Earlier Activation of Interferon and Pro-Inflammatory Response Is Beneficial to Largemouth Bass (Micropterus salmoides) against Rhabdovirus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Preparation of Crude Virus Extract
2.3. Experimental Design and Sampling
2.4. Detecting Viral Loads in the Liver, Spleen and Kidney
2.5. Enzymatic Assays
2.6. Expression of Immune-Related Genes
2.7. Statistical Analysis
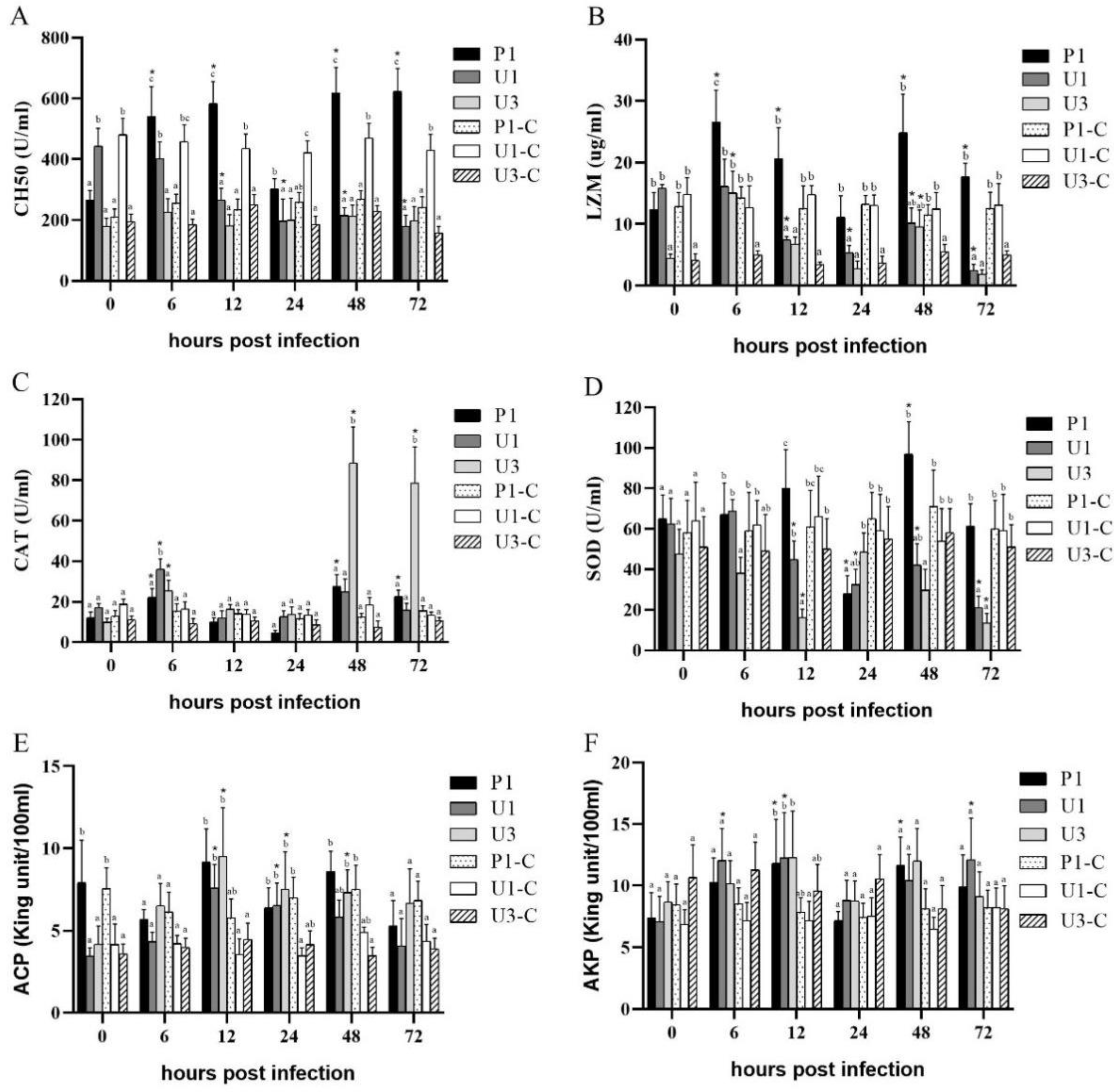
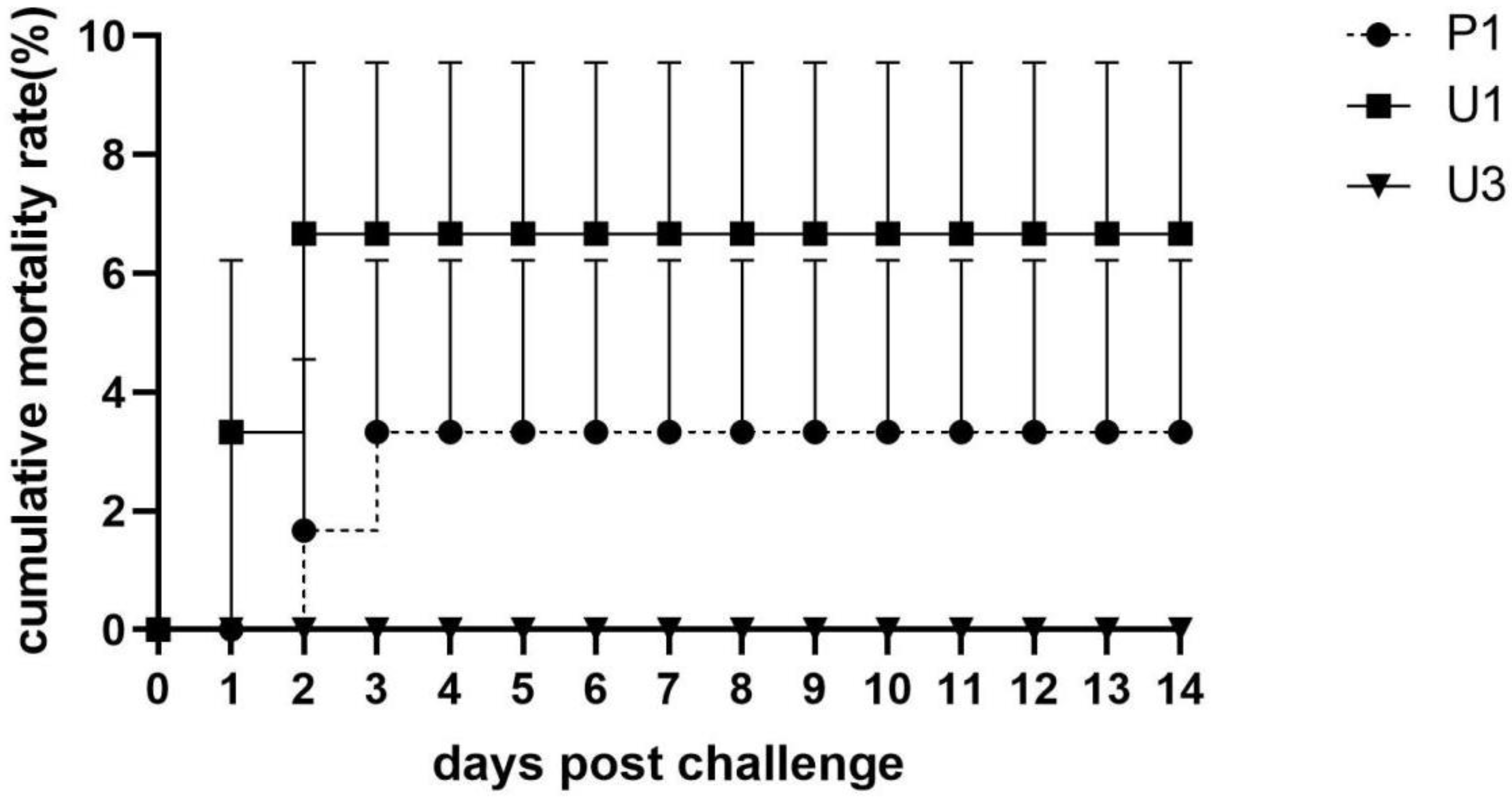
3. Results
3.1. Viral Loads in Liver, Spleen and Kidney
3.2. Enzymatic Assays
3.3. Transcripts of Some Immune-Related Genes
3.4. Cumulative Mortality Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Fishery Bureau; Ministry of Agriculture. China Fishery Statistical Yearbook; Agriculture Press: Beijing, China, 2021. (In Chinese)
- Sibley, S.D.; Finley, M.A.; Baker, B.B.; Puzach, C.; Armién, A.G.; Giehtbrock, D.; Goldberg, T.L. Novel reovirus associated with epidemic mortality in wild largemouth bass (Micropterus salmoides). J. Gen. Virol. 2016, 97, 2482–2487. [Google Scholar] [CrossRef]
- Ma, D.M.; Deng, G.C.; Bai, J.J.; Li, S.; Yu, L.; Quan, Y.; Yang, X.; Jiang, X.; Zhu, Z.; Ye, X. A strain of Siniperca chuatsi rhabdovirus causes high mortality among cultured largemouth bass in south China. J. Aquat. Anim. Health 2013, 25, 197–204. [Google Scholar] [CrossRef]
- Deng, G.C.; Li, S.J.; Xie, J.; Bai, J.J.; Chen, K.C.; Ma, D.M.; Jiang, X.Y.; Lao, H.H.; Yu, L.Y. Characterization of a ranavirus isolated from cultured largemouth bass (Micropterus salmoides) in China. Aquaculture 2011, 312, 198–204. [Google Scholar] [CrossRef]
