Abstract
Several anthropogenic products in wastewater are considered a threat to the aquatic environment. In addition to common industrial pollutants, levels of pharmaceuticals have been increasingly found in the environment in recent years, which may present a strong risk to the aquatic species that live there. The constant consumption of biologically active chemicals for human health has been matched by an increase in the leaking of these compounds in natural habitats over the last two decades. This study is aimed at evaluating the developmental toxicity of fotemustine in the ecological environment. Zebrafish embryos were exposed to doses of 25, 50 and 100 µg/mL from 4 h post-fertilization to 120 h. This study confirms that fotemustine exposure at 50 and 100 µg/mL affects the survival and hatching rate, morphology score and body length. Additionally, it significantly disturbs the antioxidant defense system and increases ROS in zebrafish larvae. From the molecular point of view, fotemustine exposure strongly induces apoptosis, endoplasmic reticulum stress (ERS) and the Wnt signaling pathway.
1. Introduction
The toxicity of chemotherapy remains an important factor in the care, management, cost of treatment, and quality of life of cancer patients with a particularly poor prognosis. In the aquatic environment, some of these medicines can be detected at ng/L−1 concentrations and have the potential to be quite persistent. Only a few anticancer medications have already had an environmental risk assessment, which has primarily been based on projected data and excluded information on their metabolites and transformation products [1,2]. Alkylating agents are one of the oldest classes of anticancer drugs used in cancer chemotherapy [3]. Although levels of fotemustine in the environment have not yet been reported on, several drugs belonging to the same class as alkylating agents have been reported in wastewater from different areas globally. Hospital wastewater effluents have the highest residue levels, ranging in some cases from <0.2 ng/L to 22.1 μg/L for some alkylating agents such as cyclophosphamide, and <0.2 ng/L to 86.2 µg/L for ifosfamide [4,5,6,7,8]. Concentrations as high as 2.9 μg/L were found in effluent from wastewater treatment plants, while up to 220 ng/L of alkylating agents were detected in aquaculture with a detection frequency of 41.7% [8,9,10,11].
Fotemustine, or diethyl{1-[3-(2-cloroethyl)-3-nitrosoureido]ethyl}phosphonate, belongs to the class of anticancer nitrosoureas and is an alkylating cytotoxic drug [12]. This chemical was developed in order to create a nitrosourea with more selective anticancer activity than previous alkylating agents. In fact, in terms of the blood–brain barrier transit and cell penetration coefficient, notably in neoplastic cells, this chemical has more appealing pharmacokinetic features than the other conventional nitrosoureas. On an outpatient basis, fotemustine chemotherapy is reported to be well tolerated. Myelosuppression with delayed reversible thrombocytopenia (40.3%) and leukopenia (46.3%) were the most common side effects of fotemustine, and they were cumulative and dose-related, similar to other nitrosoureas. Toxicity in the gastrointestinal tract was uncommon (7.7%). In 29% of patients, mild transitory elevations in serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and bilirubin were recorded, indicating moderate hepatotoxicity. The molecular processes causing the unfavorable side effects of fotemustine treatment remain unknown. The enzymes thioredoxin reductase and ribonucleotide reductase have been demonstrated to be inhibited by fotemustine [13,14], both engaged in antioxidant processes, but not the activity of glutathione reductase [15]. In rat hepatocytes, fotemustine was also shown to cause lactate dehydrogenase (LDH) leakage, glutathione (GSH) depletion, GSSG formation and lipid peroxidation (LPO) [16]. Furthermore, DEP-isocyanate has been proposed as the cause of these symptoms, meanwhile several isocyanate-related toxicities have been reported. Chromosome abnormalities, sister chromatid swaps, mutations, and/or cancer have all been linked to isocyanates [17]. Methyl isocyanate disturbs red blood cell membrane function [18], evoking alveolar damage leading to pulmonary edema [19] and immunotoxicity with concomitant disturbances in pulmonary functions in rabbits [20]. N-butyl isocyanate has been reported to cause severe histopathological lesions in rat lungs [21].
Zebrafish larvae are uniquely suited to identifying novel protective compounds through drug screening, combining the advantages of cell lines (due to their extremely small size suitable for multi-well plates and ease of chemical exposure) and vertebrate models (functional organ systems and genetic conservation) [22,23]. Because surface water contains low quantities of environmental pollutants, this is a promising technique that suggests pharmaceuticals (and other compounds such as anticancer drugs) and their toxicity processes could be monitored using fish lines at lower concentrations. Furthermore, zebrafish can quickly uncover drugs with clear developmental toxicity or absorption difficulties as a whole-animal drug screening platform [23,24].
