Growth and Microstructural Features in Otoliths of Larval and Juvenile Sinogastromyzon wui (F. Balitoridae, River Loaches) of the Upper Pearl River, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Rearing and Sampling
2.2. Otolith Preparation
2.3. Assessment of Otolith Development
2.4. Daily Increment Verification and Spacing Assessment
2.5. Statistical Analysis
3. Results
3.1. Ontogenetic Development
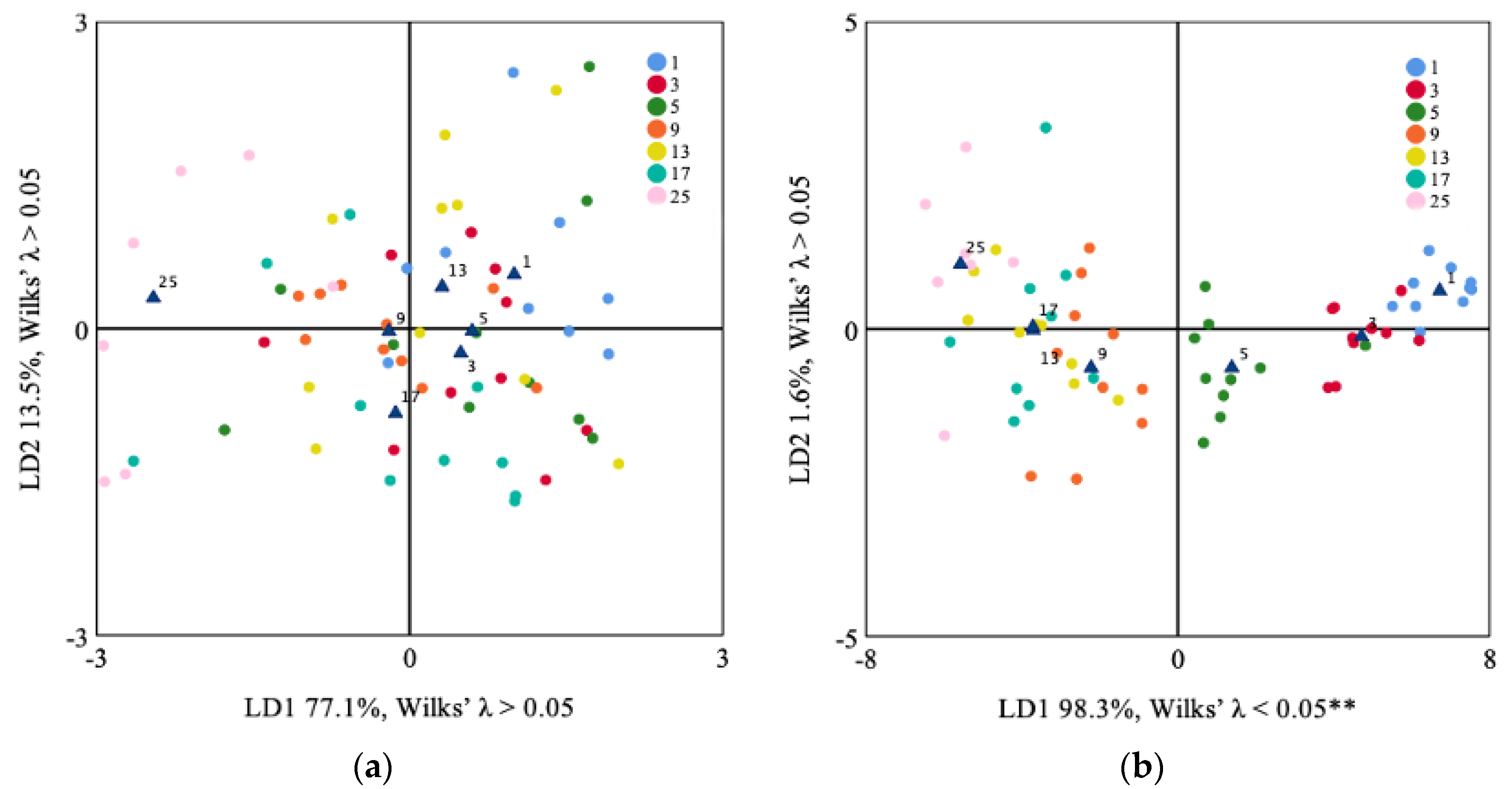
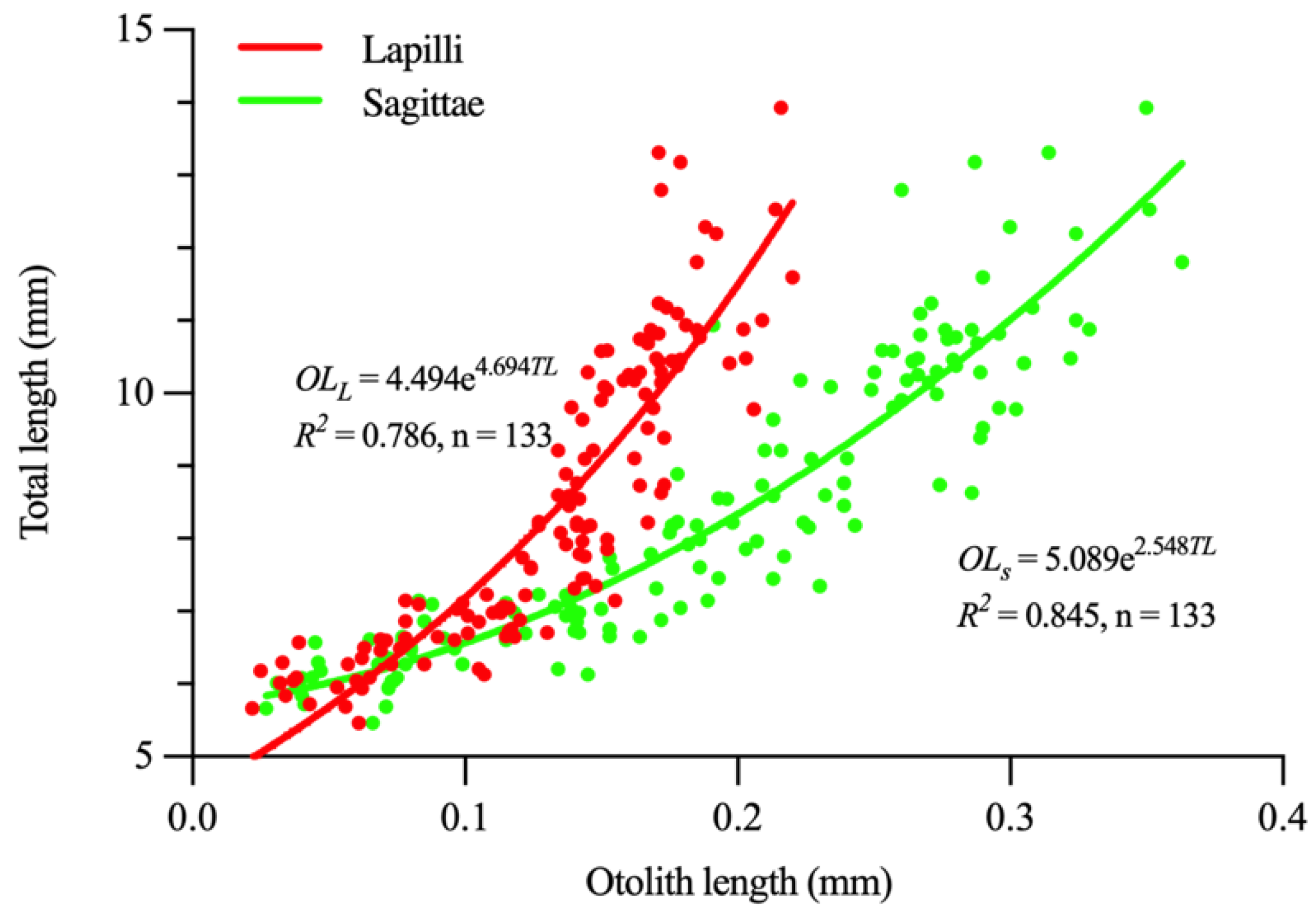
3.2. Lapilli and Sagittae Shape Analysis
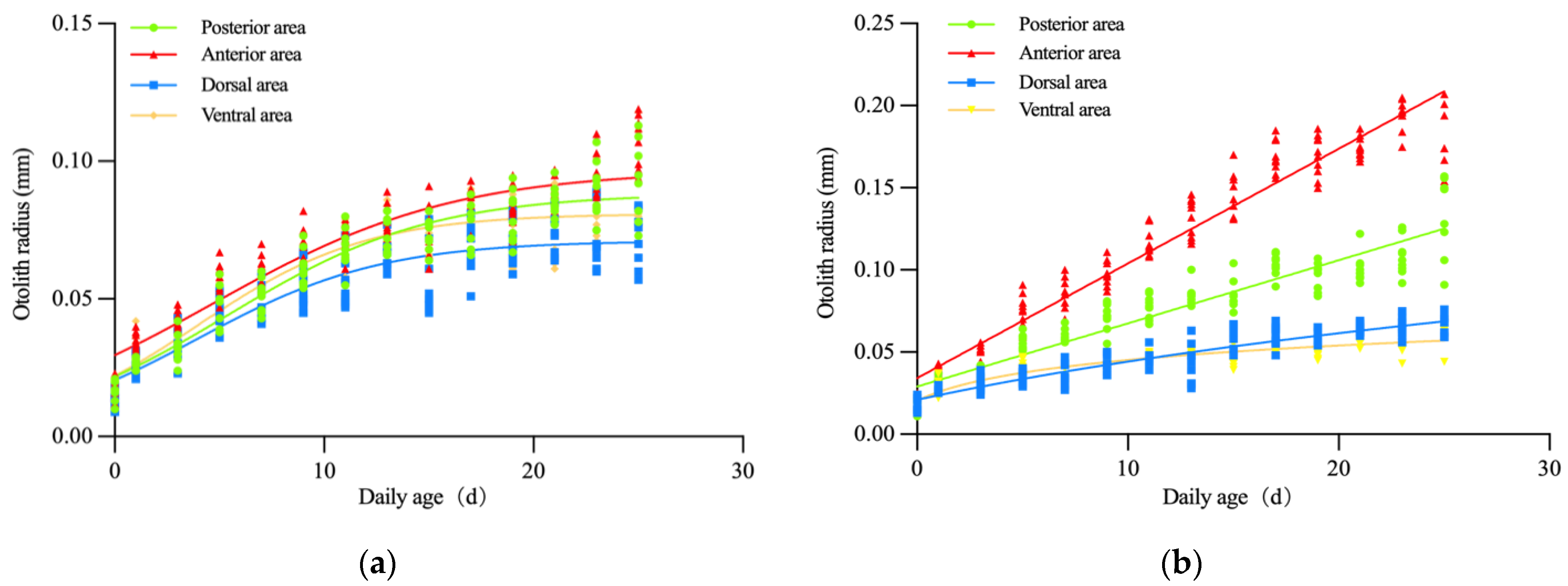
3.3. Otolith Growth
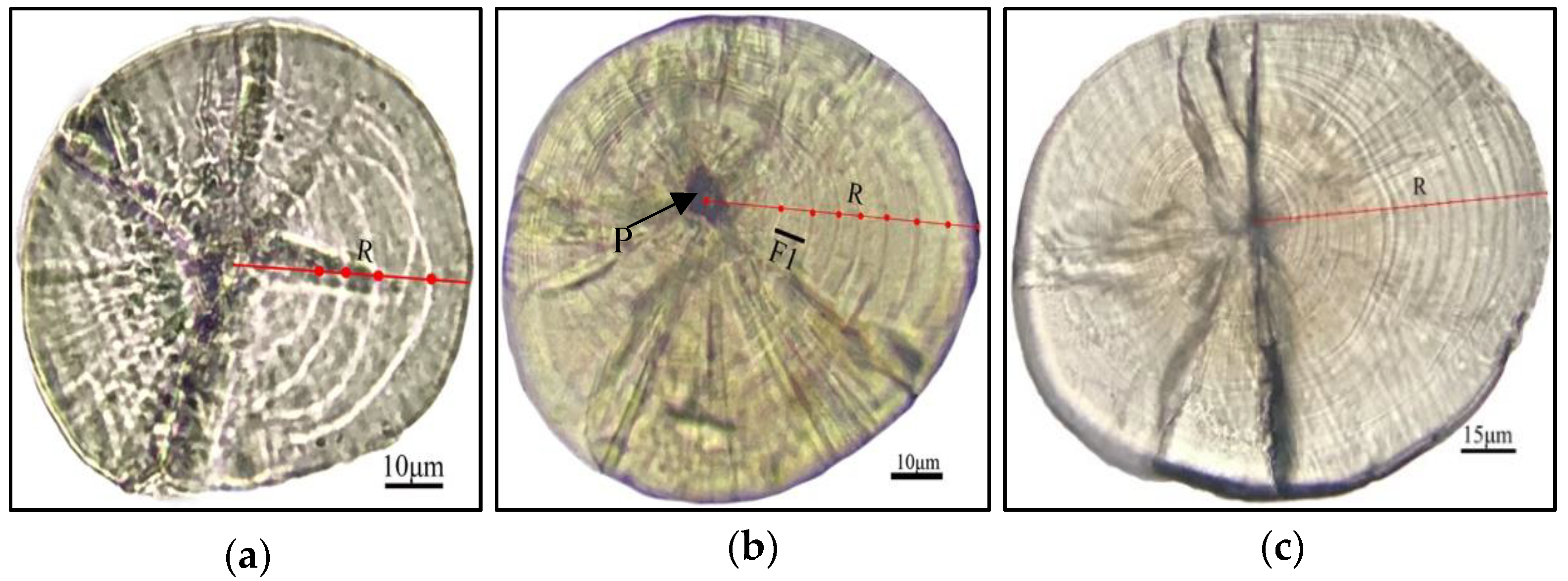
3.4. The Lapilli Otolith Microstructure
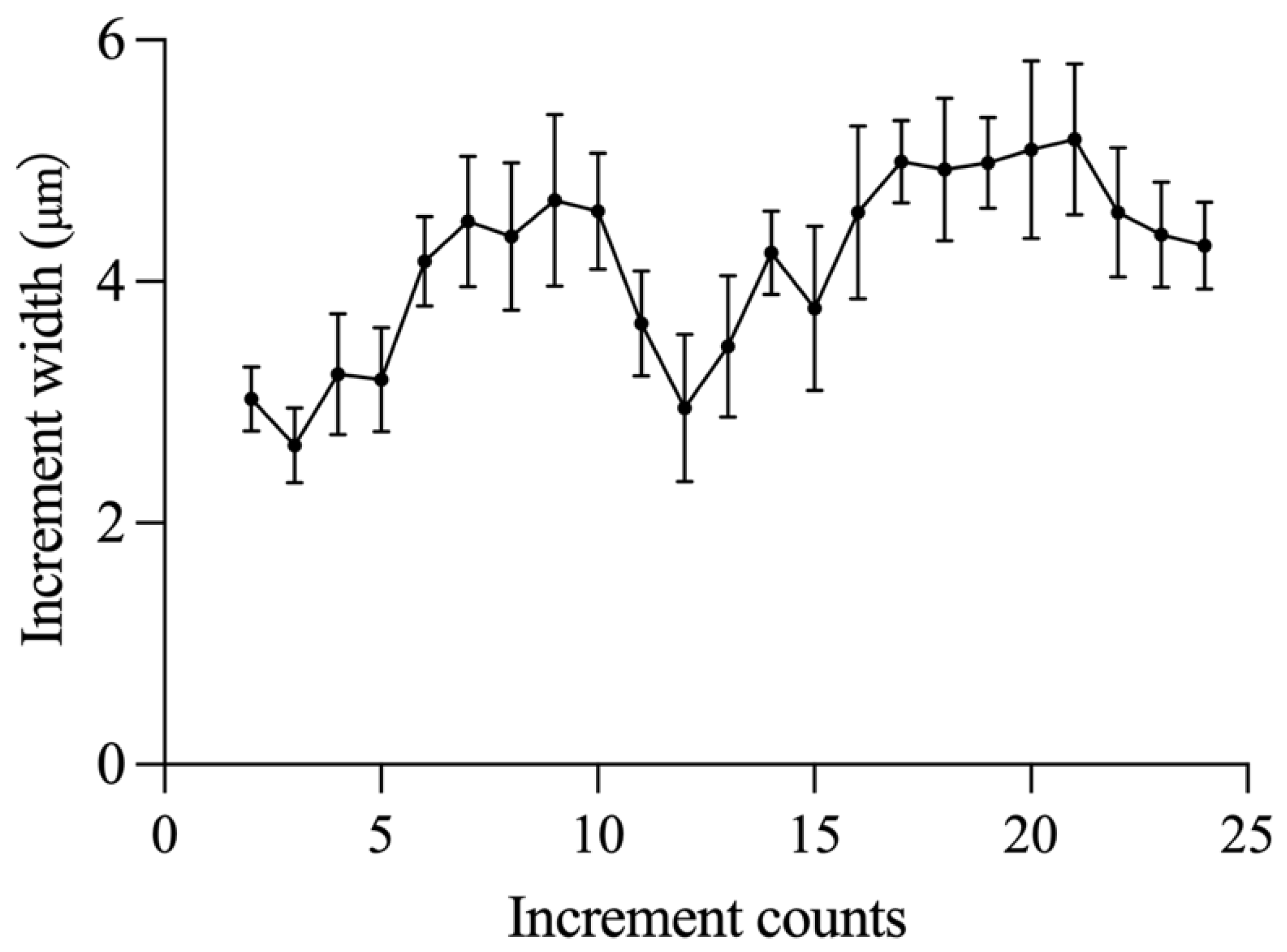
3.5. Verification of the Daily Lapilli Increment Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2021, 14, 398. [Google Scholar] [CrossRef]
- Panfili, J.; De Pontual, H.; Troadec, H.; Wrigh, P.J. Manual of Fish Sclerochronology; Ifremer-IRD: Brest, France, 2002; p. 466. [Google Scholar]
- Lundberg, Y.W.; Xu, Y.; Thiessen, K.D.; Kramer, K.L. Mechanisms of otoconia and otolith development. Dev. Dyn. 2015, 244, 239–253. [Google Scholar] [CrossRef] [Green Version]
- Panella, G. Fish otoliths: Daily growth layers and periodical patterns. Science 1971, 173, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Cieri, M.D.; McCleave, J.D. Validation of daily otolith increments in glass-phase American eels Anguilla rostrata (Lesueur) during estuarine residency. J. Exp. Mar. Biol. Ecol. 2001, 257, 219–227. [Google Scholar] [CrossRef]
