The Effect of Fishery Management on the Yield of the Critically Endangered European Eel Anguilla anguilla in Mesotrophic Rivers and Streams in Central Europe
Abstract
:1. Introductions
2. Materials and Methods
2.1. Study Area
2.2. Ecosystem Types
2.3. Fish Stocking Data
2.4. Eel Angling
2.5. Otter and Cormorant Population Data
2.6. Environmental Data
2.7. Fisheries and Biological Data
2.8. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Copp, G.H.; Daverat, F.; Bašić, T. The potential contribution of small coastal streams to the conservation of declining and threatened diadromous fishes, especially the European eel. River Res. Appl. 2020, 37, 111–115. [Google Scholar] [CrossRef]
- Baisez, A.; Laffaille, P. Strategy of recreational anglers and estimated eel (Anguilla anguilla) catches in a large European catchment basin, the Loire (France). Knowl. Manag. Aquat. Ecosyst. 2008, 390–391, 3. [Google Scholar] [CrossRef] [Green Version]
- Bernotas, P.; Vetemaa, M.; Saks, L.; Eschbaum, R.; Verliin, A.; Järvalt, A. Dynamics of European eel landings and stocks in the coastal waters of Estonia. ICES J. Mar. Sci. 2016, 73, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, H.; Crook, V. Eel Market Dynamics: An Analysis of Anguilla Production; TRAFFIC: Tokyo, Japan, 2015. [Google Scholar]
- Dekker, W. The history of commercial fisheries for European eel commenced only a century ago. Fish. Manag. Ecol. 2019, 26, 6–19. [Google Scholar] [CrossRef]
- Lyach, R.; Čech, M. A new trend in Central European recreational fishing: More fishing visits but lower yield and catch. Fish. Res. 2018, 201, 131–137. [Google Scholar] [CrossRef]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL). ICES Sci. Rep. 2019, 1, 177. [Google Scholar] [CrossRef]
- European Commission. Council Regulation (EC) No 1100/2007 of 18 September 2007 establishing measures for the recovery of the stock of European eel. Off. J. Eur. Union 2007, 248, 17–23. [Google Scholar]
- Belpaire, C.; Geeraerts, C.; Evans, D.; Ciccotti, E.; Poole, R. The European eel quality database: Towards a pan-European monitoring of eel quality. Environ. Monit. Assess. 2011, 183, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorow, M.; Beardmore, B.; Haider, W.; Arlinghaus, R. Using a novel survey technique to predict fisheries stakeholders’ support for European eel (Anguilla anguilla L.) conservation programs. Biol. Conserv. 2009, 142, 2973–2982. [Google Scholar] [CrossRef]
- Dorow, M.; Arlinghaus, R. The relationship between personal commitment to angling and the opinions and attitudes of German anglers towards the conservation and management of the European eel Anguilla anguilla. N. Am. J. Fish. Manag. 2012, 32, 466–479. [Google Scholar] [CrossRef]
