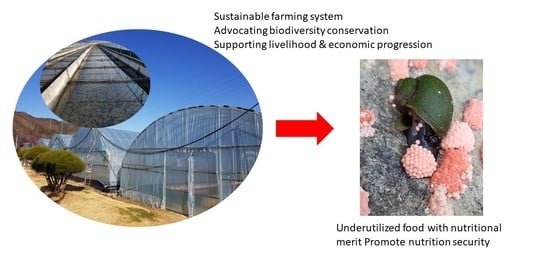
Farming the Edible Aquatic Snail Pomacea canaliculata as a Mini-Livestock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm Site
2.2. Collection of Information
2.3. Ethics Statements
3. Results
3.1. Housing
3.2. Pen
3.3. Feed
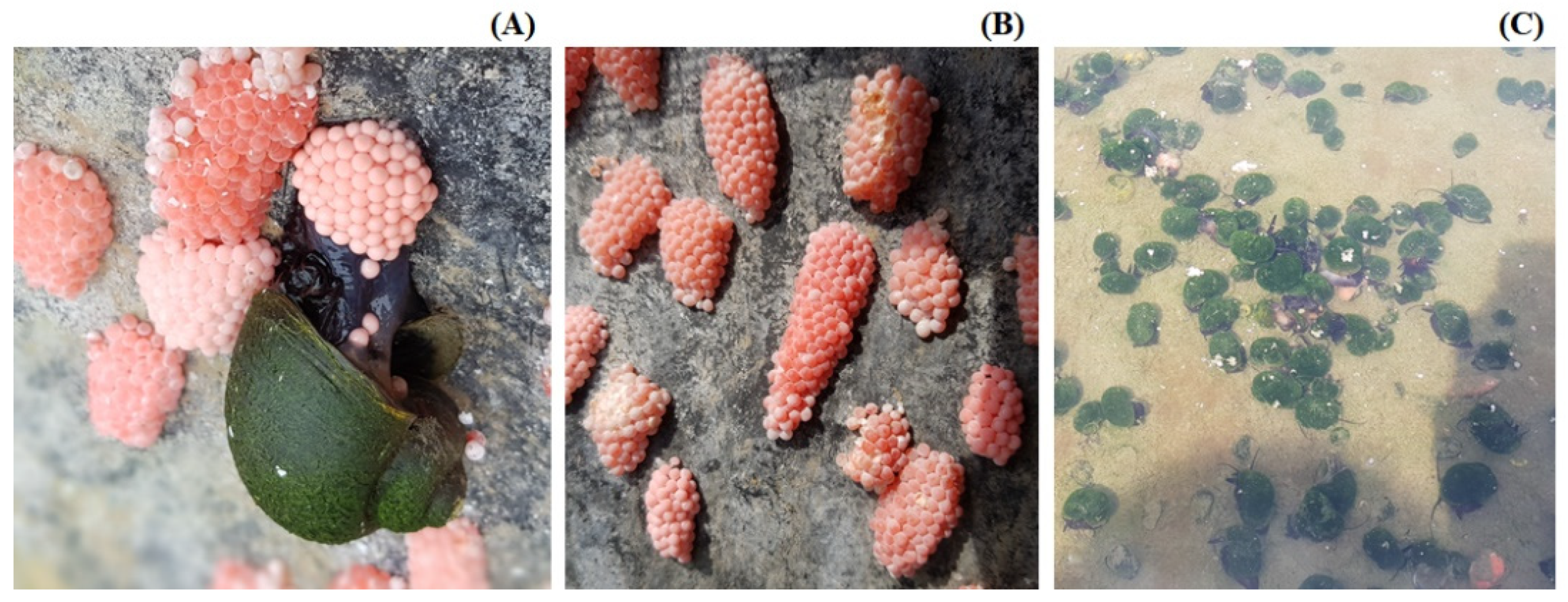
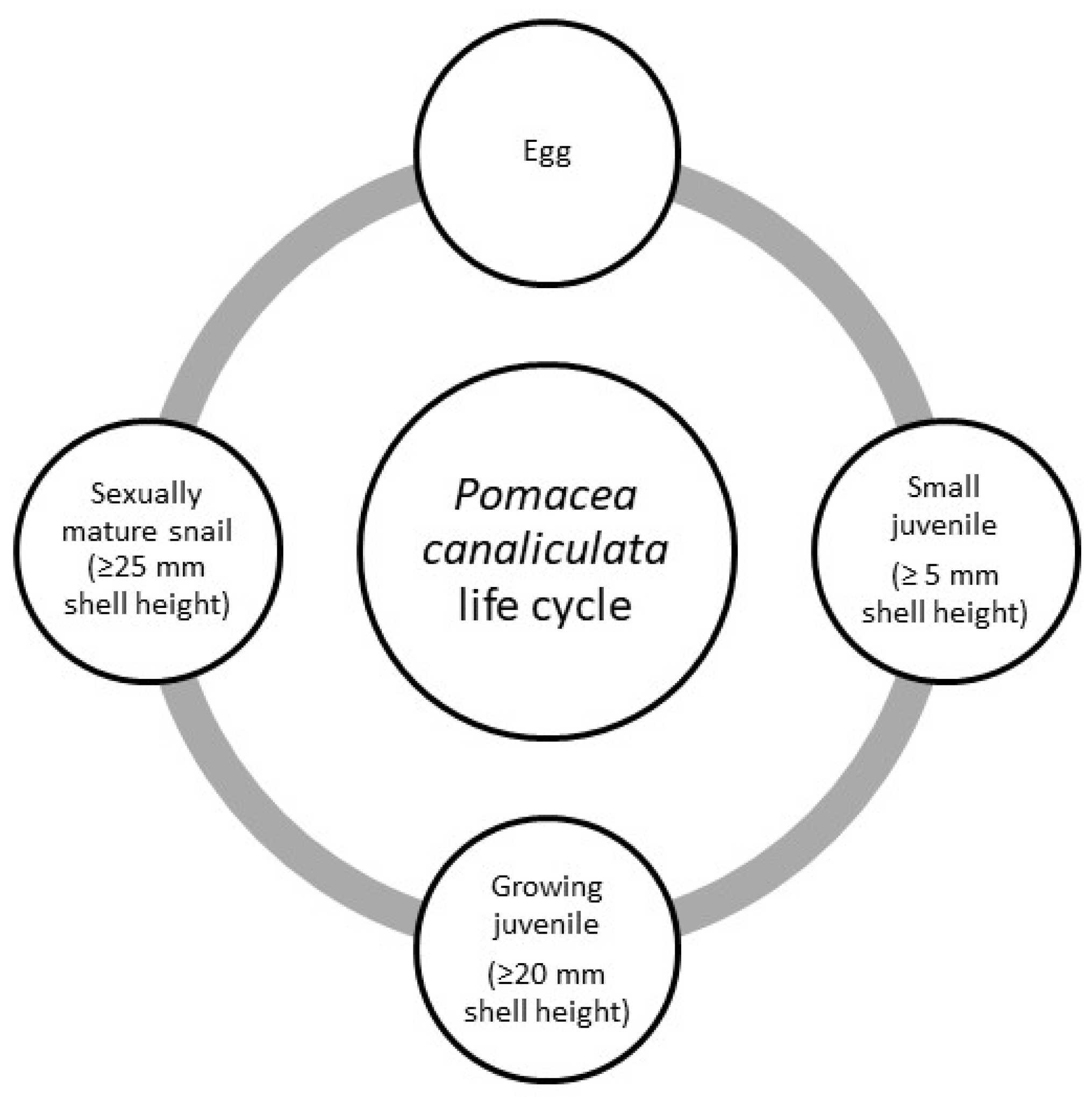
3.4. Biology and Productivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Semi-Structured Questionnaire
- The area of the snail farm.
- Housing and environmental conditions, i.e., temperature and humidity.
- Structure and measurement of pens for snail breeding and rearing.
- Reproductive age of snail.
- Fecundity, i.e., number of eggs laid by a snail at a time.
- Is there any seasonal effect on egg laying?
- Time required for hatching from egg.
- Hatchability, i.e., % of eggs likely to be hatched out of total number of eggs.
- Time taken for hatching.
- Establishment rate, i.e., % of hatchlings likely to survive.
- Growth rate in domesticated conditions.
- Requirement of feed (if any difference in feed during different stages of life).
- Requirement of water.
- Management of hatchling. Do they require being kept separately?
- Optimum density of snails in the rearing pen.
- Productivity of the farm.