- Ma, D.M.; Deng, G.C.; Bai, J.J.; Li, S.J.; Jiang, X.Y.; Yang, X.J. Pathogeny of disease characterized by swollen liver and spleen in largemouth bass (Micropterus salmoides). J. Fish. Sci. China 2011, 18, 654–659. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Gao, X.C.; Zhang, Q.Y. Whole-genome analysis of a novel fish reovirus (MsReV) discloses aquareovirus genomic structure relationship with host in saline environments. Viruses 2015, 7, 4282–4302. [Google Scholar] [CrossRef] [Green Version]
- Gao, E.B.; Chen, G.F. Micropterus salmoides rhabdovirus (MSRV) infection induced apoptosis and activated interferon signaling pathway in largemouth bass skin cells. Fish Shellfish. Immunol. 2018, 76, 161–166. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, N.Q.; Lin, Q.; Liu, L.H.; Liang, H.R.; Huang, Z.B.; Fu, X.Z. An avirulent Micropterus salmoides rhabdovirus vaccine candidate protects Chinese perch against rhabdovirus infection. Fish Shellfish. Immunol. 2018, 77, 474–480. [Google Scholar]
- Guo, Z.R.; Zhao, Z.; Zhang, C.; Jia, Y.J.; Qiu, D.K.; Zhu, B.; Wang, G.X. Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (Micropterus Salmoides). Fish Shellfish. Immunol. 2020, 99, 548–554. [Google Scholar] [CrossRef]
- Yang, F.; Song, K.; Zhang, Z.; Chen, C.; Wang, G.; Yao, J.; Ling, F. Evaluation on the antiviral activity of ribavirin against Micropterus salmoides rhabdovirus (MSRV) in vitro and in vivo. Aquaculture 2021, 543, 736975. [Google Scholar] [CrossRef]
- Kutyrev, I.; Cleveland, B.; Leeds, T.; Wiens, G.D. Proinflammatory cytokine and cytokine receptor gene expression kinetics following challenge with Flavobacterium psychrophilum in resistant and susceptible lines of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2016, 58, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yin, Z.; Weng, S.; He, J.; Li, S. Selective breeding and preliminary commercial performance of Penaeus vannamei for resistance to white spot syndrome virus (WSSV). Aquaculture 2012, 364–365, 111–117. [Google Scholar] [CrossRef]