- Feunteun, E. Management and restoration of European eel population (Anguilla anguilla): An impossible bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Garrido, F.E.; Ruiz-Ruiz, J.; Lafuente-Fernández, R.; Villafuerte, R. Exploring perceptions to manage conflicts: The case of the European eel fishing moratorium in Southern Spain. Hum. Dimens. Wildl. 2020, 25, 136–153. [Google Scholar] [CrossRef]
- Essl, K.; Gassner, H.; Schabetsberger, R.; Jagsch, A.; Kaiser, R. The development of stocked eels (Anguilla anguilla) in previously eel-free A ustrian A lpine lakes. Ecol. Freshw. Fish 2016, 25, 17–26. [Google Scholar] [CrossRef]
- Matondo, B.; Benitez, J.P.; Dierckx, A.; Rollin, X.; Ovidio, M. An Evaluation of Restocking Practice and Demographic Stock Assessment Methods for Cryptic Juvenile European Eel in Upland Rivers. Sustainability 2020, 12, 1124. [Google Scholar] [CrossRef] [Green Version]
- Van Ginneken, V.J.; Maes, G.E. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 2005, 15, 367–398. [Google Scholar] [CrossRef]
- Bernotas, P.; Öğlü, B.; Nõges, P. How do environmental factors affect the yield of European eel (Anguilla anguilla) in a restocked population? Fish. Res. 2020, 230, 105649. [Google Scholar] [CrossRef]
- Bevacqua, D.; Melià, P.; Schiavina, M.; Crivelli, A.J.; De Leo, G.A.; Gatto, M. A demographic model for the conservation and management of the European eel: An application to a Mediterranean coastal lagoon. ICES J. Mar. Sci. 2019, 76, 2164–2178. [Google Scholar] [CrossRef]
- Teichert, N.; Tétard, S.; Trancart, T.; de Oliveira, E.; Acou, A.; Carpentier, A.; Bourillon, B.; Feunteun, E. Towards transferability in fish migration models: A generic operational tool for predicting silver eel migration in rivers. Sci. Total Environ. 2020, 739, 140069. [Google Scholar] [CrossRef]
- Weltersbach, M.S.; Strehlow, H.V.; Ferter, K.; Klefoth, T.; de Graaf, M.; Dorow, M. Estimating and mitigating post-release mortality of European eel by combining citizen science with a catch-and-release angling experiment. Fish. Res. 2018, 201, 98–108. [Google Scholar] [CrossRef]
- Beardmore, B.; Dorow, M.; Haider, W.; Arlinghaus, R. The elasticity of fishing effort response and harvest outcomes to altered regulatory policies in eel (Anguilla anguilla) recreational angling. Fish. Res. 2011, 110, 136–148. [Google Scholar] [CrossRef]
- Ayllón, D.; Nicola, G.G.; Elvira, B.; Almodóvar, A. Optimal harvest regulations under conflicting tradeoffs between conservation and recreational fishery objectives. Fish. Res. 2019, 216, 47–58. [Google Scholar] [CrossRef]
- Lai, E.K.; Mueller, U.; Hyndes, G.A.; Ryan, K.L. Comparing estimates of catch and effort for boat-based recreational fishing from aperiodic access-point surveys. Fish. Res. 2019, 219, 105305. [Google Scholar] [CrossRef]