- Joh, M.; Takatsu, T.; Nakaya, M.; Higashitani, T.; Takahashi, T. Otolith microstructure and daily increment validation of marbled sole (Pseudopleuronectes yokohamae). Mar. Biol. 2005, 147, 59–69. [Google Scholar] [CrossRef]
- Higgins, R.M.; Diogo, H.; Isidro, E.J. Modelling growth in fish with complex life histories. Rev. Fish Biol. Fish. 2015, 25, 449–462. [Google Scholar] [CrossRef]
- Van Poorten, B.T.; Walters, C.J. How can bioenergetics help us predict changes in fish growth patterns? Fish. Res. 2016, 180, 23–30. [Google Scholar] [CrossRef]
- Campana, S.E. How Reliable Are Growth Back-Calculations Based on Otoliths? NRC Research Press: Ottawa, ON, Canada, 1990; Volume 47. [Google Scholar]
- Hwang, S.D.; Song, M.H.; Lee, T.W.; McFarlane, G.A.; King, J.R. Growth of larval Pacific anchovy Engraulis japonicus in the Yellow Sea as indicated by otolith microstructure analysis. J. Fish Biol. 2006, 69, 1756–1769. [Google Scholar] [CrossRef]
- Takahashi, M.; Watanabe, Y.; Kinoshita, T.; Watanabe, C. Growth of larval and early juvenile Japanese anchovy, Engraulis japonicus, in the Kuroshio-Oyashio transition region. Fish. Oceanogr. 2001, 10, 235–247. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Tian, Y.; Liu, Y.; Liu, S.; Tian, H.; Cao, C. Otolith Shape Analysis as a Tool to Identify Two Pacific Saury (Cololabis saira) Groups from a Mixed Stock in the High-Seas Fishing Ground. J. Ocean Univ. China 2021, 20, 402–408. [Google Scholar] [CrossRef]