2. Materials and Methods
2.1. Solutions Preparation
FM (fotemustine) MUPHORAN 208 mg was purchased from (ITALFARMACO S.p.A. Viale F. Testi, 330 20126 MILANO, Italy). The solution was diluted in embryo medium obtaining three concentrations ranging from 25 to 50 to 100 µg/mL and dispensed in 24-well plates (2 mL each) (Labsolute, Th. Geyer GmbH & Co. KG, Germany), one for each concentration and one plate with negative control (untreated).
2.2. Zebrafish Maintenance and Breeding
Wild-type (WT) mature zebrafish with an age of 6 month were used for embryo production. Zebrafish were reared in the fish facility of the Centre for Experimental Fish Pathology facilities (Centro di Ittiopatologia Sperimentale della Sicilia—CISS), Department of Veterinary Sciences, University of Messina, Italy. The fish were fed both with dry and live food twice a day at 3% of body weight (BW). For a successful reproduction, mature females and males were mated at 2:1 ratio. The day after, the eggs were collected and bleached and, afterwards, nonfertilized eggs were discarded. Only embryos which reached the blastula stage were used for experiments.
2.3. Zebrafish Embryo Toxicity (ZFET) Assay
The toxicity of fotemustine solutions was established following the OECD guideline (OECD, test no. 236: fish embryo acute toxicity (FET) test) [25]. Different concentrations of fotemustine (25–50–100 µg/mL) were prepared using embryo medium and placed into 24-well plates (1 embryo for each well). Fertilized eggs (n = 24 in each plate; 20 exposed to the drug and 4 used as negative control) were transferred into 24-well plates with test solutions and incubated at 26 °C at a 14:10 h day/night light regime. The experiment was repeated three times. The entire mortality and developmental abnormalities of embryos and larvae were monitored and recorded at 24, 48, 72, and 96 h post fertilization (hpf) [26]. Coagulation, lack of somites, nondetachment of the tail, and no heartbeat were considered as lethal endpoint. Furthermore, malformations of the embryos during development were evaluated as a teratogenic endpoint. In addition, the percentage of hatchability and mortality was estimated. A stereo microscope was used to capture images and video (Leica M205 C). Every 24 h, four separate endpoints were checked to see any malformations:
- (a)
- Embryo coagulation—could also occur within a few hours of the start of exposure and indicated a generic acute toxic effect;
- (b)
- Lack of somite formation—somite should be visible 12 h after fertilization; if absent, the embryo would not develop further; thus, causing its death;
- (c)
- Nondetachment of the tail—detachment of the tail from the yolk could be observed 24 h after fertilization, indicating normal growth of the embryo;
- (d)
- Absence of heartbeat—the heartbeat was easily detectable 30 h after fertilization, its absence indicated the death of the embryo; embryo coagulation and absence of heartbeat were focused on as endpoints of mortality.
Morphology scores were determined at 96 hpf as previously described. Nine endpoints, including body shape, somites, notochord, tail, fins, heart, face, brain, and pharyngeal arches/jaws, were examined to evaluate the phenotypes of the zebrafish [27].
2.4. Total RNA Extraction and RT-PCR
Total RNA was extracted from (n = 30) homogenized zebrafish larvae from 48 to 72 hpf using a NanoMag Animal and Fish RNA Isolation Kit (Shannuo Scientific Company, Tianjin, China). The synthesis of cDNA was carried out using PrimeScript RT Master Mix (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. The detailed procedure for RT-PCR was drawn from a previous work [28]. Each gene in the present study was assessed in triplicate. The sequences of primers for the real-time PCR are shown in Table 1.

Table 1.
Primers for real-time PCR.
2.5. Histopathological Analysis
Larvae were collected and fixed in buffered 4% paraformaldehyde for 24 h at 4 °C for histological investigation. After that, they were dehydrated, rinsed, and processed in an ascending order of alcohol (70–100%), followed by xylene clearing. Paraffin wax was used to embed the samples, which were then placed on wooden blocks. Microtome was used to cut 5 μm thick thin sections. Hematoxylin and eosin were used to stain the slides (H&E). Tissue ribbons were stretched by fixation on albumenized glass slides, based on the protocol already used [29,30]. Following that, the slides were inspected under a light microscope (LEICA DM6).
2.6. Data Analysis
Microsoft Excel was used to evaluate all of the raw spreadsheet data. GraphPad Prism 8.3.1 (GraphPad, San Diego, CA, USA, 2020) was used to create graphs and perform statistical analysis. To find significant differences between the mean values, a two-way ANOVA test, analysis of variance, was utilized (ANOVA-SNK). The differences were considered to be statistically significant if the p-values were less than <0.0001.