- Lyach, R.; Remr, J. Changes in recreational catfish Silurus glanis harvest rates between years 1986–2017 in Central Europe. J. Appl. Ichthyol. 2019, 35, 1094–1104. [Google Scholar] [CrossRef]
- Lyach, R. The effect of a large-scale angling restriction in minimum angling size on harvest rates, recapture rates, and average body weight of harvested common carps Cyprinus carpio. Fish. Res. 2020, 223, 105438. [Google Scholar] [CrossRef]
- Lyach, R. The effect of fishing effort, fish stocking, and population density of overwintering cormorants on the harvest and recapture rates of three rheophilic fish species in central Europe. Fish. Res. 2020, 223, 105440. [Google Scholar] [CrossRef]
- Lyach, R. The effects of fisheries management on harvest rates of native and non-native salmonid fish species. J. Appl. Ichthyol. 2020, 36, 298–314. [Google Scholar] [CrossRef]
- Jankovský, M.; Boukal, D.S.; Pivnička, K.; Kubečka, J. Tracing possible drivers of synchronously fluctuating species catches in individual logbook data. Fish. Manag. Ecol. 2011, 18, 297–306. [Google Scholar] [CrossRef]
- Post, J.R.; Mushens, C.; Paul, A.; Sullivan, M. Assessment of alternative harvest regulations for sustaining recreational fisheries: Model development and application to bull trout. N. Am. J. Fish. Manag. 2003, 23, 22–34. [Google Scholar] [CrossRef]
- Askey, P.J.; Johnston, N.T. Self-regulation of the Okanagan Lake kokanee recreational fishery: Dynamic angler effort response to varying fish abundance and productivity. N. Am. J. Fish. Manag. 2013, 33, 926–939. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Berlin, Germany, 2007. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) FishBase 2000: Concepts, Design and Data Sources; ICLARM: Los Baños, Philippines, 2000; 344p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing.; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 15 November 2021).
- Hadfield, J.D. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.; Schielzeth, H. The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Coelho, R.; Infante, P.; Santos, M.N. Comparing GLM, GLMM, and GEE modeling approaches for catch rates of bycatch species: A case study of blue shark fisheries in the South Atlantic. Fish. Oceanogr. 2020, 29, 169–184. [Google Scholar] [CrossRef]
- Humpl, M.; Pivnička, K.; Jankovský, M. Sport fishery statistics, water quality, and fish assemblages in the Berounka River in 1975–2005. Folia Zool. 2009, 58, 457–465. [Google Scholar]
