Modelling Fish Growth with Imperfect Data: The Case of Trachurus picturatus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Sampling and Analysis
2.2. Ageing Assignment and Precision
2.3. Back-Calculation of Lengths
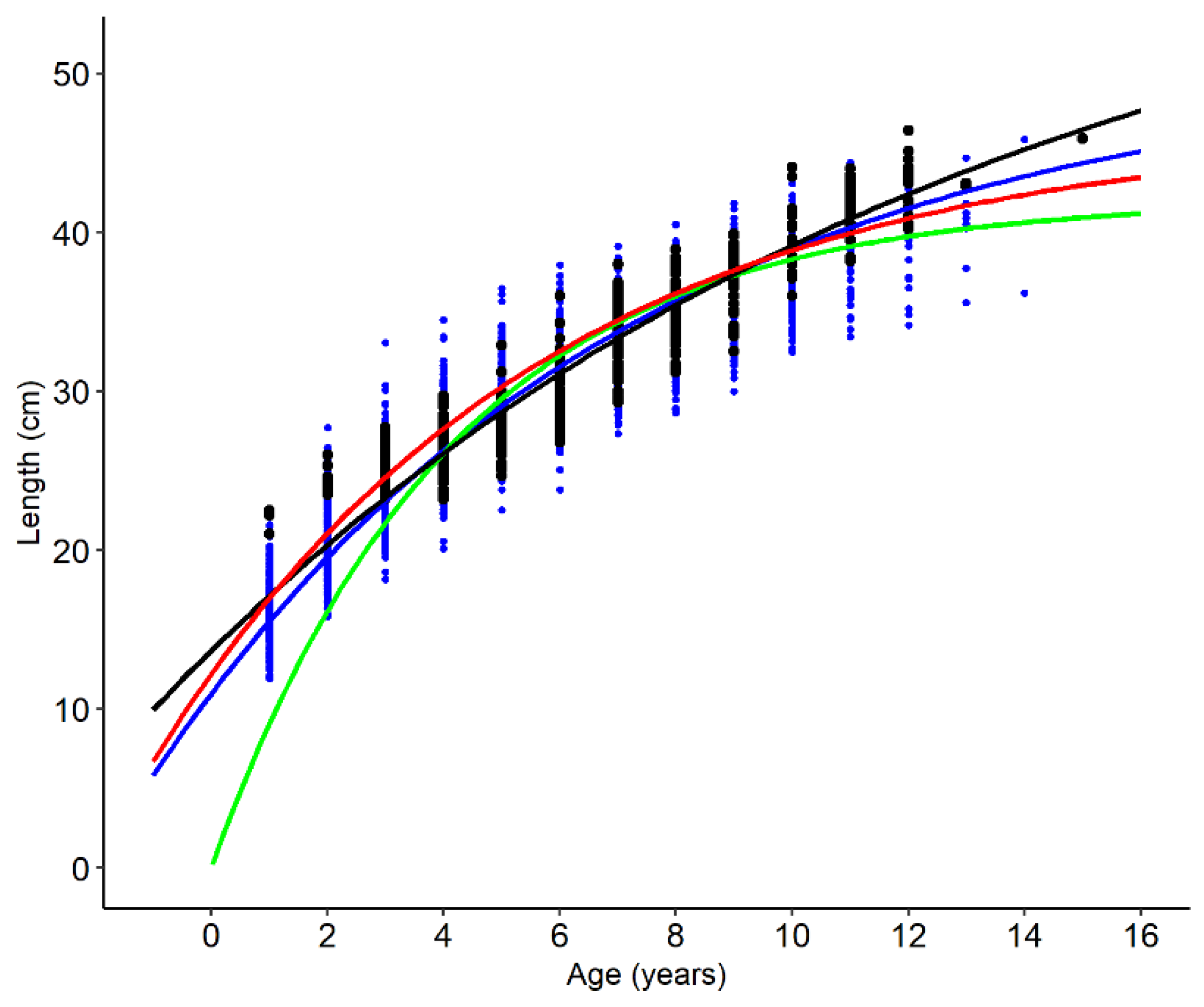
2.4. Growth Modelling
2.5. Mortality
3. Results
3.1. Ageing Precision
3.2. Growth Modelling
3.3. Mortality
4. Discussion
4.1. Ageing Precision
4.2. Growth Modelling
4.3. Age and Growth
4.4. Mortality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Labropoulou, M.; Papaconstantinou, C. Comparison of otolith growth and somatic growth in two macrourid fishes. Fish. Res. 2000, 46, 177–188. [Google Scholar] [CrossRef]
- Flinn, S.A.; Midway, S.R. Trends in Growth Modelling in Fisheries Science. Fishes 2021, 6, 1. [Google Scholar] [CrossRef]
- Gwinn, D.C.; Allen, M.S.; Rogers, M.W. Evaluation of procedures to reduce bias in fish growth parameter estimates resulting from size-selective sampling. Fish. Res. 2010, 105, 75–79. [Google Scholar] [CrossRef]
- Francis, R.I.C.C. Back-calculation of fish length: A critical review. J. Fish. Biol. 1990, 36, 883–902. [Google Scholar] [CrossRef]
- Siegfried, K.I.; Sanso, B. Two Bayesian methods for estimating parameters of the von Bertalanffy growth equation. Environ. Biol. Fishes. 2006, 77, 301–308. [Google Scholar] [CrossRef]
- Bijak, J.; Bryant, J. Bayesian demography 250 years after Bayes. Popul. Stud. 2016, 70, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doll, J.C.; Jacquemin, S.J. Introduction to Bayesian modeling and inference for fisheries scientists. Fisheries 2018, 43, 152–161. [Google Scholar] [CrossRef]
- Smart, J.J.; Grammar, G.L. Modernising fish and shark growth curves with Bayesian length-at-age models. PLoS ONE 2021, 16, e0246734. [Google Scholar] [CrossRef]
- Smith-Vaniz, W.F. Carangidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonense, E., Eds.; UNESCO: Paris, France, 1986; Volume 2, pp. 815–844. [Google Scholar]