- Zhu, J.L.; Zou, Z.Y.; Li, D.Y.; Xiao, W.; Xu, P.; Yang, H.; Xue, L.Y. Study on resistant difference among four bleeding species of tilapia Oreochromis spp. following Streptococcus agalactiae challenge. Acta Hydrobiol. Sin. 2017, 41, 1232–1241. (In Chinese) [Google Scholar]
- Bai, J.J.; Sheng, J. Current status and development trend on China largemouth bass industry. Chin. Fish. Econ. 2013, 31, 104–108. (In Chinese) [Google Scholar]
- Zhao, L.; Li, S.J.; Bai, J.J.; Liang, J.; Han, L.; Liang, X. Transfer Food from Zooplankt onto Formulated Feed in Juvenile Selectively Bred Largemouth Bass Micropterus salmoides. Fish. Sci. 2019, 38, 846–850. (In Chinese) [Google Scholar]
- Lei, Y.; Qi, R.R.; Cui, L.B.; Xiao, Y.; Zhang, W.W.; Ma, J.H.; Wang, X.P. Diagnosis of rhabdovirus disease in juvenile largemouth bass Micropterus salmonides. J. Dalian Ocean. Univ. 2015, 30, 305–308. (In Chinese) [Google Scholar]
- Ping-Yueh, H.; Omkar, B.; Wang, P.C.; Ming-An, T.; Li-Ling, L.; Chen, S.C. Identification, Molecular Cloning of IL-1β and Its Expression Profile during Nocardia seriolae Infection in Largemouth Bass, Micropterus salmoides. Int. J. Mol. Sci. 2016, 17, 1670. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (delta delta C (T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yuan, X.M.; Lv, S.J.; Shi, W.D.; Hang, X.Y.; Liu, L.; Wu, Y.L. Isolation and Egg-Yolk Antibody Preparation of Micropterus salmonides Rhabdovirus. Prog. Fish. Sci. 2020, 41, 151–157. (In Chinese) [Google Scholar]
- Chen, W.; Yi, L.; Feng, S.; Liu, X.; Asim, M.; Zhou, Y.; Lan, J.; Jiang, S.; Tu, J.; Lin, L. Transcriptomic profiles of striped snakehead fish cells (SSN-1) infected with red-spotted grouper nervous necrosis virus (RGNNV) with an emphasis on apoptosis pathway. Fish Shellfish Immunol. 2017, 60, 346–354. [Google Scholar] [CrossRef]