3. Results
3.1. Survival and Hatching Rate
Embryo cumulative mortality after exposure to fotemustine is reported in Figure 1A. In order of increasing concentration, survival rate was documented at 24, 48, 72, and 96 hpf. Figure 1B shows that 100 µg/mL of fotemustine caused 100% death at 72 hpf, resulting in a massive mortality rate compared to the control group. Moreover, also at a 50 µg/mL dose, a high peak of mortality at 96 hpf was found, which showed that also at lower concentrations fotemustine had an impact on zebrafish larvae development. Because hatching is a critical time in zebrafish embryogenesis, the hatching rate is one of the most important indices for determining fotemustine developmental toxicity in zebrafish. According to the studies conducted, embryos started to hatch by 48 hpf and finished by 96 hpf. Our results showed that approximately 50% of the 50 µg/mL group embryos had hatched by 72 hpf, while in the group with the concentration of 100 µg/mL, no embryos hatched. Therefore, as showed in Figure 1C, the embryo hatching rate was not reduced in the 25 µg/mL fotemustine exposure group. These data showed a massive dose-dependent decrease in the hatching rate in the fotemustine-treated groups compared to the control group (100% hatching rate at 72 hpf).
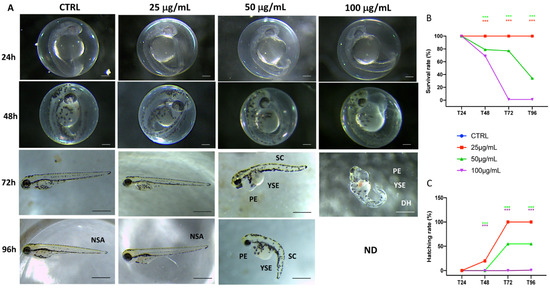
Figure 1.
The morphological abnormalities in zebrafish caused by different fotemustine dose exposures (A), survival rate (B), and hatching rate (C). Images were taken from the lateral view under a dissecting microscope (magnification 25). Scale bar, 500 mm. PE—pericardial edema; YSE—Yolk sac edema; SC—scoliosis; DH—delayed hatching; NSA—normal spine axis. *** p < 0.001 versus CTRL.
3.2. Malformation Scores and Body Length
Phenotypic defections at time points were noted after fotemustine exposure (Figure 2A). Compared to the control group, the malformation rate of the fotemustine 10 µg/mL group showed no significant change. Abnormalities, primarily modest yolk retention and pericardial edema, were found in the groups with 50 µg/mL fotemustine, but especially in the group to the concentration of 100 µg/mL (Figure 2C). The body lengths of the larvae were measured at 96 hpf to assess the degree of development (Figure 2B). The body lengths of the larvae at 96 hpf were significantly reduced in both the 50 and 100 µg/mL fotemustine groups, which indicated that in a concentration-dependent manner, fotemustine exposure significantly inhibited larval growth.
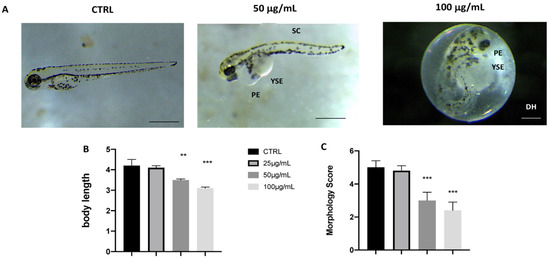
Figure 2.
Effects of fotemustine on morphological changes in zebrafish larvae at 72 hpf. Representative lateral views (A), body length (B), and morphological scoring(C) of zebrafish larvae treated with fotemustine 50 µg/mL. *** p < 0.001 versus CTRL; ** p < 0.01 versus CTRL. PE—pericardial edema; YSE—yolk sac edema; SC—scoliosis; DH—delayed hatching. Scale bars 40x magnification.
3.3. Histological Analysis
Fotemustine exposure produced noticeable effects in a dose-dependent manner on intestine and muscle tissue at 96 hpf. Indeed, frayed intestinal villi with epithelial desquamation were shown after exposure to fotemustine, a cellular degeneration that could result in increased mucosal secretions, particularly at the concentration of 100 µg/mL, as shown in Figure 3. The 25 µg/mL fotemustine group showed no significant histological alteration compared to the CTRL group, in contrast to the 50 µg/mL fotemustine group, and even more compared to the group with the highest dose of 100 µg/mL. In addition, toxicity was observed at the level of muscle tissue following exposure by fotemustine. Concentrations of 50 µg/mL and, especially, 100 µg/mL showed muscle atrophy along the spine of larvae at 96 hpf (Figure 3), with slight cell degeneration for the highest concentration. Fotemustine at the concentration of 25 µg/mL showed no histological changes in the muscle fibers, showing a very similar situation to the CTRL group (Figure 3). No alterations were seen on the eyes and brain at the different concentrations of fotemustine exposure compared to the control group (data not shown).