- INE. Instituto Nacional de Estatística—Estatísticas da Pesca; INE: Lisboa, Portugal, 2019; Available online: https://www.ine.pt/xurl/pub/435690295 (accessed on 15 July 2021).
- ICES. Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA). ICES Sci. Rep. 2019, 1, 34. [Google Scholar]
- Jurado-Ruzafa, A.; Santamaría, M.T.G. Age, growth and natural mortality of blue jack mackerel Trachurus picturatus (Carangidae) from the Canary Islands, Spain (NW Africa). Afr. J. Mar. Sci. 2018, 40, 451–460. [Google Scholar] [CrossRef]
- Beamish, R.J.; Fournier, D.A. A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- Chang, W.Y.B. A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci. 1982, 39, 1208–1210. [Google Scholar] [CrossRef]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis; R Package Version 0.8.32. 2021. Available online: https://github.com/droglenc/FSA (accessed on 20 June 2021).
- RStudio Team. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2021. [Google Scholar]
- Vigliola, L.; Meekan, M.G. The back-calculation of fish growth from otoliths. In Tropical Fish Otoliths: Information for Assessment, Management and Ecology; Green, B., Mapstone, B., Carlos, G., Begg, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 174–211. [Google Scholar]
- Sakamoto, Y.; Ishiguro, M.; Kitagawa, G. Akaike Information Criterion Statistics; D. Reidel: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Nelson, G.A. Fishmethods: Fishery Science Methods and Models; R Package Version 1.11-1. 2019. Available online: https://CRAN.R-project.org/package=fishmethods (accessed on 11 February 2020).
- Stan Development Team. RStan: The R Interface to Stan; R package version 2.21.2. 2020. Available online: https://mc-stan.org/ (accessed on 10 October 2020).
- Doll, J.C.; Jacquemin, S.J. Bayesian model selection in fisheries management and ecology. J. Fish Wildl. Manag. 2019, 10, 691–707. [Google Scholar] [CrossRef] [Green Version]
- Gabry, J.; Mahr, T. Bayesplot: Plotting for Bayesian Models; R Package Version 1.8.0. 2021. Available online: https://mc-stan.org/bayesplot (accessed on 1 February 2021).
