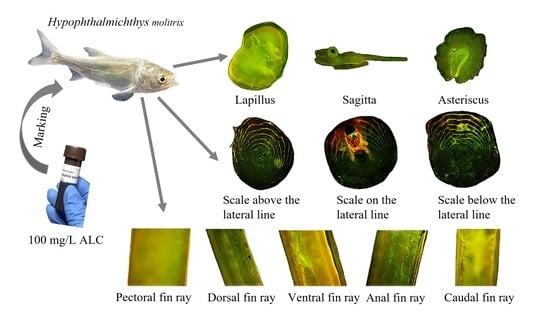
A Pilot Study Assessing a Concentration of 100 mg/L Alizarin Complexone (ALC) to Mark Calcified Structures in Hypophthalmichthys molitrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Marking Procedure
2.3. Hard Tissue Sampling
2.4. Marking Evaluation
2.5. Statistical Analysis
3. Results
3.1. Effects of ALC on Fish Survival and Growth
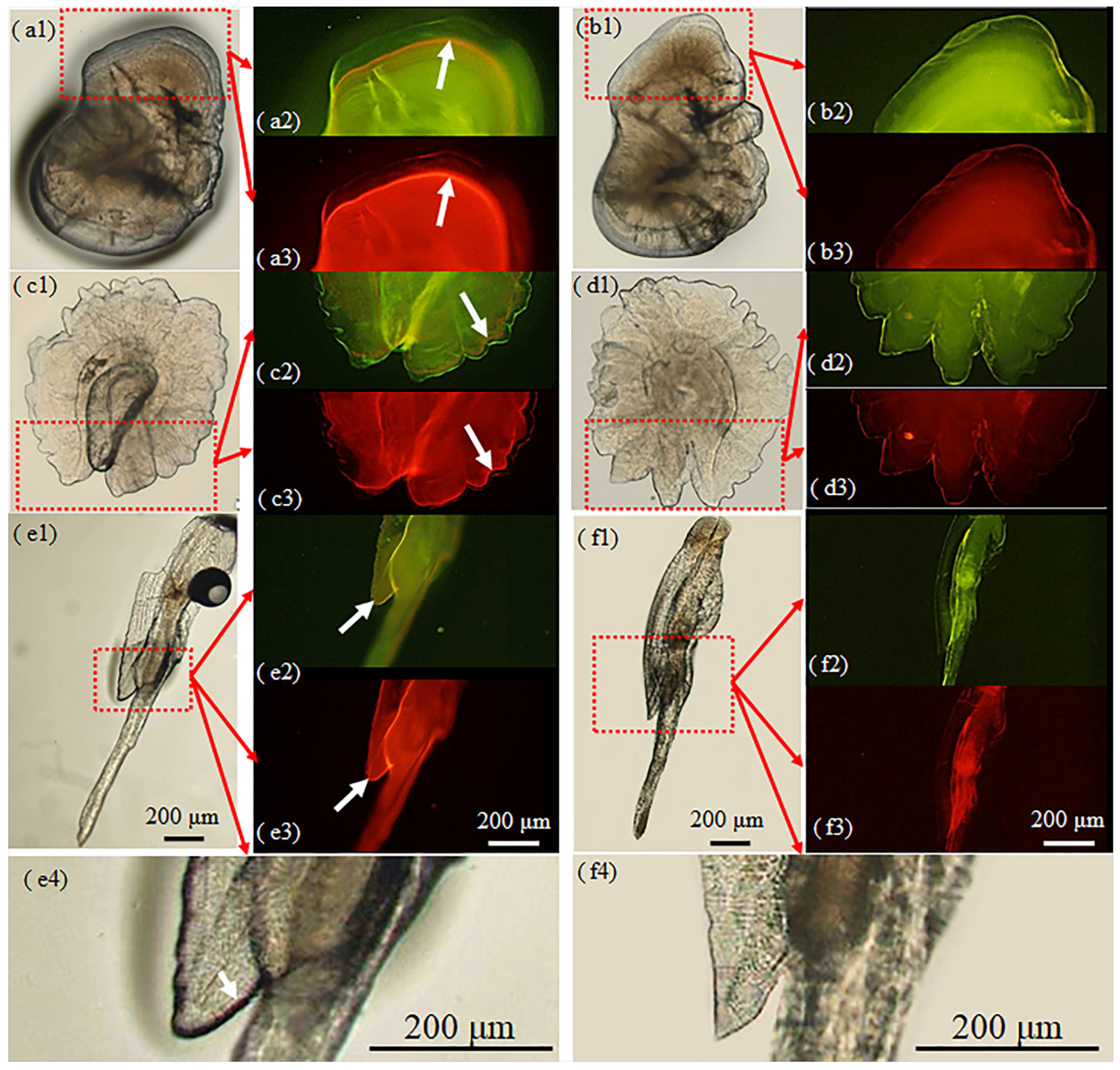
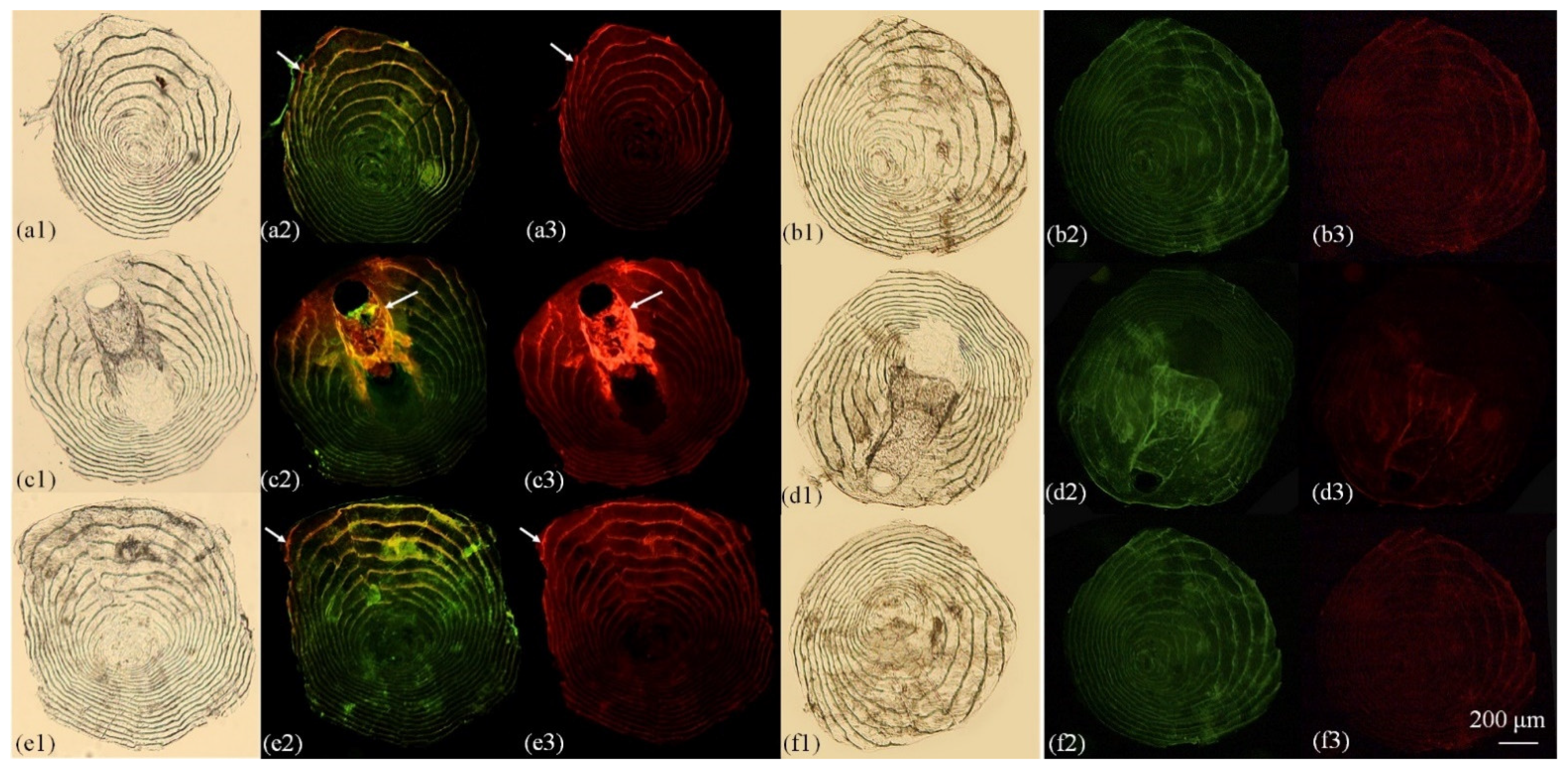
3.2. Otolith Marking
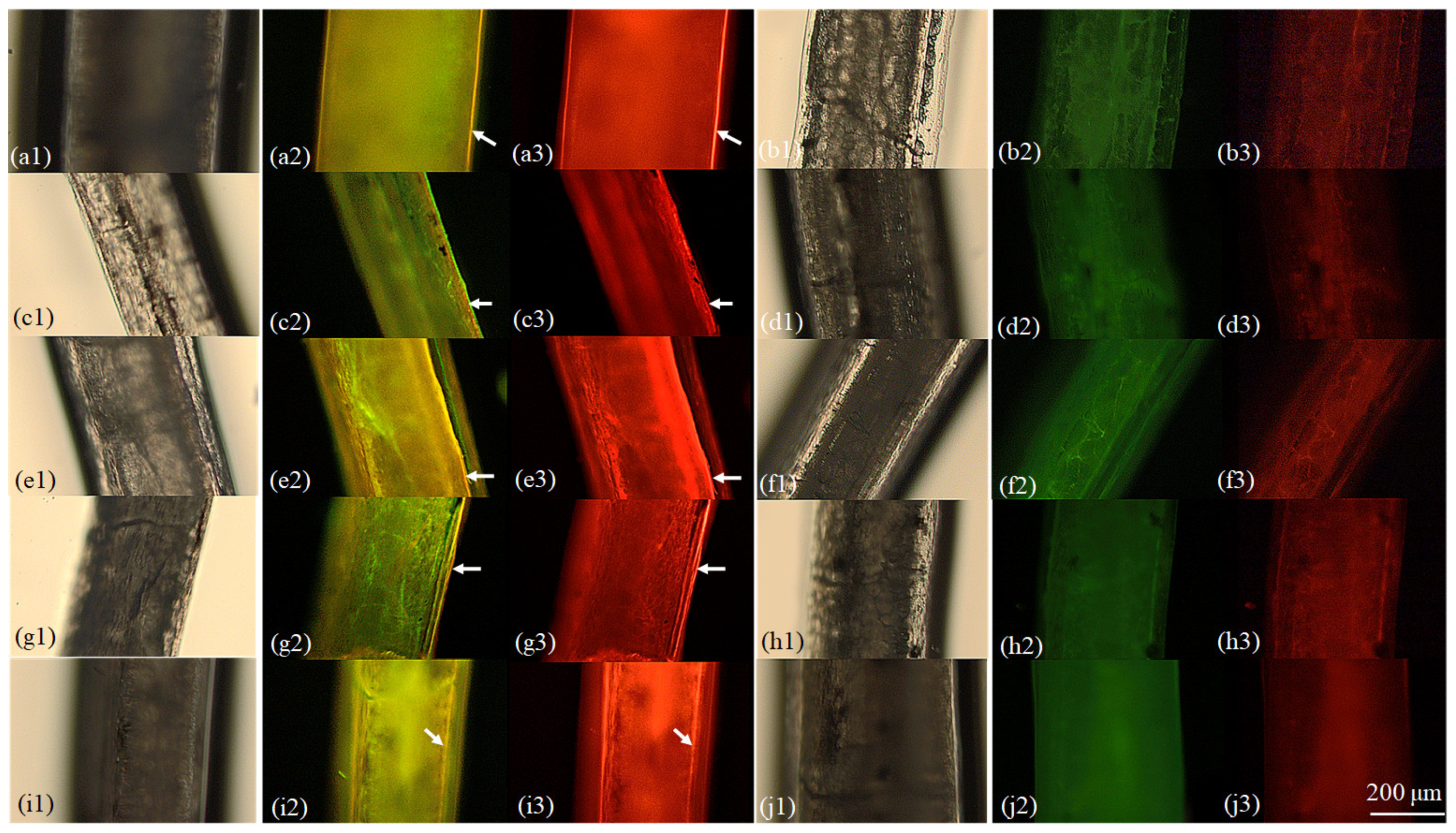
3.3. Fin Ray Marking
3.4. Marking of Scales
4. Discussion
4.1. Variation of Marking Effectiveness among Hard Structures
4.2. Optimum Marker Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren-Myers, F.; Dempster, T.; Swearer, S.E. Otolith mass marking techniques for aquaculture and restocking: Benefits and limitations. Rev. Fish Biol. Fish. 2018, 28, 485–501. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Yang, J.; Zhu, S.; Wu, Z.; Li, J.; Li, Y. Elevated temperatures shorten the spawning period of silver carp (Hypophthalmichthys molitrix) in a large subtropical river in China. Front. Mar. Sci. 2021, 8, 708109. [Google Scholar] [CrossRef]
- Lu, G.; Wang, C.; Zhao, J.; Liao, X.; Wang, J.; Luo, M.; Zhu, L.; Bernatzhez, L.; Li, S. Evolution and genetics of bighead and silver carps: Native population conservation versus invasive species control. Evol. Appl. 2020, 13, 1351–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, J.M.R. Visible implant elastomer color determination, tag visibility, and tag loss: Potential sources of error for mark-recapture studies. N. Am. J. Fish. Manag. 2006, 26, 327–337. [Google Scholar] [CrossRef]
- Phinney, D.E.; Miller, D.M.; Dahlberg, M.L. Mass-marking young salmonids with fluorescent pigment. Trans. Am. Fish. Soc. 1967, 96, 157–162. [Google Scholar] [CrossRef]