- Boukal, D.S.; Jankovský, M.; Kubečka, J.; Heino, M. Stock–catch analysis of carp recreational fisheries in Czech reservoirs: Insights into fish survival, water body productivity and impact of extreme events. Fish. Res. 2012, 119, 23–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.F.; Wang, K. Explorations of Charm Factors and Development of Fishing in Southern Taiwan Based on Miryoku Engineering and the Analytic Network Process. Sustainability 2019, 11, 737. [Google Scholar] [CrossRef] [Green Version]
- Glatzel, G. The impact of historic land use and modern forestry on nutrient relations of Central European forest ecosystems. Fertil. Res. 1991, 27, 1–8. [Google Scholar] [CrossRef]
- Van der Oost, R.; Heida, H.; Satumalay, K.; van Schooten, F.J.; Ariese, F.; Vermeulen, N.P. Bioaccumulation, biotransformation and DNA binding of PAHs in feral eel (Anguilla anguilla) exposed to polluted sediments: A field survey. Environ. Toxicol. Chem. Int. J. 1994, 13, 859–870. [Google Scholar] [CrossRef]
- Conroy, J.W.; Chanin, P.R. The status of the Eurasian otter (Lutra lutra) in Europe. A Review. J. Int. Otter Surviv. Fund 2000, 1, 7–28. [Google Scholar]
- Sures, B.; Knopf, K. Parasites as a threat to freshwater eels? Science 2004, 304, 209–211. [Google Scholar] [CrossRef]
- Marzano, M.; Carss, D.N.; Cheyne, I. Managing European cormorant-fisheries conflicts: Problems, practicalities and policy. Fish. Manag. Ecol. 2013, 20, 401–413. [Google Scholar] [CrossRef]
- Piper, A.T.; Wright, R.M.; Walker, A.M.; Kemp, P.S. Escapement, route choice, barrier passage and entrainment of seaward migrating European eel, Anguilla anguilla, within a highly regulated lowland river. Ecol. Eng. 2013, 57, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Meulenbroek, P.; Hammerschmied, U.; Schmutz, S.; Weiss, S.; Schabuss, M.; Zornig, H.; Shumka, S.; Schiemer, F. Conservation Requirements of European Eel (Anquilla anquilla) in a Balkan Catchment. Sustainability 2020, 12, 8535. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Cooke, S.J.; Lyman, J.; Policansky, D.; Schwab, A.; Suski, C.; Sutton, S.G.; Thorstad, E.B. Understanding the complexity of catch-and-release in recreational fishing: An integrative synthesis of global knowledge from historical, ethical, social, and biological perspectives. Rev. Fish. Sci. 2007, 15, 75–167. [Google Scholar] [CrossRef]
- Hunt, L.M.; Arlinghaus, R.; Lester, N.; Kushneriuk, R. The effects of regional angling effort, angler behavior, and harvesting efficiency on landscape patterns of overfishing. Ecol. Appl. 2011, 21, 2555–2575. [Google Scholar] [CrossRef]
- Storck, T.; Newman, D. Effects of size at stocking on survival and harvest of channel catfish. N. Am. J. Fish. Manag. 1988, 8, 98–101. [Google Scholar] [CrossRef]
- Michaletz, P.H.; Wallendorf, M.J.; Nicks, D.M. Effects of stocking rate, stocking size, and angler catch inequality on exploitation of stocked channel catfish in small Missouri impoundments. N. Am. J. Fish. Manag. 2008, 28, 1486–1497. [Google Scholar] [CrossRef]