- Chapman, D.G.; Robson, D.S. The analysis of a catch curve. Biometrics 1960, 16, 354–368. [Google Scholar] [CrossRef]
- Smith, M.W.; Then, A.Y.; Wor, C.; Ralph, G.; Pollock, K.H.; Hoenig, J.M. Recommendations for catch-curve analysis. N. Am. J. Fish. Manag. 2012, 32, 956–967. [Google Scholar] [CrossRef]
- Then, A.Y.; Hoenig, J.M.; Hall, N.G.; Hewitt, D.A. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Gislason, H.; Daan, N.; Rice, J.C.; Pope, J.G. Size, growth, temperature and the natural mortality of marine fish. Fish Fish. 2010, 11, 149–158. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- ICES. Workshop on Age reading of Horse Mackerel, Mediterranean Horse Mackerel and Blue Jack Mackerel (Trachurus trachurus, T. mediterraneus and T. picturatus) (WKARHOM3). ICES CM 2018/EOSG. Livorno, Italy. 2018, Volume 28. Available online: https://archimer.ifremer.fr/doc/00471/58264/60778.pdf (accessed on 10 March 2021).
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Garcia, A.; Pereira, J.G.; Canha, A.; Reis, D.; Diogo, H. Life history parameters of blue jack mackerel Trachurus picturatus (Teleostei: Carangidae) from northeast Atlantic. J. Mar. Biol. Assoc. 2015, 95, 401–410. [Google Scholar] [CrossRef]
- Gallucci, V.F.; Quinn, T.J., II. Reparameterizing, fitting, and testing a simple growth model. Trans. Am. Fish. Soc. 1979, 108, 14–25. [Google Scholar] [CrossRef]
- Isidro, H.A. Age and Growth of Trachurus picturatus (Bowdich, 1825) (Teleostei: Carangidae) from the Azores. Arquipel.—Life Earth Sci. 1990, 8, 45–54. [Google Scholar]
- Jesus, G.T. Study of the Growth and Reproduction of Trachurus picturatus (Bowdich, 1825) in Madeira; Direcção Regional das Pescas: Horta, Portugal, Doc. Nº 1991, Internal Publication; 1992. [Google Scholar]
- Vasconcelos, J.; Alves, A.; Gouveia, E.; Faria, G. Age and growth of the blue jack mackerel, Trachurus picturatus Bowdich, 1825 (Pisces: Teleostei) off Madeira archipelago. Arquipel.—Life Mar. Sci. 2006, 23A, 47–57. [Google Scholar]
- Johnson, K.F.; Monnahan, C.C.; McGilliard, C.R.; Vertpre, K.A.; Anderson, S.C.; Cunningham, C.J.; Hurtado-Ferro, F.; Licandeo, R.R.; Muradian, M.L.; Ono, K.; et al. Time varying natural mortality in fisheries stock assessment models: Identifying a default approach. ICES J. Mar. Sci. 2015, 72, 137–150. [Google Scholar] [CrossRef] [Green Version]
- Patterson, K. Fisheries for small pelagic species: An empirical approach to management targets. Rev. Fish Biol. Fish. 1992, 2, 321–338. [Google Scholar] [CrossRef]
- Beddington, J.R.; Agnew, D.J.; Clark, C.W. Current Problems in the Management of Marine Fisheries. Science 2007, 316, 1713–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICES. Blue jack mackerel (Trachurus picturatus) in Subdivision 10.a.2 (Azores grounds). In Report of the ICES Advisory Committee, 2020; ICES Advice 2020, jaa.27.10a2; ICES: Copenhagen, Denmark, 2020; Available online: https://www.ccrup.eu/wp-content/uploads/2021/01/ICES_PC_2020_Blue-jack-mackerel-Trachurus-picturatus-in-Subdivision-10.a.2-Azores-grounds_EN.pdf (accessed on 10 March 2021).