- Souza, A.T.; Soukalová, K.; Děd, V.; Šmejkal, M.; Moraes, K.; Říha, M.; Muška, M.; Frouzová, J.; Kubečka, J. Otolith shape variations between artificially stocked and autochthonous pikeperch (Sander lucioperca). Fish. Res. 2020, 231, 105708. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Savoca, S.; Panarello, G.; Di Paola, D.; Perdichizzi, A.; Rinelli, P.; Lanteri, G.; Spano, N.; et al. Intra- and interspecific variability among congeneric Pagellus otoliths. Sci. Rep. 2021, 11, 16315. [Google Scholar] [CrossRef] [PubMed]
- Hüssy, K. Otolith shape in juvenile cod (Gadus morhua): Ontogenetic and environmental effects. J. Exp. Mar. Biol. Ecol. 2008, 364, 35–41. [Google Scholar] [CrossRef]
- Fischer, P. Otolith microstructure during the pelagic, settlement and benthic phases in burbot. J. Fish Biol. 1999, 54, 1231–1243. [Google Scholar] [CrossRef]
- Zhang, Z.; Beamish, R.J.; Riddell, B.E. Differences in Otolith Microstructure between Hatchery-Reared and Wild Chinook Salmon (Oncorhynchus Tshawytscha); NRC Research Press: Ottawa, ON, Canada, 1995; Volume 52. [Google Scholar]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Campana, S.E.; Neilson, J.D. Microstructure of Fish Otoliths; NRC Research Press: Ottawa, ON, Canada, 1985; Volume 42. [Google Scholar]
