypic sex) in the opposite direction to that established genetically [9,10]. In some thermosensitive gonochoristic species (TSD or GSD + TE), the proportion of males increases with higher temperatures, generating an imbalance in the population’s sex ratio [28,30]. This higher proportion of males could be a result of the inhibition of thermosensitive genes expression that code aromatase biosynthesis (e.g., cyp19a1a in gonads). A low aromatase activity prevents the conversion of T to E2, leading to an imbalance in sexual steroids in favor of the 11-oxygenated androgens (e.g., 11-KT) and thus favoring the masculinization of undifferentiated individuals [23,30]. Aromatase activity may therefore play a key unidirectional role in sex determination since E2 acts as a natural inductor of ovary differentiation [9]. Very little data is available to date on gonad differentiation in hermaphroditic species and the effect of temperature on this differentiation [13,30,31]. However, it is assumed that ovarian differentiation may be the primary status in sequential hermaphroditic fishes, as it has been observed that all undifferentiated gonads of some protogynous or protandrous species first differentiate into immature ovaries. Subsequently, immature ovaries differentiate then into mature ovaries or testis [31,32].
4.3. Effect on Sex Change
The key factor in the physiological process of sex change in sequentially hermaphroditic species is the expression level of the genes involved in aromatase biosynthesis [23,30]. In these species, the functional female phase is characterized by high serum levels of E2, while the functional male phase sees high serum levels of 11KT [32,33]. In protandrous species, the activation of the aromatase genes induces an increase in E2 levels that favors the sex change from male to female. In contrast, in protogynous species, the deactivation of these genes provokes a decrease in aromatase and E2 levels and, by cascade effect, an increase in 11βH and 11KT levels that favors the sex change from female to male [14,22,23]. Temperature rising may impede or induce a sex change in sequential hermaphroditic species, despite the control exercised by social factors on the sexual inversion process [15,30]. Indeed, high temperatures may inhibit the expression of the aromatase genes and consequently impede sexual inversion from male to female in protandrous species. Contrarily, in protogynous species, inhibition of the aromatase genes expression may enhance sexual inversion from female to male and induce sex change at an earlier age and a smaller size [15].
5. Case Study
5.1. Studied Species
Analyses were done on 10 tropical demersal teleost species distributed in the west central Atlantic Ocean (Table 1); all are probably rather sensitive to climate change. Indeed, these species share similar complex lifecycles consisting of a succession of different stages that develop in different habitats: embryonic and larval stages occur in a three-dimensional pelagic habitat and the juvenile and adult stages in a two-dimensional demersal habitat. Each developmental stage can be disturbed in different degrees by temperature increases [4]. However, the consequences of climate change could vary according to the characteristics of each species’ reproductive cycle and the prevalent sexuality pattern in each family. The seven studied grouper and wrasse species exhibit monandric protogynous hermaphroditism; that is, individuals change sex during their lifecycle, passing from a functional female to a functional male phase and, therefore, males are termed secondary males because all are produced by sexual inversion from previous functional females. Conversely, the three analyzed snapper species are gonochoristic [34,35].

Table 1.
Tropical fish species from the Campeche Bank analyzed in the present study. a: MPH = monandric protogynous hermaphroditism; b: G = gonochorism [34,35].
5.2. Potential Change in Female’s Reproductive Cycle
The reproductive biology of Campeche Bank’s populations were analyzed: between 1988 and 1993 for red grouper [36]; 1996 and 2001 for black grouper and gag [37,38]; 1999 and 2000 for red snapper [39]; 2008 and 2010 for red hind, tiger grouper and yellowfin grouper [40,41], and yellowtail snapper and lane snapper [42,43], and between 2011 and 2015 for hogfish [44]. In the present study, species’ spawning seasonality was assessed, considering only the reproductive cycle of females, because ovaries best reflect the duration of fish spawning activity [45]. On the Campeche Bank, the grouper reproduction season is shorter (4–7 months) [36,38,40] than that of the snapper and the hogfish (11–12 months) [39,42,44]; the one exception is female black grouper, which are sexually active all year-round [37] (Figure 1). Some of the grouper begin maturing in autumn (red hind, red grouper, and gag) and others in winter (tiger grouper and yellowfin grouper), but all (including black grouper) exhibit spawning peaks in winter and/or early spring, when temperature and photoperiod increases gradually (Figure 1). None of the species spawn in summer when SST reaches maximum levels around July–September, nor in autumn (except for black grouper) when temperature and photoperiod start to decrease. In contrast, the snapper and hogfish can mature and spawn year-round, exhibiting spawning peaks in spring and autumn, when temperature and photoperiod increase or decrease, respectively (Figure 1).