- Lejk, A.M.; Radtke, G. Effect of marking salmo Trutta lacustris L. Larvae with alizarin red S on their subsequent growth, condition, and distribution as juveniles in a natural stream. Fish. Res. 2021, 234, 105786. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.M.; Zhang, P.D.; Nwafili, S.A. The use of alizarin red S and alizarin complexone for immersion marking Japanese flounder Paralichthys olivaceus (T.). Fish. Res. 2009, 98, 67–74. [Google Scholar] [CrossRef]
- Lü, H.; Fu, M.; Dai, S.; Xi, D.; Zhang, Z.-X. Experimental evaluation of calcein and alizarin red S for immersion marking of silver carp Hypophthalmichthys molitrix (Valenciennes, 1844). J. Appl. Ichthyol. 2016, 32, 83–91. [Google Scholar] [CrossRef]
- Bangs, B.L.; Falcy, M.R.; Scheerer, P.D.; Clements, S. Comparison of three methods for marking a small floodplain minnow. Anim. Biotelem. 2013, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Partridge, G.J.; Ginbey, B.M.; Woolley, L.D.; Fairclough, D.V.; Crisafulli, B.; Chaplin, J.; Prokop, N.; Dias, J.; Bertram, A.; Jenkins, G.I. Development of techniques for the collection and culture of wild-caught fertilised snapper (Chrysophrys auratus) eggs for stock enhancement purposes. Fish. Res. 2017, 186, 524–530. [Google Scholar] [CrossRef]
- Partridge, G.J.; Jenkins, G.I.; Doupé, R.G.; De Lestang, S.; Ginbey, B.M.; French, D. Factors affecting mark quality of alizarin complexone-stained otoliths in juvenile black bream Acanthopagrus butcheri and a prescription for dosage. J. Fish Biol. 2009, 75, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Gaines, P.C.; Martin, C.D. Feasibility of dual-marking age-0 Chinook salmon for mark–recapture studies. N. Am. J. Fish. Manag. 2004, 24, 1456–1459. [Google Scholar] [CrossRef]
- Schumann, D.A.; Koupal, K.D.; Hoback, W.W.; Schoenebeck, C.W. Evaluation of sprayed fluorescent pigment as a method to mass-mark fish species. Open Fish Sci. J. 2013, 6, 41–47. [Google Scholar] [CrossRef]
- Caraguel, J.-M.; Barreau, T.; Brown-Vuillemin, S.; Iglésias, S.P. In vivo staining with alizarin for ageing studies on Chondrichthyan fishes. Aquat. Living Resour. 2020, 33, 1. [Google Scholar] [CrossRef]