- Persat, H.; Mattersdorfer, K.; Charlat, S.; Schenekar, T.; Weiss, S. Genetic integrity of the European grayling (Thymallus thymallus) populations within the Vienne River drainage basin after five decades of stockings. Cybium 2016, 40, 7–20. [Google Scholar]
- Slavík, O.; Horký, P.; Randák, T.; Balvín, P.; Bílý, M. Brown trout spawning migration in fragmented Central European headwaters: Effect of isolation by artificial obstacles and the moon phase. Trans. Am. Fish. Soc. 2012, 141, 673–680. [Google Scholar] [CrossRef]
- Dekker, W.; Beaulaton, L. Climbing back up what slippery slope? Dynamics of the European eel stock and its management in historical perspective. ICES J. Mar. Sci. 2016, 73, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Winter, H.V.; Jansen, H.M.; Bruijs, M.C.M. Assessing the impact of hydropower and fisheries on downstream migrating silver eel, Anguilla anguilla, by telemetry in the River Meuse. Ecol. Freshw. Fish 2006, 15, 221–228. [Google Scholar] [CrossRef]
- Belpaire, C.G.J.; Goemans, G.; Geeraerts, C.; Quataert, P.; Parmentier, K.; Hagel, P.; De Boer, J. Decreasing eel stocks: Survival of the fattest? Ecol. Freshw. Fish 2009, 18, 197–214. [Google Scholar] [CrossRef]
- Myers, J.P. Conservation of migrating shorebirds: Staging areas, geographic bottlenecks, and regional movements. Am. Birds 1983, 37, 23–25. [Google Scholar]
- Oberhauser, K.S.; Solensky, M.J. (Eds.) Monarch Butterfly Biology & Conservation; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Harris, G.; Thirgood, S.; Hopcraft, J.G.C.; Cromsigt, J.P.; Berger, J. Global decline in aggregated migrations of large terrestrial mammals. Endanger. Species Res. 2009, 7, 55–76. [Google Scholar] [CrossRef]
- Lennox, R.J.; Paukert, C.P.; Aarestrup, K.; Auger-Méthé, M.; Baumgartner, L.; Birnie-Gauvin, K.; Boe, K.; Brink, K.; Brownscombe, J.W.; Chen, Y.; et al. One hundred pressing questions on the future of global fish migration science, conservation, and policy. Front. Ecol. Evol. 2019, 7, 286. [Google Scholar] [CrossRef] [Green Version]
- Aarestrup, K.; Økland, F.; Hansen, M.M.; Righton, D.; Gargan, P.; Castonguay, M.; Bernatchez, L.; Howey, P.; Sparholt, H.; Pedersen, M.I.; et al. Oceanic spawning migration of the European eel (Anguilla anguilla). Science 2009, 325, 1660. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, M.W.; Evans, D.W.; Briand, C.; Walker, A.M.; McElarney, Y.; Allen, M. The changing times of Europe’s largest remaining commercially harvested population of eel Anguilla anguilla L. J. Fish Biol. 2021, 99, 1201–1221. [Google Scholar] [CrossRef] [PubMed]
- Essig, R.J.; Holliday, M.C. Development of a recreational fishing survey: The marine recreational fishery statistics survey case study. Am. Fish. Soc. 1991, 12, 245–254. [Google Scholar]