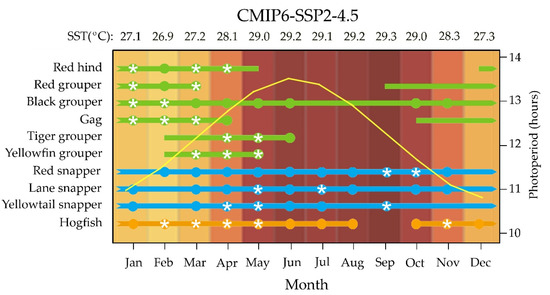
Figure 1.
Species’ natural female reproductive cycle of studied grouper (green bars) [36,37,38,40,41], snapper (blue bars) [39,42,43], and wrasse (orange bar) [44] from the Campeche Bank. Colors indicate monthly mean sea surface temperatures (SST) predicted by the Intergovernmental Panel on Climate Change, for the more-plausible CMIP6 SSP2-4.5 scenario projected for 2022 [46,47,48]. For each species, solid bars represent reproductive season reported as the months that include spawning-capable females; solid circles represent spawning season reported as the months that include actively spawning females, and white stars represent peak spawning reported as the months that include more than 50% of females in actively spawning sub phase (for reproductive phase terminology see [49]). Yellow curve indicates monthly photoperiod length (Time and Date AS 1995–2022. Available online: http://www.timeanddate.com/sun/mexico/merida, accessed on 1 March 2022).
If the water temperature is the most influential environmental factor regulating reproduction in tropical fishes, any increase that exceeds the upper limit of their physiological thermal tolerances could produce mistiming in their reproductive cycles. The upper-temperature threshold from which reproduction of grouper, snapper, or wrasse species is affected is unknown. Notwithstanding, in some tropical fishes, the thermal inhibition of reproduction typically appears at 30 °C and above [15,26]. Since the species of this study displayed a range of spawning temperatures, observed in the field or captivity, that never exceeded 30 °C (Table 2), we assumed this temperature could be the current thermal tolerance limit for their reproduction.

Table 2.
Spawning temperature ranges observed in captivity (1) and in their natural habitat (2; bottom temperature) for grouper, snapper, and wrasse species analyzed in the present study.
Sea surface temperature (SST) changes, predicted by the Intergovernmental Panel on Climate change (IPCC) Sixth Assessment Report (AR6) for the Southern Central American region [46,47], using general circulation models of the Coupled Model Intercomparison Project Phase 6 (CMIP6) [48], were taken into consideration in the present study. Mean monthly SSTs predicted for the Campeche Bank waters were used according to three possible climate change scenarios (Shared Socioeconomic Pathways: SSP2-4.5, SSP3-7.0, and SSP5-8.5) developed by the IPCC for selected 20-year time periods: 2040, 2060, 2080, and 2100 (Table 3). Monthly mean SSTs predicted for the current period (2022) do not raise the 30 °C (scenario CMIP6 SSP2-4.5). Thus, we assumed that the reproductive cycle of the studied species is not yet affected by rising water temperature (Figure 1).

Table 3.
Mean monthly sea surface temperature (SST) predicted for the Campeche Bank waters for selected 20-year time periods, according to three possible climate scenarios (SSPs, Shared Socioeconomic Pathways) developed by the Intergovernmental Panel on Climate Changes (IPCC) Sixth Assessment Report (AR6) for the Southern Central American region [46,47], using general circulation models of the Coupled Model Intercomparison Project Phase 6 (CMIP6) [48]. Gray areas indicate months in which mean SST ≥ 30 °C.
In SPP2-4.5 and SSP3-7.0 scenarios, timing in reproduction of all species would not be affected before 2060 (Figure 2 and Figure 3); while in the SSP5-8.5 scenario, the year-round reproductive cycle of black grouper and all snappers could be interrupted at the end of summer as soon as 2040 (Figure 4). From 2060 to 2100 in SPP2-4.5 and SSP3-7.0 scenarios and between 2060 and 2080 in the SSP5-8.5 scenario, a more severe mistiming in the reproductive cycle could occur for all snapper and hogfish than for grouper (Figure 2, Figure 3 and Figure 4). For 2100 in the worst SSP5-8.5 scenario, the reproductive cycle of all species could be dramatically affected (Figure 4).