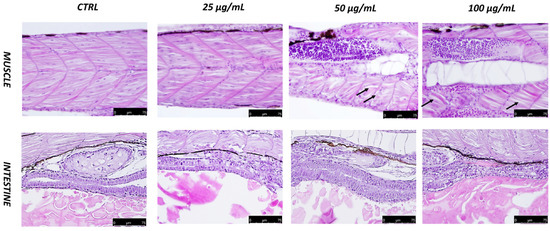
Figure 3.
Histopathological changes in the intestines and muscles of zebrafish larvae exposed to fotemustine at 96 hpf. Muscle atrophy is indicated by a dotted black arrow (40× magnification).
3.4. Gene Expression
To investigate the possible mechanisms of the toxic effects induced by fotemustine, we carried out an RT-PCR test to examine the mRNA expression levels of larvae (n = 30) exposed by different fotemustine concentrations from 6 to 72 hpf. The RT-PCR results showed that the expression levels of the oxidative stress-related genes (cat, sod1, and gstp2) were upregulated in the fotemustine exposure group (50 and 100 µg/mL) compared to the control group, while they were downregulated in the other groups (Figure 4A). The mRNA expression levels of ER stress-related genes (chop, hspa5, hsp90b1, and perk) and apoptosis-related genes (caspase-3, p53, and bax) increased with the increased fotemustine exposure dose (50 and 100 µg/mL) (Figure 4B,C). The mRNA expression level of bcl-2 was downregulated with increasing fotemustine exposure doses.
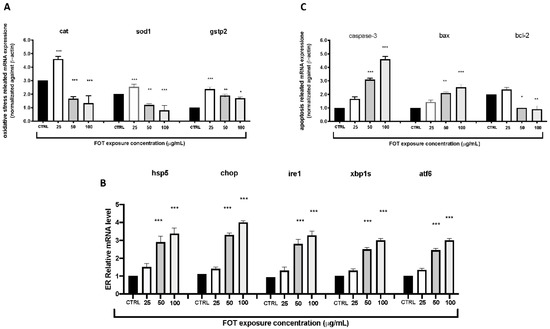
Figure 4.
The fotemustine exposure effects on oxidative (A), apoptosis (B), and ER stress (C) pathway-related genes on zebrafish embryos. The results are expressed as mean of three independent experiment data. The expression levels of mRNA are represented as the fold change from the CTRL group. * p < 0.05, ** p < 0.01, *** p < 0.001 versus CTRL.
As the fotemustine exposure dose rose, the mRNA expression levels of Wnt signaling pathway-related genes (b-catenin, wnt3a, and wnt8a) increased at first, but then dropped; nevertheless, the expression of wnt11 increased substantially (Figure 5).
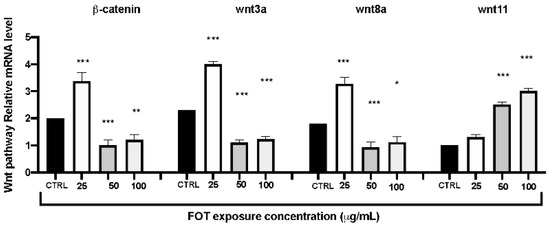
Figure 5.
The fotemustine exposure effects on Wnt pathway related genes on zebrafish embryos. The results are expressed as mean of three independent experiment data. The expression levels of mRNA are represented as the fold change from the CTRL group. * p < 0.05, ** p < 0.01, *** p < 0.001 versus CTRL.
4. Discussion
Recent results demonstrate that pharmaceuticals are widely distributed into marine and coastal ecosystems; therefore, additional research into the possible effects on aquatic organisms are important [31]. Pharmaceuticals differ from usual pollutants, being designed to react with specific pathways at low doses, and can induce significant alterations in aquatic species. In our study, we aimed to analyze the toxic action of fotemustine in a concentration-dependent manner. We showed that fotemustine induced developmental toxicity in zebrafish embryos, particularly delaying hatching and causing morphological abnormalities. The 25, 50 and 100 µg/mL doses chosen for the toxicity studies ranged from no injury to a noticeable toxic effect on development. Fotemustine treatment at a dose of 25 µg/mL did not show clear signs of toxicity, while doses of 50 and 100 µg/mL strongly reduced the survival and hatching rates at 96 and 72 hpf, respectively. For the first time, our investigations employed zebrafish to study the developmental toxicity of fotemustine. Hatching is a crucial moment of zebrafish embryogenesis; consequently, the decreased hatching rate was induced by functional and structural disturbances during embryonic development [32,33]. In addition, the suppression of embryogenesis, the inhibition of mitosis [34] or the incapability of the embryonic larvae to open the eggshell [35] also likely caused the developmental delay. Our data showed a significant dose-dependent reduction in the hatching rate, which is also a critical indicator of developmental toxicity. Fotemustine exposure at 50 and 100 µg/mL resulted in embryonic teratogenesis, characterized by spinal curvature, pericardial edema, bent tails, and uninflated swim bladders. Moreover, the reduction in body length indicated that fotemustine exposure could affect the growth of the larvae, which could be explained by the delayed maturation. All these data agreed in proving that developmental toxicity is indeed induced by fotemustine exposure.