- Watanabe, K.-I.; Ohta, K. Efficacy of a fluorescent tagging technique on otolith and scales using alizarin complexone in artificial seawater for Red Sea bream and Japanese flounder juveniles. Aquac. Sci. 2009, 57, 627–630. [Google Scholar] [CrossRef]
- Lü, H.; Fu, M.; Zhang, Z.; Su, S.; Yao, W. Marking fish with fluorochrome dyes. Rev. Fish. Sci. Aquac. 2020, 28, 117–135. [Google Scholar] [CrossRef]
- Mansur, L.; Catalán, D.; Plaza, G.; Landaeta, M.F.; Ojeda, F.P. Validations of the daily periodicity of increment deposition in rocky intertidal fish otoliths of the South-Eastern Pacific Ocean. Rev. Biol. Mar. Oceanogr. 2013, 48, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Cottingham, A.; Hall, N.G.; Loneragan, N.R.; Jenkins, G.I.; Potter, I.C. Efficacy of restocking an estuarine-resident species demonstrated by long-term monitoring of cultured fish with alizarin complexone-stained otoliths. A case study. Fish. Res. 2020, 227, 105556. [Google Scholar] [CrossRef]
- Stötera, S.; Degen-Smyrek, A.K.; Krumme, U.; Stepputtis, D.; Bauer, R.; Limmer, B.; Hammer, C. Marking otoliths of Baltic cod (Gadus morhua Linnaeus, 1758) with tetracycline and strontium chloride. J. Appl. Ichthyol. 2019, 35, 427–435. [Google Scholar] [CrossRef]
- Taylor, M.D.; Fielder, D.S.; Suthers, I.M. Batch marking of otoliths and fin spines to assess the stock enhancement of Argyrosomus japonicus. J. Fish Biol. 2005, 66, 1149–1162. [Google Scholar] [CrossRef]
- Romanek, C.S.; Gauldie, R.W. A Predictive model of otolith growth in fish based on the chemistry of the endolymph. Comp. Biochem. Physiol. 1996, 114, 71–79. [Google Scholar] [CrossRef]
- Fisher, M.; Hunter, E. Digital imaging techniques in otolith data capture, analysis and interpretation. Mar. Ecol. Prog. Ser. 2018, 598, 213–231. [Google Scholar] [CrossRef] [Green Version]