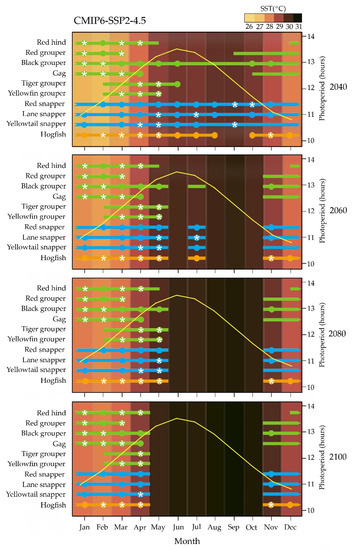
Figure 2.
Hypothetical effect of climate change on reproductive cycle of studied grouper (green bars), snapper (blue bars), and wrasse (orange bars) species from the Campeche Bank, in relation to 2040, 2060, 2080, and 2100 monthly changes in mean sea surface water temperature (SST) predicted by the CMIP6 SSP2-4.5 scenario of the Intergovernmental Panel on Climate change [46,47,48]. For each species, solid bars represent reproductive season reported as the months that include spawning capable females; solid circles represent spawning season reported as the months that include actively spawning females, and white stars represent peak spawning reported as the months that include more than 50% of females in actively spawning sub phase (for reproductive phase terminology see [49]). Yellow curve indicates monthly photoperiod length (Time and Date AS 1995–2022. Available online: http://www.timeanddate.com/sun/mexico/merida, accessed on 1 March 2022).
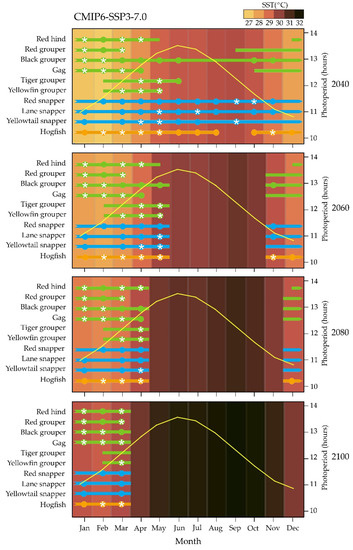
Figure 3.
Hypothetical effect of climate change on reproductive cycle of studied grouper (green bars), snapper (blue bars), and wrasse (orange bars) species from the Campeche Bank, in relation to 2040, 2060, 2080, and 2100 monthly changes in mean sea surface water temperature (SST) predicted by the CMIP6 SSP3-7.0 scenario of the Intergovernmental Panel on Climate change [46,47,48]. For each species, solid bars represent reproductive season reported as the months that include spawning capable females; solid circles represent spawning season reported as the months that include actively spawning females, and white stars represent peak spawning reported as the months that include more than 50% of females in actively spawning sub phase (for reproductive phase terminology see [49]). Yellow curve indicates monthly photoperiod length (Time and Date AS 1995–2022. Available online: http://www.timeanddate.com/sun/mexico/merida, accessed on 1 March 2022).

Figure 4.
Hypothetical effect of climate change on reproductive cycle of studied grouper (green bars), snapper (blue bars), and wrasse (orange bars) species from the Campeche Bank, in relation to 2040, 2060, 2080, and 2100 monthly changes in mean sea surface water temperature (SST) predicted by the CMIP6 SSP5-8.5 scenario of the Intergovernmental Panel on Climate change [46,47,48]. For each species, solid bars represent reproductive season reported as the months that include spawning capable females; solid circles represent spawning season reported as the months that include actively spawning females, and white stars represent peak spawning reported as the months that include more than 50% of females in actively spawning sub (for reproductive phase terminology see [49]). Yellow curve indicates monthly photoperiod length (Time and Date AS 1995–2022. Available online: http://www.timeanddate.com/sun/mexico/merida, accessed on 1 March 2022).
5.3. Potential Change in Population Structure
5.3.1. Effect on Sex Determination
Snapper populations generally exhibit characteristics of gonochoristic species such as balanced sex ratios, with a similar average size and size range between sexes [65], such as the one observed for red snapper from the Campeche Bank [39] (Figure 5A). On the other hand, the monandric protogynous hermaphroditic sexuality of grouper and hogfish display a biased sex ratio towards females and bimodal size–frequency distributions: minor females’ average size than average males’ size (all secondary males); size-range of females barely overlapping with that of males [66], such as the one observed for tiger grouper of the Campeche Bank [41] (Figure 5B).
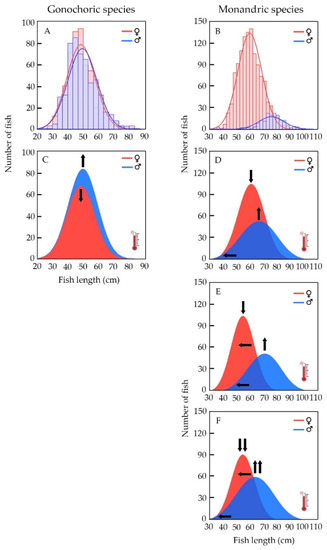
Figure 5.