- Pleic, I.L.; Buselic, I.; Trumbic, Z.; Bočina, I.; Šprung, M.; Mladineo, I. Expression analysis of the Atlantic bluefin tuna (Thunnus thynnus) pro-inflammatory cytokines, IL-1b, TNFa1 and TNFa2 in response to parasites Pseudocycnus appendiculatus (Copepoda) and Didymosulcus katsuwonicola (Digenea). Fish Shellfish. Immunol. 2015, 45, 946–954. [Google Scholar] [CrossRef]
- Watkins, L.R.; Nguyen, K.T.; Lee, J.E.; Maier, S.F. Dynamic regulation of proinflammatory cytokines. Adv. Exp. Med. Biol. 1999, 461, 153–178. [Google Scholar]
- Feng, H.; Zhang, Y.B.; Zhang, Q.M.; Li, Z.; Zhang, Q.Y.; Gui, J.F. Zebrafish IRF1 regulates IFN antiviral response through binding to IFNϕ1 and IFNϕ3 promoters downstream of MyD88 signaling. J. Immunol. 2015, 194, 1225–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Huang, X.; Cai, J.; OuYang, Z.; Wei, S.; Wei, J.; Qin, Q. Identification of orange-spotted grouper (Epinephelus coioides) interferon regulatory factor 3 involved in antiviral immune response against fish RNA virus. Fish Shellfish Immunol. 2015, 42, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.B.; Ohira, T.; Hirono, I.; Aoki, T. Use of a cDNA microarray to study immunity against viral hemorrhagic septicemia (VHS) in Japanese flounder (Paralichthys olivaceus) following DNA vaccination. Fish Shellfish. Immunol. 2005, 18, 135–147. [Google Scholar]
- Kinkelin, P.D.; Dorson, M.; Hattenberger-Baudouy, A.M. Interferon synthesis in trout and carp after viral infection. Dev. Comp. Immunol. 1982, 2, 167–174. [Google Scholar]
- Lopez, M.A.; Roca, F.J.; Meseguer, J.; Mulero, V. New insights into the evolution of IFNs: Zebrafish group II IFNs induce a rapid and transient expression of IFN-dependent genes and display powerful antiviral activities. J. Immunol. 2009, 182, 3440–3449. [Google Scholar] [CrossRef] [Green Version]


| Primer Name | Sequence (5′-3′) | GenBank ID |
|---|---|---|
| MSRV-qF | CACCAGCCACATCAATCCC | MK397811.2 |
| MSRV-qR | CCCGTCCGTCGCTTGA | |
| actin-F | AAAGGGAAATCGTGCGTGAC | XM_038695351.1 |