References
- Gomot, A. Biochemical Composition of Helix Snails: Influence of Genetic and Physiological Factors. J. Molluscan Stud. 1998, 64, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Adeyeye, E.; Afolabi, E. Amino acid composition of three different types of land snails consumed in Nigeria. Food Chem. 2004, 85, 535–539. [Google Scholar] [CrossRef]
- Fagbuaro, O.; Oso, J.A.; Edward, J.B.; Ogunleye, R.F. Nutritional status of four species of giant land snails in Nigeria. J. Zhejiang Univ. Sci. B 2006, 7, 686–689. [Google Scholar] [CrossRef] [Green Version]
- Babalola, O.O.; Akinsoyinu, A.O. Proximate Composition and Mineral Profile of Snail Meat from Different Breeds of Land Snail in Nigeria. Pak. J. Nutr. 2009, 8, 1842–1844. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Snail as mini-livestock: Nutritional potential of farmed Pomacea canaliculata (Ampullariidae). Agric. Nat. Resour. 2017, 51, 504–511. [Google Scholar] [CrossRef]
- Envin, A.J.B.; Ekissi, G.S.E.; Sea, B.T.; Kouame, P.L. Biochemical and nutritional composition of garden snail (Limicolariaflammea) flesh consumed in Côte d’Ivoire. J. Basic Appl. Res. 2018, 4, 63–70. [Google Scholar]
- Çelik, M.Y.; Duman, M.B.; Sariipek, M.; Gören, G.U.; Öztürk, D.K.; Kocatepe, D.; Karayücel, S. Comparison of Proximate and Amino Acid Composition between Farmed and Wild Land Snails (Cornuaspersum Müller, 1774). J. Aquat. Food Prod. Technol. 2020, 29, 383–390. [Google Scholar] [CrossRef]
- Hardouin, J.; Stiévenart, C.; Codjia, J.T.C. L’Achatiniculture; FAO: Rome, Italy, 1995; Available online: http://www.fao.org/3/V6200T/v6200T0b.htm#TopOfPage (accessed on 10 February 2021).
- Ngenwi, A.A.; Mafeni, J.M.; Etchu, K.A.; Oben, F.T. Chracteristics of snail farmers and constraints to increased production in west and central Africa. Afr. J. Environ. Sci. Technol. 2010, 4, 274–278. [Google Scholar]
- Yildirim, M.Z.; Kebapçi, Ü.; Gümüş, A. Edible snails (terrestrial) of Turkey. Turk. J. Zool. 2004, 28, 329–335. [Google Scholar]
- Gheoca, V. Can Heliciculture Act as a Tool for Edible Land Snails’ Natural Populations’ Management in Romania? Manag. Sustain. Dev. 2013, 5, 21–25. [Google Scholar] [CrossRef]
- 1Ghosh, S.; Meyer-Rochow, V.B.; Jung, C. Importance of neglected traditional food to ensure health and well being. Nutr. Food Sci. Int. J. 2018, 8, 555729. [Google Scholar]
- Cunningham, S.A.; Shaikh, N.I.; Datar, A.; Chernishkin, A.E.; Patil, S.S. Food subsidies, nutrition transition, and dietary patterns in a remote Indian district. Glob. Food Secur. 2021, 29, 100506. [Google Scholar] [CrossRef]
- Elmqvist, T.; Zipperer, W.; Güneralp, B. Urbanization, habitat loss, biodiversity decline: Solution pathways to break the cycle. In The Routledge Handbook of Urbanization and Global Environmental Change; Seto, K.C., Solecki, W.D., Griffith, C.A., Eds.; Routledge: Abingdon, UK, 2015; pp. 139–151. [Google Scholar]
- Forte, A.; Zucaro, A.; DE Vico, G.; Fierro, A. Carbon footprint of heliciculture: A case study from an Italian experimental farm. Agric. Syst. 2016, 142, 99–111. [Google Scholar] [CrossRef]
- Halwart, M. The golden apple snail Pomacea canaliculatain Asian rice farming systems: Present impact and future threat. Int. J. Pest Manag. 1994, 40, 199–206. [Google Scholar] [CrossRef]
- Lee, M.-K.; Moon, J.-H.; Ryu, H.-S. Nutrient composition and protein quality of giant snail products. J. Korean Soc. Food Sci. Nutr. 1994, 23, 453–458. [Google Scholar]
- Park, I.W.; Kim, C.K. Fatty acid composition of Achatina fulica Bowdich and Ampullarius insularus. J. Korean Soc. Food Sci. Nutr. 1992, 21, 36–42. [Google Scholar]
- Lee, M.-H.; Kim, Y.-K.; Moon, H.S.; Kim, Y.A.; Yoon, N.Y.; Lim, C.-W.; Park, H.-Y.; Kim, D.-H. Antioxidant activities of five Melania snails of the genus Semisulcospirain Korea. Korean J. Fish. Aqua. Sci. 2010, 43, 188–194. [Google Scholar]
- Chang, H.; Hwang, Y. Product development and market testing of ready-to-eat mandu with pond-snail as a health food. Korean J. Community Nutr. 2006, 115, 650–660. [Google Scholar]