Hypothetical effect of climate change on population structure (size and sex-ratio) of gonochoristic (snapper) and hermaphroditic (grouper and wrasse) species. (A) Size–frequency distributions observed for female and male red snapper from the Campeche Bank [39], a representative gonochoristic fish. (B) Size–frequency distributions observed for female and male tiger grouper from the Campeche Bank [41], a representative monandric protogynous hermaphroditic fish. (C) Expected change in red snapper sex–size–frequency distributions caused by the effect of increasing water temperature on sex determination. (D) Expected change in tiger grouper sex–size–frequency distributions caused by the effect of increasing water temperature on sex determination. (E) Expected change in tiger grouper sex–size–frequency distributions caused by the effect of increasing water temperature on sex change. (F) Expected change in tiger grouper sex–size–frequency distributions caused by the effect of increasing water temperature on sex determination and sex change. Vertical arrows indicate an increase (↑) or a decrease (↓) in male or female proportion, respectively. Horizontal arrows (←) indicate an increase or a decrease in male or female size range, respectively.
In snapper, the reduction of aromatase activity induced by ocean warming due to climate change could lead to the masculinization of a larger proportion of undifferentiated individuals, diminishing in consequence the proportion of females. The sex ratio in these populations could then become imbalanced, in favor of males without a notable modification in average size or individual size ranges for both sexes (Figure 5C).
If assumed that a rise in temperature produces the same physiological effect in steroidogenesis as observed in gonochoristic snapper, previously explained, in monandric protogynous hermaphroditic grouper and hogfish, the reduction or inhibition of aromatase activity could also cause early masculinization of a fraction of undifferentiated individuals. These would result in the appearance of small-sized males in the populations (i.e., primary males) and consequently diminish the whole proportion of females. Under this scenario, hermaphroditic species would exhibit a less biased sex ratio towards females, or perhaps a balanced one, depending on the magnitude of the early masculinization process. Average male size, including a mix of primary and secondary males, would decline and eventually match that of females. The male size range would then widen and, in time, overlap that of the females (Figure 5D). Therefore, ocean warming due to climate change could modify the sexuality pattern of these protogynous hermaphroditic species from monandry (only secondary males) to diandry (primary and secondary males).
5.3.2. Effect on Sex Change
For populations of monandric species such as grouper and hogfish, ocean warming could inhibit aromatase biosynthesis or activity leading to a sex ratio change in favor of males due to the premature transition of females to males. Therefore, the average size of males and females would be reduced, the size range of males would expand, and as a consequence, the female-size range would narrow (Figure 5E). Considering a hypothetical early masculinization in a fraction of the undifferentiated individuals, the early sexual inversion of females could skew the sex ratio further in favor of males, with smaller average sizes and a wider size range for males, and even smaller average sizes and narrower size range of females (Figure 5F).
6. Conclusions
The physiological stress and phenological alterations caused by ocean warming due to climate change in tropical fish species may have repercussions in recruitment mechanisms, marine population abundance, and distribution [5]. At the organism level, it is probable that disturbances in the aromatase synthesis and/or activity involved in steroidogenesis will always be the physiological key factor responsible for the changes that could occur in the female reproductive cycle, in sex differentiation, and, in the case of sequential hermaphroditic species, in the sexual inversion process.
These changes may affect the temporal dynamic of maturation and gamete release, as well as causing significant modifications in a population’s sex ratio and the size structure of males and females. Reductions in the proportion of females experienced by gonochoristic and protogynous hermaphroditic fishes could diminish the population fecundity in all the analyzed species. In addition, the predicted decreases in the proportion of larger-sized females (the most prolific) in protogynous hermaphroditic species could contribute to further lowering the population fecundity in grouper species and hogfish. However, the magnitude of the potential effects of temperature changes on disturbances in aromatase synthesis and/or activity can be extremely different between species, even those that belong to the same genus or family and share the same ecosystem. It is important to consider that the impact of higher temperatures depends on organisms’ thermal tolerance range, their capacity to acclimate and adapt to environmental changes, and their habitat requirements, which varies widely among species [2,4].