- Tzadik, O.E.; Curtis, J.S.; Granneman, J.E.; Kurth, B.N.; Pusack, T.J.; Wallace, A.A.; Hollander, D.J.; Peebles, E.B.; Stallings, C.D. Chemical archives in fishes beyond otoliths: A review on the use of other body parts as chronological recorders of microchemical constituents for expanding interpretations of environmental, ecological, and life-history changes. Limnol. Oceanogr. 2017, 15, 238–263. [Google Scholar] [CrossRef]
- Seibert, J.R.; Phelps, Q.E. Evaluation of aging structures for silver carp from midwestern U.S. rivers. N. Am. J. Fish. Manag. 2013, 33, 839–844. [Google Scholar] [CrossRef]
- Ding, C.; He, D.; Chen, Y.; Jia, Y.; Tao, J. Otolith microstructure analysis based on wild young fish and its application in confirming the first annual increment in Tibetan Gymnocypris selincuoensis. Fish. Res. 2020, 221, 105386. [Google Scholar] [CrossRef]
- Durham, B.W.; Wilde, G.R. Validation of daily growth increment formation in the otoliths of juvenile Cyprinid fishes from the Brazos River, Texas. N. Am. J. Fish Manag. 2008, 28, 442–446. [Google Scholar] [CrossRef]
- Gao, M.H.; Wu, Z.Q.; Huang, L.L.; Tan, X.C.; Liu, H.; Rad, S. Otolith shape analysis and growth characteristics in larval and juvenile Squalidus argentatus. Environ. Biol. Fishes 2021, 104, 937–945. [Google Scholar] [CrossRef]
- Elle, S.F.; Koenig, M.K.; Meyer, K.A. Evaluation of calcein as a mass mark for rainbow trout raised in outdoor hatchery raceways. N. Am. J. Fish. Manag. 2011, 30, 1408–1412. [Google Scholar] [CrossRef]
- Brusher, J.H.; Schull, J. Non-lethal age determination for juvenile goliath grouper Epinephelus itajara from Southwest Florida. Endang. Species Res. 2009, 7, 205–212. [Google Scholar] [CrossRef]
- Lü, H.; Fu, M.; Xi, D.; Yao, W.-Z.; Su, S.-Q.; Wu, Z.-L. Experimental evaluation of using calcein and alizarin red S for immersion marking of bighead carp Aristichthys nobilis (Richardson, 1845) to assess growth and identification of marks in otoliths, scales and fin rays. J. Appl. Ichthyol. 2015, 31, 665–674. [Google Scholar] [CrossRef]