- Wu, L.; Liu, J.S.; Wang, X.L.; Zhang, G.; Zhang, Z.Y.; Murphy, B.R.; Xie, S.G. Identification of individuals born in different spawning seasons using otolith microstructure to reveal life history of Neosalanx taihuensis. Fish. Sci. 2011, 77, 321–327. [Google Scholar] [CrossRef]
- Gao, M. Fish Resource in the Early Life Stages and Its Relation to Environmental Factors in Laibin Section of Xijiang River; Guilin University of Technology: Guilin, China, 2018. [Google Scholar]
- Kendall, A.W.; Ahlstrom, E.H.; Moser, H.G. Early Life History Stages of Fishes and Their Characters; Allen Press Inc.: Lawrence, KS, USA, 1984. [Google Scholar]
- Taiming, Y.; Jiaxiang, H.; Ting, Y.; Liulan, Z.; Zhi, H. Study on the otolith development and the formation of increments in larvae and juvenile of Chuanchia labiosa. Acta Hydrobiol. Sin. 2014, 38, 764–771. [Google Scholar] [CrossRef]
- Zhu, Q.; Xia, L.; Chang, J. Computer identification on otolith microstructure of fish. Acta Hydrobiol. Sin. 2002, 26, 600–604. [Google Scholar]
- Vignon, M. Ontogenetic trajectories of otolith shape during shift in habitat use: Interaction between otolith growth and environment. J. Exp. Mar. Biol. Ecol. 2012, 420–421, 26–32. [Google Scholar] [CrossRef]
- Bounket, B.; Gibert, P.; Gennotte, V.; Argillier, C.; Carrel, G.; Maire, A.; Logez, M.; Morat, F. Otolith shape analysis and daily increment validation during ontogeny of larval and juvenile European chub Squalius cephalus. J. Fish Biol. 2019, 95, 444–452. [Google Scholar] [CrossRef]