Climate change more-plausible trajectories should include scenarios such as SSP2-4.5 and SSP3-7.0 [67]. Whichever the scenario contemplated, seasonal spawning of hogfish, all snapper, and most of the grouper of the Campeche Bank could be affected by 2060, if 30 °C is considered as the current thermal tolerance limit for their reproduction. All species are of high commercial value and are intensively exploited throughout the Gulf of Mexico and the tropical western Atlantic. Overfishing is the main threat for all these species. Red grouper, gag, red snapper, and hogfish are currently listed as Vulnerable, whereas black grouper, yellowfin grouper, and lane snapper are assessed as Near Threatened [68]. The level of fishing exploitation could enhance species’ sensitivity to environmental changes. Fish stocks subject to heavy exploitation would therefore be more vulnerable to the effects of climate change than those that experience a low fishing pressure. An excessive fishing pressure could modify the population age and size structure, and reduce their size and genetic variability, thus limiting their evolutionary response to environmental changes and their recovery capacity in case of collapse [4]. As a consequence, the overexploited Campeche Bank red grouper stock could be more affected by temperature increments than other species in the region. In contrast, the appearance of primary males by the early masculinization of some undifferentiated individuals and the early sexual inversion of females in the populations of hermaphroditic grouper and wrasse could enhance their resilience towards the selective fishing of larger and older fish, which are commonly male.
Ocean warming due to climate change may affect fisheries by altering organism physiology, but it may also modify other aspects such as species distribution and/or abundance as individuals can migrate toward areas with more propitious conditions, consequently lowering the availability in zones where they were exploited traditionally [2,4]. In this case, a predicted rise of 2 °C over pre-industrial temperatures could cause a drop of 30 to 70% in the species richness of tropical fisheries, threatening food security in some countries. In socioeconomic terms, developing countries in the tropics could be particularly vulnerable to climate change; for example, an increasing scarcity of fishing resources would result in reduced food security, lower financial income, and loss of jobs in the fishing industry [2].
Author Contributions
T.B., writing—original draft preparation; X.R. and T.C.-M., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Reproductive studies of grouper, snapper and wrasse Campeche Bank’s populations were funded by: European Community (EC) grants No. CI*042 ME (JR); the Science and Technology Council of the French-Embassy in Mexico (CST) grant P65000 ME; the National Science and Technology Council of Mexico (CONACYT) grants 2184P-B9507, 37606-B, 254556; the CONACYT-SISIERRA grant 19990706020; the SEP-CONACYT grant 49963/2411, and the Yucatan Department of Rural and Fishery Development, Yucatan State Government.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Munday, P.L.; Jones, G.P.; Sheaves, M.; Williams, A.J.; Goby, G. Vulnerability of fishes of the Great Barrier Reef to climate change. In Climate Change and the Great Barrier Reef. A Vulnerability Assessment; Johnson, J.E., Marshall, P.A., Eds.; Great Barrier Marine Park Authority and Australian Greenhouse Office: Townsville, Australia, 2007; pp. 357–391. [Google Scholar]
- Pörtner, H.O.; Karl, D.M.; Boyd, P.W.; Cheung, W.W.L.; Lluch-Cota, S.E.; Nojiri, Y.; Schmidt, D.N.; Zavialov, P.O. Ocean systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 411–484. [Google Scholar]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Rijnsdorp, A.D.; Peck, M.A.; Engelhard, G.H.; Möllman, C.; Pinnegar, J.K. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 2009, 66, 1570–1583. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Farrell, A.P. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Demarée, G.R.; This Rutishauser. Origins of the word “Phenology”. Eos Trans. Am. Geophys. Union 2009, 90, 291. [Google Scholar] [CrossRef] [Green Version]
- Puppi, G. Origin and development of phenology as a science. Ital. J. Agrometeorol. 2007, 3, 24–29. [Google Scholar]
- Bruslé, J.; Quignard, J.P. Les poisons et leur environnement. In Écophysiologie et Comportement Adaptifs [Fishes and Their Environment. Ecophysiology and Adaptive Behavior]; Tec & Doc, Ed.; Lavoisier: Paris, France, 2004. [Google Scholar]
- Wootton, R.J.; Smith, C. Reproductive Biology of Teleost Fishes; Wiley Blackwell: Oxford, UK, 2015; p. 469. [Google Scholar]
- Lam, T.J. Environmental influences on gonadal activity in fish. In Fish Physiology; Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds.; Academic Press: New York, NY, USA, 1983; Volume IXB, pp. 65–116. [Google Scholar]
- Pankhurst, N.W.; Porter, M.J.R. Cold and dark or warm and light: Variations on the theme of environmental control of reproduction. Fish Physiol. Biochem. 2003, 28, 385–389. [Google Scholar] [CrossRef]
- Chan, S.T.H.; Yeung, W.S.B. Sex control and sex reversal in fish under natural conditions. In Fish Physiology; Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds.; Academic Press: New York, NY, USA, 1983; Volume IXB, pp. 171–222. [Google Scholar]
- Delvin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Munday, P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Anuario Estadísitco de Pesca 2014 [Annual Aquaculture and Fisheries Statistics 2014]; National Commission of Aquaculture and Fishery: Mazatlán, Mexico, 2015; Available online: www.gob.mx/conapesca/documentos/anuario-estadistico-de-acuacultura-y-pesca (accessed on 1 June 2018).
- SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación). Acuerdo por el que se da a conocer la actualización de la Carta Nacional Pesquera [Agreement to update the Nacional Fisheries Chart]. In Official Diary of the Federation; López-González, A., Ed.; Second Section; Secretaría de Gobernación: Mexico City, Mexico, 2012; Volume DCCVII 18, pp. 21–128. [Google Scholar]
- Burgos, R.; Defeo, O. Long-term population structure, mortality and modeling of a tropical multi-fleet fishery: The red grouper Epinephelus morio of the Campeche Bank, Gulf of Mexico. Fish. Res. 2004, 66, 325–335. [Google Scholar] [CrossRef]
- Arreguín-Sánchez, F.; del Monte Luna, P.; Zetina-Rejón, M.J.; Tripp-Valdez, A.; Albañez-Lucero, M.O.; Ruiz-Barreira, T.M. Building an ecosystems-type fisheries management approach for the Campeche Bank, subarea in the Gulf of Mexico Large Marine Ecosystem. Environ. Dev. 2017, 22, 143–149. [Google Scholar] [CrossRef]
- Nagahama, Y. Endocrine regulation of gametogenesis ion fish. Int. J. Dev. Biol. 1994, 38, 217–229. [Google Scholar] [PubMed]
- Strüssmann, C.A.; Nakamura, M. Morphology, endocrinology, and environmental modulation of gonad sex differentiation in teleost fishes. Fish Physiol. Biochem. 2002, 26, 13–29. [Google Scholar] [CrossRef]
- Frisch, A. Sex-change and gonadal steroids in sequentially-hermaphroditic teleost fish. Rev. Fish Biol. Fish 2004, 14, 481–499. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonad sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Miranda, L.A.; Chalde, T.; Elisio, M.; Strüssmann, C.A. Effects of global warming on fish reproductive endocrine axis, with special emphasis in pejerrey Odontesthes bonariensis. Gen. Comp. Endocrinol. 2013, 192, 45–54. [Google Scholar] [CrossRef]
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From gametogenesis to spawning: How climate-driven warming affects teleost reproductive biology. J. Fish Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Dahlke, F.T.; Wohlrab, S.; Butzin, M.; Pörtner, H.O. Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 2020, 369, 65–70. [Google Scholar] [CrossRef]
- Valenzuela, N.; Adams, D.C.; Janzen, F. Pattern does not equal process: Exactly when is sex environmentally determined? Am. Nat. 2003, 161, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Ospina-Álvarez, N.; Piferrer, F. Temperature-dependent sex determination in fish revisited: Prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS ONE 2008, 3, e2837. [Google Scholar] [CrossRef] [Green Version]
- Penman, D.J.; Piferrer, F. Fish gonadogenesis. Part I: Genetic and environmental mechanisms of sex determination. Rev. Fish. Sci. 2008, 16, 16–34. [Google Scholar] [CrossRef]
- Baroiller, J.F.; Guiguen, Y.; Fostier, A. Endocrine and environmental aspects of sex differentiation in fish. Cell. Mol. Life Sci. 1999, 55, 910–931. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nagahama, Y.; Nakamura, M. Diversity and plasticity of sex determination and differentiation in fishes. Sex. Dev. 2012, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kobayashi, Y.; Miura, S.; Alam, M.A.; Bhandari, R.K. Sex change in coral reef fish. Fish Physiol. Biochem. 2005, 31, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Bhandari, K.; Higa, M. The role estrogens play in sex differentiation and sex changes of fish. Fish Physiol. Biochem. 2003, 28, 113–117. [Google Scholar] [CrossRef]
- García-Cagide, A.; Claro, R.; Koshelev, B.V. Reproductive patterns of fishes of the Cuban shelf. In Ecology of the Marine Fishes of Cuba; Claro, R., Lindeman, K.C., Parenti, L.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2001; pp. 73–114. [Google Scholar]
- Claro, R.; Lindeman, K.C. Biología y Manejo de los Pargos (Lutjanidae) en el Atlántico Occidental [Biology and Management of Snapper (Lutjanidae) in the Western Atlantic]; Instituto de Oceanología, CITMAR: La Habana, Cuba, 2004; Available online: www.redciencia.cu/cdoceano (accessed on 15 June 2018).