- Wood, R.S.; Chakoumakos, B.C.; Fortner, A.M.; Gillies-Rector, K.; Frontzek, M.D.; Ivanov, I.N.; Kah, L.C.; Kennedy, B.; Pracheil, B.M. Quantifying fish otolith mineralogy for trace-element chemistry studies. Sci. Rep. 2022, 12, 2727. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.D.; Telmer, K.H.; Shrimpton, J.M. Elemental analysis of otoliths, fin rays and scales: A comparison of bony structures to provide population and life-history information for the Arctic grayling (Thymallus arcticus). Ecol. Freshw. Fish 2010, 16, 354–361. [Google Scholar] [CrossRef]
- Lü, H.; Zhang, X.; Xi, D.; Gao, T. Use of calcein and alizarin red S for immersion marking of black rockfish Sebastes schlegelii Juveniles. Chin. J. Oceanol. Limnol. 2014, 32, 88–98. [Google Scholar] [CrossRef]
- Katakura, S.; Ohta, M.; Jin, M.; Sakurai, Y. Otolith-marking experiments of juvenile walleye pollock Theragra chalcogramma using oxytetracycline, alizarin complexone, and alizarin red S. Aquac. Sci. 2003, 51, 327–335. [Google Scholar] [CrossRef]
- Able, K.W.; Sakowicz, G.P.; Lamonaca, J.C. Scale formation in selected fundulid and cyprinodontid fishes. Ichthyol. Res. 2009, 56, 1–9. [Google Scholar] [CrossRef]
- Meyer, S.; Sørensen, S.R.; Peck, M.A.; Støttrup, J.G. Sublethal effects of alizarin complexone marking on Baltic cod (Gadus morhua) eggs and larvae. Aquaculture 2012, 324, 158–164. [Google Scholar] [CrossRef]
- Jurgelėnė, Ž.; Montvydienė, D.; Stakėnas, S.; Poviliūnas, J.; Račkauskas, S.; Taraškevičius, R.; Skrodenytė-Arbačiauskienė, V.; Kazlauskienė, N. Impact evaluation of marking Salmo trutta with alizarin red S produced by different manufacturers. Aquat. Toxicol. 2022, 242, 106051. [Google Scholar] [CrossRef]
- Lü, H.; Chen, H.; Fu, M.; Peng, X.; Xi, D.; Zhang, Z. Experimental evaluation of calcein and alizarin red S for immersion marking grass carp Ctenopharyngodon idellus. Fish. Sci. 2015, 81, 653–662. [Google Scholar] [CrossRef]