- Crampton, J.S. Elliptic Fourier Shape Analysis of Fossil Bivalves: Some Practical Considerations; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Iwata, H.; Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Fu, Z.; Li, J.; Yue, B. Validation of daily otolith increments in larval and juvenile Chinese sucker, Myxocyprinus asiaticus. Environ. Biol. Fishes 2007, 82, 165–171. [Google Scholar] [CrossRef]
- Gao, M.H.; Wu, Z.Q.; Huang, L.L.; Tan, X.C.; Liu, H.; Rad, S. Otolith shape analysis and growth characteristics in larval and juvenile Squalidus argentatus. Environ. Biol. Fishes 2021, 104, 937–945. [Google Scholar] [CrossRef]
- Wilson, R.R., Jr. Depth-related changes in Sagitta morphology in six macrourid fishes of the Pacific and Atlantic oceans. Copeia 1985, 1985, 1011–1017. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, G.; Lin, X. Otolith ontogeny and increment of larval Macropodus opercularis. J. Fish. Sci. China 2010, 17, 1364–1370. [Google Scholar]
- Ji, Y.; Zhao, F.; Yang, Q.; Ma, R.; Yang, G.; Zhang, T.; Zhuang, P. Sagittal otolith morphology and the relationship between its mass and the age of Liza haematocheila in the Yangtze Estuary, China. Chin. J. Appl. Ecol. 2018, 29, 953–960. [Google Scholar]
- Campana, S.; Stevenson, D.K. Otolith Microstructure Examination and Analysis. Copeia 1992, 1197. [Google Scholar] [CrossRef]