Some previous studies have shown that chronic exposure to fotemustine may exhibit toxicity in human lungs, or damage on isolated rat hepatocytes through an imbalance of antioxidant defenses [16,34,35]. In our study, we showed, for the first time, damage related to fotemustine exposure during the early stages of zebrafish embryonic development. Indeed, fotemustine, in a concentration-dependent manner, caused damage to muscle and intestine tissue at 96 hpf, two organs often subject to alteration following exposure to toxic substances. The highest concentration of fotemustine caused muscle atrophy accompanied by cell degeneration, the latter also present in the intestine, as well as a fraying of intestinal villi with epithelial desquamation. No alterations were found in other organs such as the brain or the eyes. It is still not very clear how fotemustine caused damage to some organs and not to all, with the development of many organs in the early stages of the embryonic development of zebrafish being unclear, as well as the same innate immunity compared to adults, which creates a limitation in the analysis of organ damage. Critical pathways involved in developmental toxicity are oxidative stress and inflammation [36,37]. Regarding drug-induced oxidative stress, two main mechanisms are involved: the reduction in the cellular antioxidant defenses and the ROS overproduction [38,39]. ROS are the main promotors of oxidative stress [40] because they excessively combine with CAT, GSH, and SOD, unbalancing the antioxidant protection mechanism. SOD is an important enzyme in the endogenous antioxidant system, thanks to its ability to prevent lipid peroxidation and the removal of ROS [41]. Another key antioxidant factor is gstp2, a member of the GST Pi family, which is involved with glutathione in removing ROS [42]. Our experiments showed a significant decrease in ROS content in zebrafish larvae. The mRNA levels of sod1, cat, and gstp2 decreased in fotemustine treatments at doses of 50 and 100 µg/mL, showing that the antioxidant defenses were impaired in the high-exposure groups. Our data demonstrated what was previously shown about the involvement of ROS in the toxic action of fotemustine. In fact, it has been shown that fotemustine, in a dose-dependent manner, can cause an imbalance in normal antioxidant defenses [43]. ROS can also damage ER [44], dysregulating calcium homeostasis, hindering the folding and transport of proteins, and stimulating ER stress [45]. Consequently, we investigated the expression of ERS-related genes. Severe and prolonged ERS induces the increased expression of chop [46] and of the molecular chaperones hspa5 and hsp90b1, which are the zebrafish homologues of grp78 and grp94 [47,48]. These chaperones are important factors for ER homeostasis and antiapoptotic pathways [49,50]. During ERS, the grp78–kinase complexes dissociate and activate perk, atf6, and ire1 [51]. Perk is an ERS kinase that induces the transcription of other unfolded protein reaction UPR-dependent genes [52]. In this paper, we showed, for the first time in zebrafish early life stages, the upregulated expression of chop, hspa5, hsp90b1, and perk, indicating the key role of ERS in the developmental toxicity of fotemustine. Chop was also involved in ERS-induced apoptosis. The increased expression of chop caused the activation of caspase-3, p53, and bax, while reducing the expression of bcl-2 [53]. P53 is a multifunctional regulation factor. Previous studies showed that the action of fotemustine could be linked to the production of ROS and increase in the apoptotic process [54,55]. In our study, we aimed to confirm whether the toxic action of fotemustine was related to oxidative stress and apoptosis in a zebrafish model. Our data showed that the exposure of fotemustine at 50 and 100 µg/mL doses increased the expression of the apoptosis-inducing target genes and reduced the expression of the antiapoptotic factor. These results suggested that apoptosis induction has a key role in the developmental toxicity of fotemustine.
Finally, we wanted to investigate the toxicity of fotemustine on the Wnt pathway, which is often linked to zebrafish development. In fact, the Wnt signaling pathway is an important signaling pathway involved in the vertebrate embryo development. Wnt signaling includes canonical and noncanonical pathways. The classical pathway is regulated by b-catenin and is related to developmental malformations [56]. This pathway also comprehends wnt8a and wnt3a, which are responsible for the posterior structure of the zebrafish body [57,58]. Our results showed that the mRNA expression levels of b-catenin, wnt3a, and wnt8a were significantly decreased after fotemustine exposure at 50 and 100 µg/mL doses. The noncanonical pathway comprehends wnt11, which regulates cardiac differentiation and regulation, and is involved in late-stage heart development [59]. Our data displayed that wnt11 expression was increased by fotemustine exposure, indicating that the wnt canonical and noncanonical pathways have a key role in the developmental toxicity of fotemustine.