- Pollock, K.H.; Jones, C.M.; Brown, T.L. Angler Survey Methods and Their Applications in Fisheries Management; American Fisheries Society Special Publication 25: Bethesda, MD, USA, 1994; 371p. [Google Scholar]
- Cooke, S.J.; Dunlop, W.I.; McLennan, D.M.; Power, G. Applications and characteristics of angler diary programs in Ontario, Canada. Fish. Manag. Ecol. 2000, 7, 473–487. [Google Scholar] [CrossRef] [Green Version]
- Bray, G.S.; Schramm, H.L. Evaluation of a state-wide volunteer angler diary program for use as a fishery assessment tool. N. Am. J. Fish. Manag. 2001, 21, 606–615. [Google Scholar] [CrossRef]
- Mosindy, T.E.; Duffy, M.J. The use of angler diary surveys to evaluate long-term changes in muskellunge populations on Lake of the Woods, Ontario. Environ. Biol. Fishes 2007, 7, 71–83. [Google Scholar] [CrossRef]
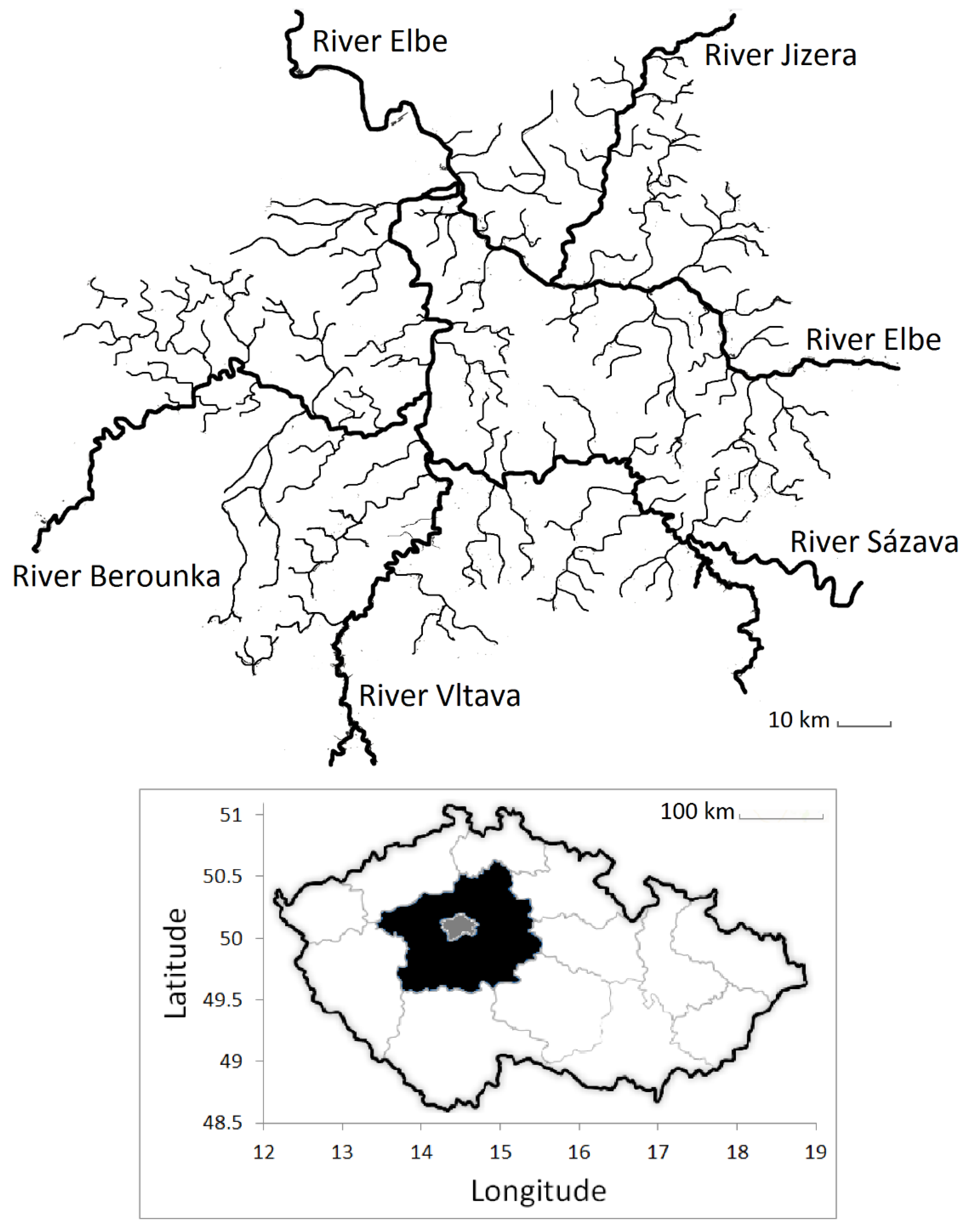
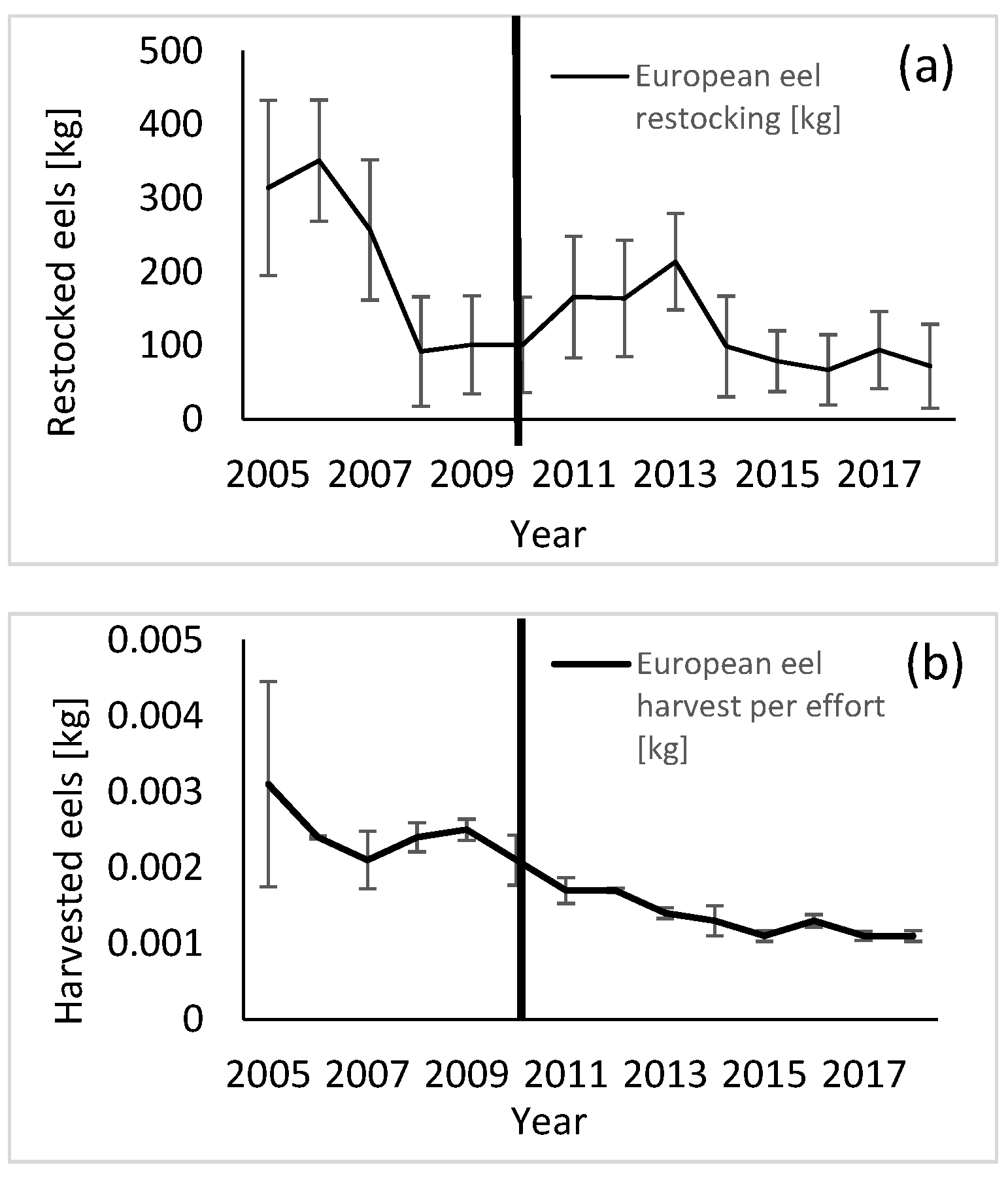
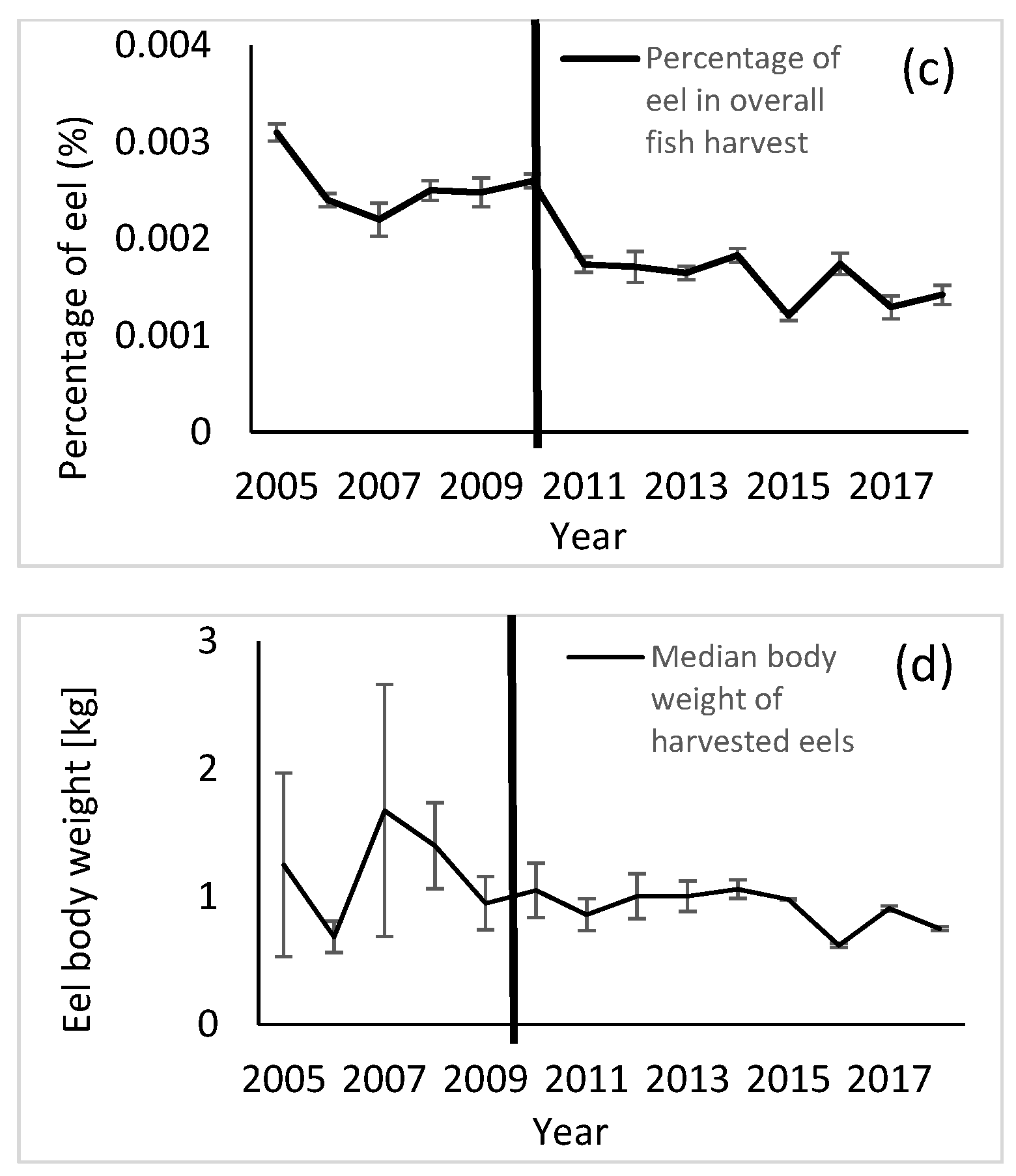
| Parameter | Study Group (Median) | Control Group (Median) | p-Value (Medians) | F-Value (Variances) | p-Value (Variances) |
|---|---|---|---|---|---|
| Eel yield (kg per effort) | 0.0007 | 0.0009 | 0.84 | 0.76 | 0.09 |
| Regulated fishes yield (kg per effort) | 0.027 | 0.018 | 0.79 | 1.09 | 0.21 |
| Non-regulated fishes yield (kg per effort) | 0.74 | 0.68 | 0.06 | 1.79 | 0.06 |
| Angling effort (anglers per ha) | 53.8 | 61.2 | 0.12 | 0.62 | 0.11 |
| Size of stocked eels (kg per eel) | 0.002 | 0.002 | 0.48 | 8.08 | 0.08 |
| Stocking intensity of elver eels (kg per ha) | 0.18 | 0.14 | 0.31 | 1.27 | 0.18 |
| Stocking intensity of yellow eels (kg per ha) | 0.31 | 0.26 | 0.07 | 1.96 | 0.23 |
| Fish biomass (kg per ha) | 215 | 238 | 0.63 | 1.01 | 0.86 |
| Nutrient intake (kg per ha) | 101 | 89 | 0.77 | 1.03 | 0.64 |
| Water discharge (m3 per s) | 31.6 | 29.5 | 0.73 | 0.94 | 0.38 |
| Otter population density (n per ha) | 1.5 | 1.1 | 0.65 | 0.98 | 0.76 |
| Cormorant population density (n per ha) | 4.2 | 4.7 | 0.19 | 1.03 | 0.66 |
| Biomass of eels (kg per ha) | 0.38 | 0.43 | 0.06 | 1.95 | 0.07 |
| River obstacles (n per 100 m) | 0.12 | 0.08 | 0.74 | 1.02 | 0.83 |
| Bank to surface ratio | 5.1 | 5.4 | 0.68 | 0.99 | 0.92 |
| The % of eel in the overall fish yield | 0.006 | 0.004 | 0.41 | 0.71 | 0.58 |
| Fisheries Parameter | Value over Years 2005–2018 |
|---|---|
| angling visits | 9,524,561.000 |
| angling visits per hectare | 177.399 |
| biomass of harvested fish (kg) | 6,451,829.000 |
| biomass of harvested fish per hectare (kg) | 120.168 |
| biomass of harvested fish per effort per hectare (kg) | 0.677 |
| biomass of harvested European eel (kg) | 41,276.000 |
| biomass of harvested European eel per hectare (kg) | 0.769 |