- Karakiri, M.H.C. Preliminary notes on the formation of daily increments in otoliths of Oreochromis aureus. J. Appl. Ichthyol. 1989, 5, 53–60. [Google Scholar] [CrossRef]
- Smith, B.B.W.; Keith, F. Validation of the ageing of 0+ carp (Cyprinus carpio L.). Mar. Freshw. Res. 2003, 54, 1005–1008. [Google Scholar] [CrossRef]
- Huang, W.-B.; Chiu, T.-S. Daily Increments in Otoliths and Growth Equation of Black Porgy, Acanthopagrus schlegeli, larvae. Acta Zool. Taiwanica 1997, 8, 121–130. [Google Scholar]
- Akira, N.; Juro, Y. Age and Growth of Larval and Juvenile Walleye Pollock, Theragra Chalcogramma (Pallas), as Determined by Otolith Daily Growth Increments; Elsevier: Amsterdam, The Netherlands, 1984; Volume 82. [Google Scholar]
- Huang, Y.; Chen, F.; Tang, W.; Lai, Z.; Li, X. Validation of daily increment deposition and early growth of mud carp Cirrhinus molitorella. J. Fish Biol 2017, 90, 1517–1532. [Google Scholar] [CrossRef]
- Brothers, E.B.; Mathews, C.P.; Lasker, R. Daily growth increments in otoliths from larval and adult fishes. Fish. Bull. 1976, 74, 1–8. [Google Scholar]
- Jenkins, G.P. Age and growth of co-occurring larvae of two flounder species, Rhombosolea tapirina andAmmotretis rostratus. Mar. Biol. 1987, 95, 157–166. [Google Scholar] [CrossRef]
- Lagardère, F.; Troadec, H. Age estimation in common sole Solea solea larvae: Validation of daily increments and evaluation of a pattern recognition technique. Mar. Ecol. Prog. Ser. 1997, 155, 223–237. [Google Scholar] [CrossRef]