5. Conclusions
We can conclude by stating that fotemustine exposure, in a concentration-dependent manner, causes toxicity in the early stages of zebrafish embryonic development, causing alterations both at the morphological and histological levels to some tissues such as muscle and intestine. In addition, fotemustine toxicity seems to be accompanied by an increase in oxidative and apoptotic pathways, as well as an increase in ERS. In addition, the analysis of gene expression also showed the involvement of the Wnt pathway in the toxic action of fotemustine. Although the organ-damaging action of fotemustine is not fully understood, our work showed a first approach on toxicity exposure at different concentrations. Further studies are needed to investigate the mechanism by which fotemustine causes toxicity on these organs and related cell types, also by analysis on adult zebrafish.
Author Contributions
Conceptualization, S.C.; methodology D.I., R.C. and D.D.P.; validation, M.C., R.S. and E.G.; formal analysis and investigation, A.F.P. and C.I.; resources, R.F.; data curation G.L. and R.D.; writing—original draft preparation, A.F.P.; writing—review and editing, A.F.P.; visualization, E.G.; supervision, D.D.P.; project administration, S.C. and N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to experiment on zebrafish larvae up to five days (120 h) post fertilization and particularly ZFET are paired with alternative according to Directive 2010/63/EU and relating Italian DL 26/2014 on the protection of animals used for scientific purposes.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Toolaram, A.P.; Kümmerer, K.; Schneider, M. Environmental risk assessment of anti-cancer drugs and their transformation products: A focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res. Mutat. Res. 2014, 760, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Guichard, N.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Antineoplastic drugs and their analysis: A state of the art review. Analyst 2017, 142, 2273–2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brock, N. Oxazaphosphorine cytostatics: Past-present-future. Seventh Cain Memorial Award lecture. Cancer Res. 1989, 49, 1–7. [Google Scholar] [PubMed]
- Česen, M.; Kosjek, T.; Laimou-Geraniou, M.; Kompare, B.; Širok, B.; Lambropolou, D.; Heath, E. Occurrence of cyclophosphamide and ifosfamide in aqueous environment and their removal by biological and abiotic wastewater treatment processes. Sci. Total Environ. 2015, 527–528, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Catastini, C.; Mullot, J.-U.; Boukari, S.; Mazellier, P.; Lévi, Y.; Cervantes, P.; Ormsby, J.-N. Identification de molécules anticancéreuses dans les effluents hospitaliers. Eur. J. Water Qual. 2008, 39, 171–180. [Google Scholar] [CrossRef]
- Azuma, T.; Arima, N.; Tsukada, A.; Hirami, S.; Matsuoka, R.; Moriwake, R.; Ishiuchi, H.; Inoyama, T.; Teranishi, Y.; Yamaoka, M.; et al. Detection of pharmaceuticals and phytochemicals together with their metabolites in hospital effluents in Japan, and their contribution to sewage treatment plant influents. Sci. Total Environ. 2016, 548–549, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Steger-Hartmann, T.; Kümmerer, K.; Schecker, J. Trace analysis of the antineoplastics ifosfamide and cyclophosphamide in sewage water by twostep solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 1996, 726, 179–184. [Google Scholar] [CrossRef]
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.-G.; Xie, L. Anticancer drugs in the aquatic ecosystem: Environmental occurrence, ecotoxicological effect and risk assessment. Environ. Int. 2021, 153, 106543. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.W.-P.; Lin, Y.-C.; Wang, Y.-H.; Guo, Y.L.; Lin, A.Y.-C. Occurrence of Emerging Contaminants in Aquaculture Waters: Cross-Contamination between Aquaculture Systems and Surrounding Waters. Water Air Soil Pollut. 2018, 229, 249. [Google Scholar] [CrossRef]
- Buerge, I.J.; Buser, H.-R.; Poiger, T.; Müller, M.D. Occurrence and Fate of the Cytostatic Drugs Cyclophosphamide and Ifosfamide in Wastewater and Surface Waters. Environ. Sci. Technol. 2006, 40, 7242–7250. [Google Scholar] [CrossRef] [PubMed]
- Busetti, F.; Linge, K.; Heitz, A. Analysis of pharmaceuticals in indirect potable reuse systems using solid-phase extraction and liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 5807–5818. [Google Scholar] [CrossRef]
- De Rossi, A.; Rossi, L.; Laudisi, A.; Sini, V.; Toppo, L.; Marchesi, F.; Tortorelli, G.; Leti, M.; Turriziani, M.; Aquino, A.; et al. Focus on Fotemustine. J. Exp. Clin. Cancer Res. 2006, 25, 461. [Google Scholar]