| Light Source | Wavelength (nm) | ||
|---|---|---|---|
| Excitation Filter | Suppression Filter | Dichromatic Mirror | |
| Brightfield light | – | – | – |
| Blue excitation light | 460–495 | 510–550 | 505 |
| Green excitation light | 530–550 | 575–625 | 570 |
| Hard Tissue Type | Sample Size | Marking Grade | ||
|---|---|---|---|---|
| BF | WBS | WGS | ||
| Otolith (sagitta) | 5 | 3 | 3 | 3 |
| Otolith (lapillus) | 5 | 3 | 3 | 3 |
| Otolith (asteriscus) | 5 | 2 | 2 | 2 |
| Fin ray (pectoral) | 5 | 1 | 3 | 3 |
| Fin ray (dorsal) | 5 | 1 | 3 | 3 |
| Fin ray (ventral) | 5 | 1 | 3 | 3 |
| Fin ray (anal) | 5 | 1 | 3 | 3 |
| Fin ray (caudal) | 5 | 1 | 3 | 3 |
| Scales (above lateral line) | 25 | 0 | 2 | 2 |
| Scales (on lateral line) | 25 | 0 | 3 | 3 |
| Scales (below lateral line) | 25 | 0 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Jiang, T.; Chen, X.; Liu, H.; Phelps, Q.; Yang, J. A Pilot Study Assessing a Concentration of 100 mg/L Alizarin Complexone (ALC) to Mark Calcified Structures in Hypophthalmichthys molitrix. Fishes 2022, 7, 66. https://doi.org/10.3390/fishes7020066
Zhu Y, Jiang T, Chen X, Liu H, Phelps Q, Yang J. A Pilot Study Assessing a Concentration of 100 mg/L Alizarin Complexone (ALC) to Mark Calcified Structures in Hypophthalmichthys molitrix. Fishes. 2022; 7(2):66. https://doi.org/10.3390/fishes7020066
Chicago/Turabian StyleZhu, Yahua, Tao Jiang, Xiubao Chen, Hongbo Liu, Quinton Phelps, and Jian Yang. 2022. "A Pilot Study Assessing a Concentration of 100 mg/L Alizarin Complexone (ALC) to Mark Calcified Structures in Hypophthalmichthys molitrix" Fishes 7, no. 2: 66. https://doi.org/10.3390/fishes7020066
APA StyleZhu, Y., Jiang, T., Chen, X., Liu, H., Phelps, Q., & Yang, J. (2022). A Pilot Study Assessing a Concentration of 100 mg/L Alizarin Complexone (ALC) to Mark Calcified Structures in Hypophthalmichthys molitrix. Fishes, 7(2), 66. https://doi.org/10.3390/fishes7020066