- Durham, B.W.; Wilde, G.R. Validation of Daily Growth Increment Formation in the Otoliths of Juvenile Cyprinid Fishes from the Brazos River, Texas. N. Am. J. Fish. Manag. 2008, 28, 442–446. [Google Scholar] [CrossRef]
- Ding, C.; Chen, Y.; He, D.; Tao, J. Validation of daily increment formation in otoliths for Gymnocypris selincuoensis in the Tibetan Plateau, China. Ecol. Evol. 2015, 5, 3243–3249. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, F.; Zhao, S.; Xie, S. Ontogenetic development and otolith microstructure in the larval and juvenile stages of mandarin fish Siniperca chuatsi. Ichthyol. Res. 2018, 66, 57–66. [Google Scholar] [CrossRef]
- Souza, A.; Soukalová, K.; Děd, V.; Šmejkal, M.; Blabolil, P.; Říha, M.; Jůza, T.; Vašek, M.; Čech, M.; Peterka, J.; et al. Ontogenetic and interpopulation differences in otolith shape of the European perch (Perca fluviatilis). Fish. Res. 2020, 230, 105673. [Google Scholar] [CrossRef]
- Molina-Valdivia, V.; Landaeta, M.F.; Castillo, M.I.; Alarcón, D.; Plaza, G. Short-term variations in the early life history traits of common sardine Strangomera bentincki and anchoveta Engraulis ringens off central Chile. Fish. Res. 2020, 224. [Google Scholar] [CrossRef]


| DPH | N | Increment Count (Mean ± SD, Days) | DGR (Mean ± SD, μm·day–1) | TL (Mean ± SD, mm) |
|---|---|---|---|---|
| 2 | 10 | 1 ± 1 | 3.02 ± 0.26 | 6.02 ± 0.25 |
| 3 | 10 | 2 ± 1 | 2.64 ± 0.31 | 6.68 ± 0.26 |