- Brulé, T.; Déniel, C.; Colás-Marrufo, T.; Sánchez-Crespo, M. Red grouper reproduction in the southern Gulf of Mexico. Trans. Am. Fish. Soc. 1999, 128, 385–402. [Google Scholar] [CrossRef]
- Brulé, T.; Renán, X.; Colás-Marrufo, T.; Hauyon, Y.; Tuz-Sulub, A.; Déniel, C. Reproduction in the protogynous grouper Mycteroperca bonaci (Poey) from the southern Gulf of Mexico. Fish. Bull. 2003, 101, 463–475. [Google Scholar]
- Brulé, T.; Déniel, C.; Colás-Marrufo, T.; Renán, X. Reproductive biology of gag in the southern Gulf of Mexico. J. Fish Biol. 2003, 63, 1505–1520. [Google Scholar] [CrossRef]
- Brulé, T.; Colás-Marrufo, T.; Pérez-Díaz, E.; Sámano-Zapata, J.C. Red snapper reproductive biology in the southern Gulf of Mexico. Trans. Am. Fish. Soc 2010, 139, 957–968. [Google Scholar] [CrossRef]
- Caballero-Arango, D. Estrategia Reproductiva de tres Especies de Mero (Epinephelus guttatus, Mycteroperca tigris y Mycteroperca venenosa) en Arrecifes Coralinos del Banco de Campeche, México [Reproductive Strategy of Three Grouper Species (Epinephelus guttatus, Mycteroperca tigris and Mycteroperca venenosa) in Campeche Bank Coral Reefs]. Ph.D. Thesis, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Unidad Mérida, Mérida, Mexico, 2013. [Google Scholar]
- Caballero-Arango, D.; Brulé, T.; Nóh-Quiñones, V.; Colás-Marrufo, T.; Pérez-Díaz, E. Reproductive biology of the tiger grouper in the southern Gulf of Mexico. Trans. Am. Fish. Soc. 2013, 142, 282–299. [Google Scholar] [CrossRef]
- Trejo-Martínez, J.; Brulé, T.; Mena-Loría, A.; Colás-Marrufo, T.; Sanchez-Crespo, M. Reproductive aspects of the yellowtail snapper Ocyurus chrysurus from the southern Gulf of Mexico. J. Fish Biol. 2011, 79, 915–936. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Martínez, J.; Brulé, T.; Morales-López, N.; Colás-Marrufo, T.; Sanchez-Crespo, M. Reproductive strategy of a continental shelf lane snapper population from the southern Gulf of Mexico. Mar. Coast. Fish 2021, 13, 140–156. [Google Scholar] [CrossRef]
- Nóh-Quiñones, V.E. Estrategia Reproductiva del Labridae de Importancia Comercial: La Doncella de Pluma Lachnolaimus maximus, en la Costa de Yucatán, México [Reproductive Strategy of the Commercial Labridae: The Hogfish Lachnolaimus maximus, from the Yucatan Coast, Mexico]. Ph.D. Thesis, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Unidad Mérida, Mérida, Mexico, 2017. [Google Scholar]
- Sadovy, Y.J. Reproduction of reef fishery species. In Reef Fisheries; Polunin, N.V.C., Roberts, C.M., Eds.; Chapman and Hall: London, UK, 1996; pp. 15–59. [Google Scholar]
- Gutiérrez, J.M.; Jones, R.G.; Narisma, G.T.; Alves, L.M.; Amjad, M.; Gorodetskaya, I.V.; Grose, M.; Klutse, N.A.B.; Krakovska, S.; Li, J.; et al. Atlas. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; Available online: http://interactive-atlas.ipcc.ch/ (accessed on 1 March 2022).