| biomass of harvested European eel per effort per hectare (kg) | 0.004 |
| median size of harvested fish (kg) | 1.010 |
| median size of harvested European eel (kg) | 0.610 |
| % of European eel in overall fish harvest | 0.006 |
| biomass of stocked fish (kg) | 4,518,967.000 |
| biomass of stocked fish per hectare (kg) | 84.168 |
| biomass of stocked European eel (kg) | 26,288.000 |
| biomass of stocked European eel per hectare (kg) | 0.490 |
| % of European eel in overall biomass of stocked fish | 0.006 |
| average size of stocked European eel (kg) | 0.002 |
| % of fishing sites with harvest of European eel | 0.570 |
| (A) | ||||||
| Response Variable | Fixed Variables | Estimate | 95 CI: Low | 95 CI: Up | SD (Slope) | p-Value |
| Yield of European eel (kg per effort) | (intercept) | 1.60 × 10−1 | −7.04 × 10−2 | −4.78 × 10−2 | 6.43 × 10−2 | 0.01 * |
| Angling effort (anglers per ha) | −8.15 × 10−6 | −1.12 × 10−5 | −5.86 × 10−6 | 4.46 × 10−6 | 0.03 * | |
| Size of stocked eels (kg per eel) | 1.24 × 10−1 | 8.92 × 10−2 | 1.70 × 10−1 | 5.27 × 10−2 | 0.08 | |
| Stocking intensity of elver eels (kg per ha) | 1.56 × 10−4 | 1.13 × 10−4 | 2.14 × 10−4 | 1.32 × 10−4 | 0.38 | |
| Stocking intensity of yellow eels (kg per ha) | 1.36 × 10−5 | 9.81 × 10−6 | 1.87 × 10−5 | 1.12 × 10−5 | 0.26 | |
| Fish biomass (kg per ha) | −2.96 × 10−4 | −4.02 × 10−4 | −2.13 × 10−4 | 1.76 × 10−4 | 0.09 | |
| Nutrient intake (kg per ha) | −3.23 × 10−4 | −4.40 × 10−4 | −2.33 × 10−4 | 5.15 × 10−4 | 0.53 | |
| Water discharge (m3 per s) | −3.08 × 10−5 | −4.18 × 10−5 | −2.22 × 10−5 | 6.37 × 10−4 | 0.51 | |
| Otter population density (n per ha) | −8.01 × 10−3 | −1.09 × 10−2 | −5.77 × 10−3 | 6.95 × 10−3 | 0.25 | |
| Cormorant population density (n per ha) | −4.14 × 10−3 | −5.63 × 10−3 | −2.98 × 10−3 | 2.45 × 10−3 | 0.09 | |
| Yield of non-regulated fishes (kg per effort) | −6.40 × 10−6 | −8.71 × 10−6 | −4.61 × 10−6 | 2.49 × 10−4 | 0.09 | |
| Yield of regulated fishes (kg per effort) | 5.81 × 10−7 | 4.18 × 10−7 | 7.90 × 10−7 | 3.50 × 10−6 | 0.19 | |
| Biomass of eels (kg per ha) | −7.22 × 10−6 | −9.82 × 10−6 | −5.20 × 10−6 | 1.11 × 10−5 | 0.52 | |
| River obstacles (n per 100 m) | −5.10 × 10−4 | −6.94 × 10−4 | −3.67 × 10−4 | 1.41 × 10−3 | 0.72 | |
| Bank to surface ratio | −2.23 × 10−6 | −3.04 × 10−6 | −1.61 × 10−6 | 3.10 × 10−4 | 0.31 | |
| (B) | ||||||
| Response Variable | Fixed Variables | Estimate | 95 CI: Low | 95 CI: Up | SD (Slope) | p-Value |
| Yield of European eel (kg per effort) | (intercept) | 3.23 × 10−2 | 1.45 × 10−2 | 4.91 × 10−2 | 2.41 × 10−2 | 0.18 |
| Angling effort (anglers per ha) | −1.31 × 10−6 | −2.83 × 10−6 | 2.06 × 10−7 | 1.52 × 10−6 | 0.03 * | |
| Size of stocked eels (kg per eel) | 2.87 × 10−1 | −1.19 × 10+00 | 1.76 × 10+00 | 1.48 × 10+00 | 0.86 | |