| 4 | 10 | 3 ± 1 | 3.23 ± 0.49 | 6.75 ± 0.31 |
| 7 | 10 | 6 ± 2 | 4.49 ± 0.54 | 6.92 ± 0.40 |
| 13 | 10 | 12 ± 2 | 3.46 ± 0.58 | 8.46 ± 0.73 |
| 17 | 10 | 16 ± 2 | 4.99 ± 0.34 | 9.95 ± 0.67 |
| 23 | 10 | 22 ± 3 | 4.38 ± 0.43 | 11.31 ± 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Wu, Z.; Huang, L.; Tan, X.; Li, M.; Huang, H. Growth and Microstructural Features in Otoliths of Larval and Juvenile Sinogastromyzon wui (F. Balitoridae, River Loaches) of the Upper Pearl River, China. Fishes 2022, 7, 57. https://doi.org/10.3390/fishes7020057
Gao M, Wu Z, Huang L, Tan X, Li M, Huang H. Growth and Microstructural Features in Otoliths of Larval and Juvenile Sinogastromyzon wui (F. Balitoridae, River Loaches) of the Upper Pearl River, China. Fishes. 2022; 7(2):57. https://doi.org/10.3390/fishes7020057
Chicago/Turabian StyleGao, Minghui, Zhiqiang Wu, Liangliang Huang, Xichang Tan, Mingsi Li, and Haibo Huang. 2022. "Growth and Microstructural Features in Otoliths of Larval and Juvenile Sinogastromyzon wui (F. Balitoridae, River Loaches) of the Upper Pearl River, China" Fishes 7, no. 2: 57. https://doi.org/10.3390/fishes7020057
APA StyleGao, M., Wu, Z., Huang, L., Tan, X., Li, M., & Huang, H. (2022). Growth and Microstructural Features in Otoliths of Larval and Juvenile Sinogastromyzon wui (F. Balitoridae, River Loaches) of the Upper Pearl River, China. Fishes, 7(2), 57. https://doi.org/10.3390/fishes7020057