- Meyer-Rochow, V.B. Snails (Terrestrial and Freshwater) as Human Food. In Encyclopedia of Food Security and Sustainability; Elsevier BV: Amsterdam, The Netherlands, 2019; Volume 1, pp. 376–378. [Google Scholar] [CrossRef]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Snail farming: An Indian perspective of a potential tool for food security. Ann. Aquacult. Res. 2016, 3, 1024. [Google Scholar]
- Joshi, N.; Pandey, S. Meat demand-snailed it: A comprehensive review on snail rearing, to meet the meat demand in future India. J. Entomol. Zool. Stud. 2019, 7, 396–400. [Google Scholar]
- Salleh, N.H.M.; Arbain, D.; Daud, M.Z.M.; Pilus, N.; Nawi, R. Distribution and Management of Pomacea Canaliculata in the Northern Region of Malaysia: Mini Review. APCBEE Procedia 2012, 2, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Smallholders Foundation. Snail Farming as an Enterprise, Traning Pamphlet; Version 1; Smallholders Foundation: Owerri, Nigeria, 2013. [Google Scholar]
- Cobbinah, J.R.; Vink, A.; Onwuka, B. Snail Farming: Production, Processing and Marketing; Agrodok 47; Agromisa: Wageningen, The Netherlands, 2008. [Google Scholar]
- Tripoli, F.F.D.; Genecera, J.; Matela, M.N.; Fanuga, K.J.D.; Velasco, D.G.M.; Landero, R.S.; Cataluña, R.B.; Torres, M.A.J.; Requieron, E.A.; Bigsang, R.T. Sexual dimorphism in the shell shape of the golden apple snail, Pomacea canaliculata. Aquacult. Aquar. Conserv. Legis. Int. J. Bioflux Soc. 2015, 8, 910–923. [Google Scholar]
- Arfan, A.G.; Muhamad, R.; Omar, D.; Nor Azwady, A.A.; Manjeri, G. Comparative life cycle studies of Pomacea maculate and Pomacea canaliculata on rice (Oryza sativa). Pak. J. Agric. Sci. 2015, 52, 1079–1083. [Google Scholar]
- Estebenet, A.L.; Cazzaniga, N.J. Growth and demography of Pomacea canaliculata (Gastropoda: Ampullariidae) under laboratory conditions. Malacol. Rev. 1992, 25, 1–12. [Google Scholar]
- Yoshida, K.; Hoshikawa, K.; Wada, T.; Yusa, Y. Life cycle of the apple snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) inhabiting Japanese paddy fields. Appl. Èntomol. Zool. 2009, 44, 465–474. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Meyer-Rochow, V.B. An investigation into the morphological and behavioral adaptations of the aquatic larvae of Aquaticaleii (Coleoptera: Lampyridae) to prey upon freshwater snails that serve as intermediate hosts for the liver fluke. Biol. Control. 2012, 62, 127–134. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Liu, H.-X.; Zhang, C.-W.; Steinmann, P.; Zhou, X.-N.; Utzinger, J. Angiostrongylus cantonensis: Morphological and behavioral investigation within the freshwater snail Pomacea canaliculata. Parasitol. Res. 2009, 104, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-P.; Wu, Z.-D.; Wei, J.; Owen, R.L.; Lun, Z.-R. Human Angiostrongylus cantonensis: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 389–395. [Google Scholar] [CrossRef]
- Tsai, H.-C.; Chen, Y.-S.; Yen, C.-M. Human Parasitic Meningitis Caused by Angiostrongylus cantonensis Infection in Taiwan. Hawai’i J. Med. Public Health 2013, 72, 26–27. [Google Scholar]
- Hollyer, J.R.; Troegner, V.A.; Cowie, R.H.; Hollingsworth, R.G.; Nakamura-Tengan, L.C.; Castro, L.F.; Buchholz, A.E. Best on-farmfood Safety Practices: Reducing Risks Associated with Rat Lungworm Infection and Human Eosinophilic Meningitis. Food Safety Technol. 2010, 39. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.512.8840&rep=rep1&type=pdf (accessed on 4 November 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Meyer-Rochow, V.B.; Jung, C. Farming the Edible Aquatic Snail Pomacea canaliculata as a Mini-Livestock. Fishes 2022, 7, 6. https://doi.org/10.3390/fishes7010006
Ghosh S, Meyer-Rochow VB, Jung C. Farming the Edible Aquatic Snail Pomacea canaliculata as a Mini-Livestock. Fishes. 2022; 7(1):6. https://doi.org/10.3390/fishes7010006
Chicago/Turabian StyleGhosh, Sampat, Victor Benno Meyer-Rochow, and Chuleui Jung. 2022. "Farming the Edible Aquatic Snail Pomacea canaliculata as a Mini-Livestock" Fishes 7, no. 1: 6. https://doi.org/10.3390/fishes7010006
APA StyleGhosh, S., Meyer-Rochow, V. B., & Jung, C. (2022). Farming the Edible Aquatic Snail Pomacea canaliculata as a Mini-Livestock. Fishes, 7(1), 6. https://doi.org/10.3390/fishes7010006