- Raymond, E.; Boaziz, C.; Coste, M. Logistic regression model of fotemustine toxicity combining independent phase II studies. Cancer 1996, 78, 1980–1987. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Gleason, F.K.; Wood, J.M. The mechanism of action of the nitrosourea anti-tumor drugs on thioredoxin reductase, glutathione reductase and ribonucleotide reductase. Biochim. Biophys. Acta 1990, 1054, 14–20. [Google Scholar] [CrossRef]
- Boutin, J.A.; Norbeck, K.; Moldeus, P.; Genton, A.; Paraire, M.; Bizzari, J.-P.; Lavielle, G.; Cudennec, C.A. Effects of the new nitrosourea derivative, fotemustine, on the glutathione reductase activity in rat tissues in vivo and in isolated rat hepatocytes. Eur. J. Cancer Clin. Oncol. 1989, 25, 1311–1316. [Google Scholar] [CrossRef]
- Vermeulen, N.; Commandeur, J.; Groot, E.; Wormhoudt, L.; Ramnatshing, S.; Li, Q.; Brakenhoff, J. Toxicity of fotemustine in rat hepatocytes and mechanism-based protection against it. Chem. Interact. 1998, 110, 139–158. [Google Scholar] [CrossRef]
- Lee, M.S. Oxidative conversion by rat liver microsomes of 2-naphthyl isothiocyanate to 2-naphthyl isocyanate, a genotoxicant. Chem. Res. Toxicol. 1992, 5, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Jeevaratnam, K.; Vaidyanathan, C.S. Acute toxicity of methyl isocyanate in rabbit: In vitro and in vivo effects on rabbit erythrocyte membrane. Arch. Environ. Contam. Toxicol. 1992, 22, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Kennedy, A.; Stock, M.; Brown, W.; Alarie, Y. Uptake and distribution of 14C during and following exposure to [14C]methyl isocyanate. Toxicol. Appl. Pharmacol. 1988, 94, 104–117. [Google Scholar] [CrossRef]
- Karol, M.H.; Jin, R. Mechanisms of immunotoxicity to isocyanates. Chem. Res. Toxicol. 1991, 4, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Pauluhn, J.; Eben, A. Altered lung function in rats after subacute exposure to n-butyl isocyanate. Arch. Toxicol. 1992, 66, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Mac, R.C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T. Discovery of therapeutic targets by phenotype-based zebrafish screens. Drug Discov. Today Technol. 2004, 1, 49–54. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Parenti, C.C.; Ghilardi, A.; Della Torre, C.; Magni, S.; Del Giacco, L.; Binelli, A. Evaluation of the infiltration of polystyrene nanobeads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut. 2019, 254, 112947. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Liu, K.; He, Q.; Sun, C.; Han, J.; Han, L.; Tian, Q. Xiaoaiping Induces Developmental Toxicity in Zebrafish Embryos Through Activation of ER Stress, Apoptosis and the Wnt Pathway. Front. Pharmacol. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, K.; Hassan, H.M.; Guo, H.; Ding, P.; Han, L.; He, Q.; Chen, W.; Hsiao, C.-D.; Zhang, L.; et al. Liver Fatty Acid Binding Protein Deficiency Provokes Oxidative Stress, Inflammation, and Apoptosis-Mediated Hepatotoxicity Induced by Pyrazinamide in Zebrafish Larvae. Antimicrob. Agents Chemother. 2016, 60, 7347–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabbri, E.; Franzellitti, S. Human pharmaceuticals in the marine environment: Focus on exposure and biological effects in animal species. Environ. Toxicol. Chem. 2016, 35, 799–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samaee, S.-M.; Rabbani, S.; Jovanović, B.; Mohajeri-Tehrani, M.R.; Haghpanah, V. Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol. Environ. Saf. 2015, 116, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Coelhan, M.; Chan, H.M.; Ma, W.; Liu, L. Relative developmental toxicity of short-chain chlorinated paraffins in Zebrafish (Danio rerio) embryos. Environ. Pollut. 2016, 219, 1122–1130. [Google Scholar] [CrossRef]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Papiya, S.; Kanamadi, R. Effect of mercurial fungicide Emisan®-6 on the embryonic developmental stages of zebrafish, Brachydanio (Danio) rerio. J. Adv. Zool. 2000, 21, 12–18. [Google Scholar]
- Jacquillat, C.; Khayat, D.; Banzet, P.; Weil, M.; Fumoleau, P.; Avril, M.-F.; Namer, M.; Bonneterre, J.; Kerbrat, P.; Bonerandi, J.J.; et al. Final report of the french multicenter phase II study of the nitrosourea fotemustine in 153 evaluable patients with disseminated malignant melanoma including patients with cerebral metastases. Cancer 1990, 66, 1873–1878. [Google Scholar] [CrossRef]