- Vasconcelos, J.; Sousa, R.; Riera, R.; Delgado, J.; Faria, G.; Gordo, L. Stock assessment of the blue jack mackerel, Trachurus picturatus, in the North-eastern Atlantic. Fish Manag. Ecol. 2018, 25, 233–239. [Google Scholar] [CrossRef]
| Reader1 vs. Reader2 | Reader1 vs. Reader3 | Reader2 vs. Reader3 | |
|---|---|---|---|
| APE | 4.05 | 3.33 | 4.42 |
| CV | 5.72 | 4.71 | 6.25 |
| PAgree | 56.81 | 62.08 | 53.75 |
| PAgree ± 1 | 92.03 | 95.34 | 91.47 |
| N | 459 | 490 | 427 |
| N | Mean (cm) | Range (cm) | ||
|---|---|---|---|---|
| DR | Females | 276 | 32.0 | 23.4–46.4 |
| Males | 299 | 31.9 | 21.0–44.6 | |
| BC | Females | 176 | 33.3 | 23.5–46.4 |
| Males | 200 | 33.9 | 22.5–44.6 |
| VBGF | Gompertz | Logistic | ||
|---|---|---|---|---|
| AIC | AIC | AIC | ||
| DR | Females | 1301 | 1315 | 1324 |
| Males | 1135 | 1154 | 1167 | |
| Overall | 2432 | 2464 | 2485 | |
| BC | Females | 5223 | 5253 | 5289 |
| Males | 4837 | 4860 | 4887 | |
| Overall | 10,070 | 10,123 | 10,185 | |
| BC | DR | DRfix | DRBayes | ||
|---|---|---|---|---|---|
| Parameter | nls Estimates | Prior | MCMC Posterior | ||
| L∞ | 46.68 (45.52–47.83) | 63.31 (56.33–70.29) | 42.03 (41.19–42.88) | N(50, 5) | 50.89 (48.78–53.21) |
| k | 0.15 (0.14–0.16) | 0.07 (0.06–0.09) | 0.24 (0.23–0.25) | U(0, 0.5) | 0.12 (0.11–0.13) |
| t0 | −2.04 (−2.19–−1.89) | −3.37 (−3.93–−2.80) | 0 | N(0, 0.2) | −2.01 (−1.73–−2.29) |
| σ | 2.412 | 2.03 | 2.734 | U(0, 5) | 2.06 (1.94–2.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, A.; Vieira, A.R.; Sequeira, V.; Silva, E.; Silva, F.; Duarte, A.M.; Mendes, S.; Ganhão, R.; Assis, C.; Rebelo, R.; et al. Modelling Fish Growth with Imperfect Data: The Case of Trachurus picturatus. Fishes 2022, 7, 52. https://doi.org/10.3390/fishes7010052
Neves A, Vieira AR, Sequeira V, Silva E, Silva F, Duarte AM, Mendes S, Ganhão R, Assis C, Rebelo R, et al. Modelling Fish Growth with Imperfect Data: The Case of Trachurus picturatus. Fishes. 2022; 7(1):52. https://doi.org/10.3390/fishes7010052
Chicago/Turabian StyleNeves, Ana, Ana Rita Vieira, Vera Sequeira, Elisabete Silva, Frederica Silva, Ana Marta Duarte, Susana Mendes, Rui Ganhão, Carlos Assis, Rui Rebelo, and et al. 2022. "Modelling Fish Growth with Imperfect Data: The Case of Trachurus picturatus" Fishes 7, no. 1: 52. https://doi.org/10.3390/fishes7010052
APA StyleNeves, A., Vieira, A. R., Sequeira, V., Silva, E., Silva, F., Duarte, A. M., Mendes, S., Ganhão, R., Assis, C., Rebelo, R., Magalhães, M. F., Gil, M. M., & Gordo, L. S. (2022). Modelling Fish Growth with Imperfect Data: The Case of Trachurus picturatus. Fishes, 7(1), 52. https://doi.org/10.3390/fishes7010052