| Stocking intensity of elver eels (kg per ha) | −1.36 × 10−4 | −2.58 × 10−4 | −1.47 × 10−5 | 1.22 × 10−4 | 0.30 | |
| Stocking intensity of yellow eels (kg per ha) | 1.82 × 10−5 | 1.07 × 10−5 | 2.57 × 10−5 | 7.50 × 10−6 | 0.39 | |
| Fish biomass (kg per ha) | −2.35 × 10−6 | −6.83 × 10−5 | 6.36 × 10−5 | 6.59 × 10−5 | 0.25 | |
| Nutrient intake (kg per ha) | −2.40 × 10−4 | −4.32 × 10−4 | −4.79 × 10−5 | 1.92 × 10−4 | 0.21 | |
| Water discharge (m3 per s) | 6.51 × 10−5 | −1.75 × 10−4 | 3.05 × 10−4 | 2.40 × 10−4 | 0.79 | |
| Otter population density (n per ha) | −2.11 × 10−3 | −4.74 × 10−3 | 5.18 × 10−4 | 2.63 × 10−3 | 0.42 | |
| Cormorant population density (n per ha) | −3.13 × 10−4 | −1.25 × 10−3 | 6.21 × 10−4 | 9.34 × 10−4 | 0.74 | |
| Yield of non-regulated fishes (kg per effort) | −2.90 × 10−5 | −1.68 × 10−4 | 1.10 × 10−4 | 1.39 × 10−4 | 0.71 | |
| Yield of regulated fishes (kg per effort) | −8.58 × 10−7 | −2.27 × 10−6 | 5.56 × 10−7 | 1.41 × 10−6 | 0.54 | |
| Biomass of eels (kg per ha) | −1.83 × 10−6 | −6.27 × 10−6 | 2.62 × 10−6 | 4.45 × 10−6 | 0.68 | |
| River obstacles (n per ha) | −2.64 × 10−5 | −5.56 × 10−4 | 5.04 × 10−4 | 5.30 × 10−4 | 0.42 | |
| Bank to surface ratio | 1.03 × 10−4 | 9.15 × 10−5 | 1.14 × 10−4 | 1.15 × 10−5 | 0.37 | |
| Response Variable | Fixed Variables | Estimate | 95 CI: Low | 95 CI: Up | SD (Slope) | p-Value |
| Yield of European eel (kg per effort) | (intercept) | 2.08 × 10−2 | 1.24 × 10−2 | 2.70 × 10−2 | 8.11 × 10−3 | <0.01 * |
| time | −1.52 × 10−2 | −2.03 × 10−2 | −9.30 × 10−3 | 9.07 × 10−3 | 0.04 * | |
| management | −2.12 × 10−2 | −2.70 × 10−2 | −1.24 × 10−2 | 1.70 × 10−2 | <0.01 * | |
| time: management | −2.57 × 10−2 | −3.38 × 10−2 | −1.55 × 10−2 | 1.80 × 10−2 | <0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyach, R. The Effect of Fishery Management on the Yield of the Critically Endangered European Eel Anguilla anguilla in Mesotrophic Rivers and Streams in Central Europe. Fishes 2022, 7, 42. https://doi.org/10.3390/fishes7010042
Lyach R. The Effect of Fishery Management on the Yield of the Critically Endangered European Eel Anguilla anguilla in Mesotrophic Rivers and Streams in Central Europe. Fishes. 2022; 7(1):42. https://doi.org/10.3390/fishes7010042
Chicago/Turabian StyleLyach, Roman. 2022. "The Effect of Fishery Management on the Yield of the Critically Endangered European Eel Anguilla anguilla in Mesotrophic Rivers and Streams in Central Europe" Fishes 7, no. 1: 42. https://doi.org/10.3390/fishes7010042
APA StyleLyach, R. (2022). The Effect of Fishery Management on the Yield of the Critically Endangered European Eel Anguilla anguilla in Mesotrophic Rivers and Streams in Central Europe. Fishes, 7(1), 42. https://doi.org/10.3390/fishes7010042