| actin-R | AAGGAAGGCTGGAAGAGGG | |
| TNF-α-F | CTTCGTCTACAGCCAGGCATCG | XM_038710731.1 |
| TNF-α-R | TTTGGCACACCGACCTCACC | |
| IL-1β-F | TGGACTTGGAGATTGCCCA | [17] |
| IL-1β-R | AAACCGCACCATGTCGCTG | |
| Akt-F | GACAACGAGGAACAGAGTGGG | XM_038701566.1 |
| Akt-R | ATGACGCAGGCTTGGTAATG | |
| mTOR-F | ACCCTACCGCAAGTACCCC | XM_038723321.1 |
| mTOR-R | GTCAATCATTCCGATGTTCACC | |
| IFNγ-F | TCAAATCCCTCTGAAGATGAACA | XM_038707474.1 |
| IFNγ-R | ACGCCACCCATAAACACCA | |
| IRF3-F | TCTCATCTTTAAGGCGTGGGC | XM_038735465.1 |
| IRF3-R | GGGGTTAGCGGTGTCGTTC | |
| IRF7-F | AGAAAGCTGCCCCAGAATACC | XM_038706685.1 |
| IRF7-R | GAGGGACCACCTTGACTACGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Liang, Q.; Zhu, N.; Zheng, X.; Chen, X.; Zhou, F.; Ding, X. Earlier Activation of Interferon and Pro-Inflammatory Response Is Beneficial to Largemouth Bass (Micropterus salmoides) against Rhabdovirus Infection. Fishes 2022, 7, 90. https://doi.org/10.3390/fishes7020090
He R, Liang Q, Zhu N, Zheng X, Chen X, Zhou F, Ding X. Earlier Activation of Interferon and Pro-Inflammatory Response Is Beneficial to Largemouth Bass (Micropterus salmoides) against Rhabdovirus Infection. Fishes. 2022; 7(2):90. https://doi.org/10.3390/fishes7020090
Chicago/Turabian StyleHe, Runzhen, Qianrong Liang, Ningyu Zhu, Xiaoye Zheng, Xiaoming Chen, Fan Zhou, and Xueyan Ding. 2022. "Earlier Activation of Interferon and Pro-Inflammatory Response Is Beneficial to Largemouth Bass (Micropterus salmoides) against Rhabdovirus Infection" Fishes 7, no. 2: 90. https://doi.org/10.3390/fishes7020090
APA StyleHe, R., Liang, Q., Zhu, N., Zheng, X., Chen, X., Zhou, F., & Ding, X. (2022). Earlier Activation of Interferon and Pro-Inflammatory Response Is Beneficial to Largemouth Bass (Micropterus salmoides) against Rhabdovirus Infection. Fishes, 7(2), 90. https://doi.org/10.3390/fishes7020090