- Iturbide, M.; Fernández, J.; Gutiérrez, J.M.; Bedia, J.; Cimadevilla, E.; Díez-Sierra, J.; Manzanas, R.; Casanueva, A.; Baño-Medina, J.; Milovac, J.; et al. Repository Supporting the Implementation of FAIR Principles in the IPCC-WG1 Atlas (v2.0); Zenodo: Geneve, Switzerland, 2021. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef] [Green Version]
- Brown-Peterson, N.J.; Wyansky, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A Standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Tucker, J.W., Jr. Marine Fish Culture; Kluwer Academic Publishers: Norwell, MA, USA, 1998; p. 750. [Google Scholar]
- Nemeth, R.S.; Blondeau, J.; Herzlieb, S.; Kadison, E. Spatial and temporal patterns of movement and migration at spawning aggregations of red hind, Epinephelus guttatus, in the U.S. Virgin Islands. Environ. Biol. Fishes 2007, 78, 365–381. [Google Scholar] [CrossRef]
- Sedberry, G.R.; Pashuk, O.; Wyansky, D.M.; Stephen, J.A.; Weinbach, P. Spawning Locations for Atlantic Reef Fishes off the Southeastern, U.S. Proc. Gulf Caribb. Fish. Inst. 2006, 57, 464–514. [Google Scholar]
- Sadovy, Y.; Colin, P.L.; Domeier, M.L. Aggregation and spawning in the tiger grouper, Mycteroperca tigris (Pisces: Serranidae). Copeia 1994, 2, 511–516. [Google Scholar] [CrossRef]
- Tuz-Sulub, A.; Brulé, T. Spawning aggregations of three protogynous groupers in the southern Gulf of Mexico. J. Fish Biol. 2015, 86, 162–185. [Google Scholar] [CrossRef]
- Arnold, C.R.; Wakeman, J.M.; Williams, T.D.; Treece, G.D. Spawning of red snapper (Lutjanus campechanus) in captivity. Aquaculture 1978, 15, 301–302. [Google Scholar] [CrossRef]
- Minton, R.V.; Hawke, J.P.; Tatum, W.M. Hormone induced spawning of red snapper Lutjanus campechanus. Aquaculture 1983, 30, 363–368. [Google Scholar] [CrossRef]
- Collins, L.A.; Fitzhugh, G.R.; Mourand, L.; Lombardi-Carlson, L. Preliminary results from a continuing study of spawning and fecundity of the red snapper (Lutjanidae: Lutjanus campechanus) from the Gulf of Mexico, 1998–1990. Proc. Gulf Caribb. Fish. Inst. 2001, 52, 34–47. [Google Scholar]
- Watanabe, W.O.; Benetti, D.D.; Feeley, M.W.; Davis, D.A.; Phelps, R.P. Status of Artificial Propagation of mutton, yellowtail, and red snapper (family Lutjanidae) in the Southeastern United States. Am. Fish. Soc. Symp. 2005, 46, 517–540. [Google Scholar]
- Papanikos, N.; Phelps, R.P.; Davis, D.A.; Ferry, A.; Maus, D. Spontaneous spawning of captive red snapper, Lutjanus campechanus, and dietary lipid effect on reproductive performance. J. World Aquac. Soc. 2008, 39, 324–338. [Google Scholar] [CrossRef]
- Luckhurst, B.L.; Dean, J.M.; Reichert, M. Age, growth and reproduction of the lane snapper Lutjanus synagris (Pisces: Lutjanidae) at Bermuda. Mar. Ecol. Prog. Ser. 2000, 203, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Claro, R.; Lindeman, K.C. Spawning aggregation sites of snapper and grouper species (Lutjanidae and Serranidae) on the insular shelf of Cuba. Gulf Caribb. Res. 2003, 14, 91–106. [Google Scholar] [CrossRef]
- Wallace, R.K., Jr. Thermal acclimation, upper temperature tolerance, and preferred temperature of juvenile yellowtail snappers, Ocyurus chrysurus (Bloch) (Pisces: Lutjanidae). Bull. Mar. Sci. 1977, 27, 292–298. [Google Scholar]
- Turano, M.J.; Davis, D.A.; Arnold, C.R. Observations and techniques for maturation, spawning, and larval rearing of the yellowtail snapper Ocyurus chrysurus. J. World Aquac. Soc. 2000, 31, 59–68. [Google Scholar] [CrossRef]
- Colin, P.L. Spawning and larval development of the hogfish, Lacholaimus maximus (Pisces: Labridae). Bull Fish. 1982, 80, 853–862. [Google Scholar]
- Grimes, C.B. Reproductive biology of the Lutjanidae: A review. In Tropical Snappers and Groupers: Biology and Fisheries Management; Polovina, J.J., Ralston, S., Eds.; Westview Press: Boulder, CO, USA, 1987; pp. 239–294. [Google Scholar]
- Sadovy, Y.; Shapiro, D.Y. Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1987, 1, 136–156. [Google Scholar] [CrossRef]
- Hausfather, Z.; Peters, G.P. Emissions—The ‘business as usual’ story is misleading. Nature 2020, 577, 618–620. [Google Scholar] [CrossRef]
- IUCN (International Union for Conservation of Nature). The IUCN Red List of Threatened Species. Version 2017-3. 2018. Available online: www.iucnredlist.org (accessed on 22 June 2018).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).