- Bertrand, M.; Wémeau-Stervinou, L.; Gauthier, S.; Auffret, M.; Mortier, L. New toxicity of fotemustine: Diffuse interstitial lung disease. Ann. De Dermatol. Et De Venereologie 2012, 139, 277–281. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Gao, D.; Zhang, Y.; Chen, X.; Xia, Q.; Jin, M.; Sun, C.; He, Q.; Wang, R.; et al. Developmental toxicity caused by sanguinarine in zebrafish embryos via regulating oxidative stress, apoptosis and wnt pathways. Toxicol. Lett. 2021, 350, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Q.; Li, W.; Li, H.; Bao, J.; Yang, C.; Wang, A.; Wei, J.; Chen, S.; Jin, H. Role of Nrf2 in the antioxidation and oxidative stress induced developmental toxicity of honokiol in zebrafish. Toxicol. Appl. Pharmacol. 2019, 373, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Baillie, T.A.; Rettie, A.E. Role of Biotransformation in Drug-Induced Toxicity: Influence of Intra- and Inter-Species Differences in Drug Metabolism. Drug Metab. Pharmacokinet. 2011, 26, 15–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, L.; Kalgutkar, A.S.; Obach, R.S. Metabolic activation in drug-induced liver injury. Drug Metab. Rev. 2012, 44, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Kim, E.-A.; Kang, M.-C.; Lee, W.-W.; Lee, H.-S.; Vairappan, C.S.; Jeon, Y.-J. Assessment of anti-inflammatory effect of 5beta-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ. Toxicol. Pharmacol. 2014, 37, 110–117. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Liu, F.; Ye, Y.; Peng, T.; Fu, Z. Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicol. Teratol. 2015, 48, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhu, L.; Shao, B.; Zhu, S.; Wang, J.; Xie, H.; Wang, J.; Wang, F. The effects of endosulfan on cytochrome P450 enzymes and glutathione S-transferases in zebrafish (Danio rerio) livers. Ecotoxicol. Environ. Saf. 2013, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.K.-W.; Chen, Y.-J.; Fu, X.-Q.; Su, T.; Li, T.; Guo, H.; Zhu, P.-L.; Kwan, H.Y.; Cheng, B.C.-Y.; Cao, H.-H.; et al. Sensitization of melanoma cells to alkylating agent-induced DNA damage and cell death via orchestrating oxidative stress and IKKβ inhibition. Redox Biol. 2017, 11, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Landau, G.; Kodali, V.K.; Malhotra, J.D.; Kaufman, R.J. Detection of Oxidative Damage in Response to Protein Misfolding in the Endoplasmic Reticulum. Methods Enzymol. 2013, 526, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Cycle or a Double-Edged Sword? Antioxid. Redox Signal. 2007, 9, 2277–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, J.D.; Kaufman, R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007, 18, 716–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, W.; Li, F.; Zhang, J.; Wang, B.; Xiang, J. Cloning and expression of glucose regulated protein 78 (GRP78) in Fenneropenaeus chinensis. Mol. Biol. Rep. 2007, 36, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffenbach, K.T.; Lee, A.S. The critical role of GRP78 in physiologic and pathologic stress. Curr. Opin. Cell Biol. 2011, 23, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Fan, Q.; Mao, H.; Liu, Y.; Hu, C. GRP78 from grass carp (Ctenopharyngodon idella) provides cytoplasm protection against thermal and Pb2+ stress. Fish Shellfish Immunol. 2013, 34, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kaufman, R.J. The impact of the unfolded protein response on human disease. J. Cell Biol. 2012, 197, 857–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2003, 11, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Naumann, S.C.; Roos, W.; Jöst, E.; Belohlavek, C.; Lennerz, V.; Schmidt, C.; Christmann, M.; Kaina, B. Temozolomide- and fotemustine-induced apoptosis in human malignant melanoma cells: Response related to MGMT, MMR, DSBs, and p53. Br. J. Cancer 2009, 100, 322–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passagne, I.; Evrard, A.; Winum, J.-Y.; Depeille, P.; Cuq, P.; Montero, J.-L.; Cupissol, D.; Vian, L. Cytotoxicity, DNA Damage, and Apoptosis Induced by New Fotemustine Analogs on Human Melanoma Cells in Relation to O6-Methylguanine DNA-Methyltransferase Expression. J. Pharmacol. Exp. Ther. 2003, 307, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinison, J.; Aguilar, J.S.; Avalos, A.; Huang, Y.; Wang, Z.; Cameron, D.J.; Hao, J. Triptonide Effectively Inhibits Wnt/beta-Catenin Signaling via C-terminal Transactivation Domain of beta-catenin. Sci. Rep. 2016, 6, 32779. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Bae, Y.-K.; Muraoka, O.; Hibi, M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev. Biol. 2005, 279, 125–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, X.W.; Teh, C.; Korzh, V.; Wohland, T. The Secreted Signaling Protein Wnt3 Is Associated with Membrane Domains In Vivo: A SPIM-FCS Study. Biophys. J. 2016, 111, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Pandur, P.; Läsche, M.; Eisenberg, L.M.; Kühl, M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 2002, 418, 636–641. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).