Ontogeny of the Respiratory Area in Relation to Body Mass with Reference to Resting Metabolism in the Japanese Flounder, Paralichthys olivaceus (Temminck & Schlegel, 1846)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Specimens
2.2. Measurement of Various Body Areas
2.3. Filament Length
2.4. Spacing
2.5. Lamellar Area
2.6. Measurement of Cutaneous Area
2.7. Data Analysis
3. Results
3.1. Gill Development
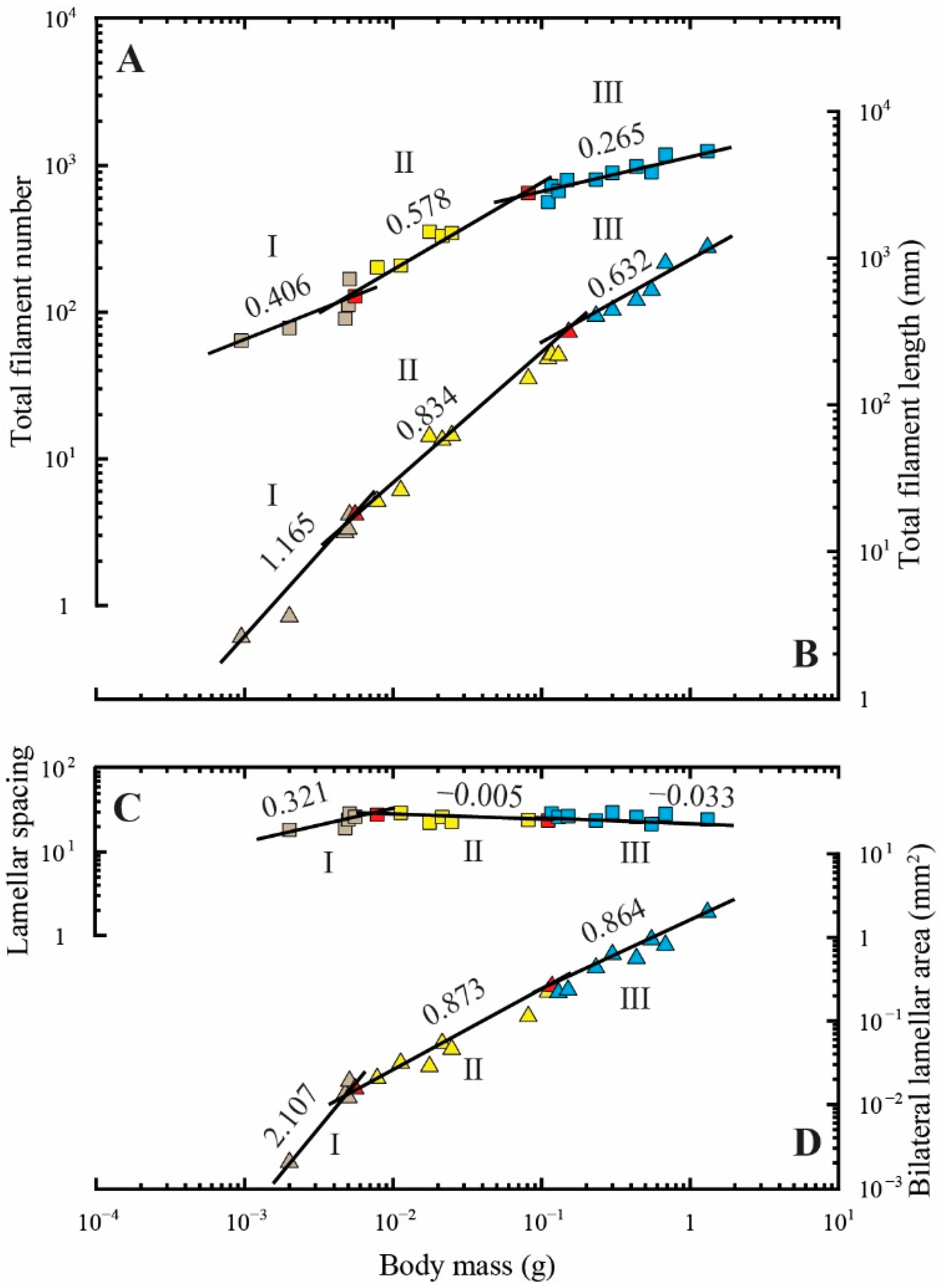
3.2. Regression Analyses of Gill Area Measurements
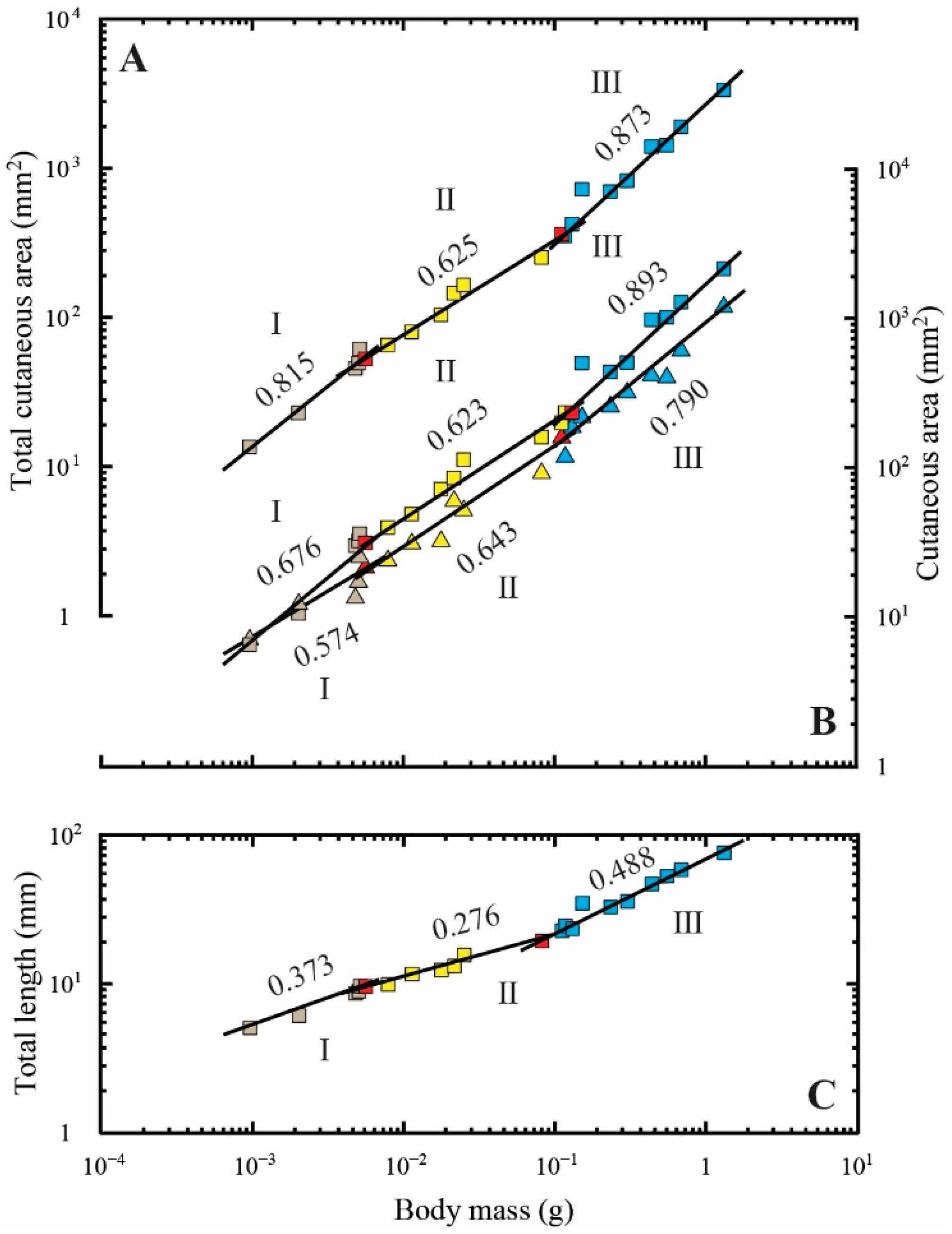
3.3. Regression Analyses of Cutaneous Area Measurements
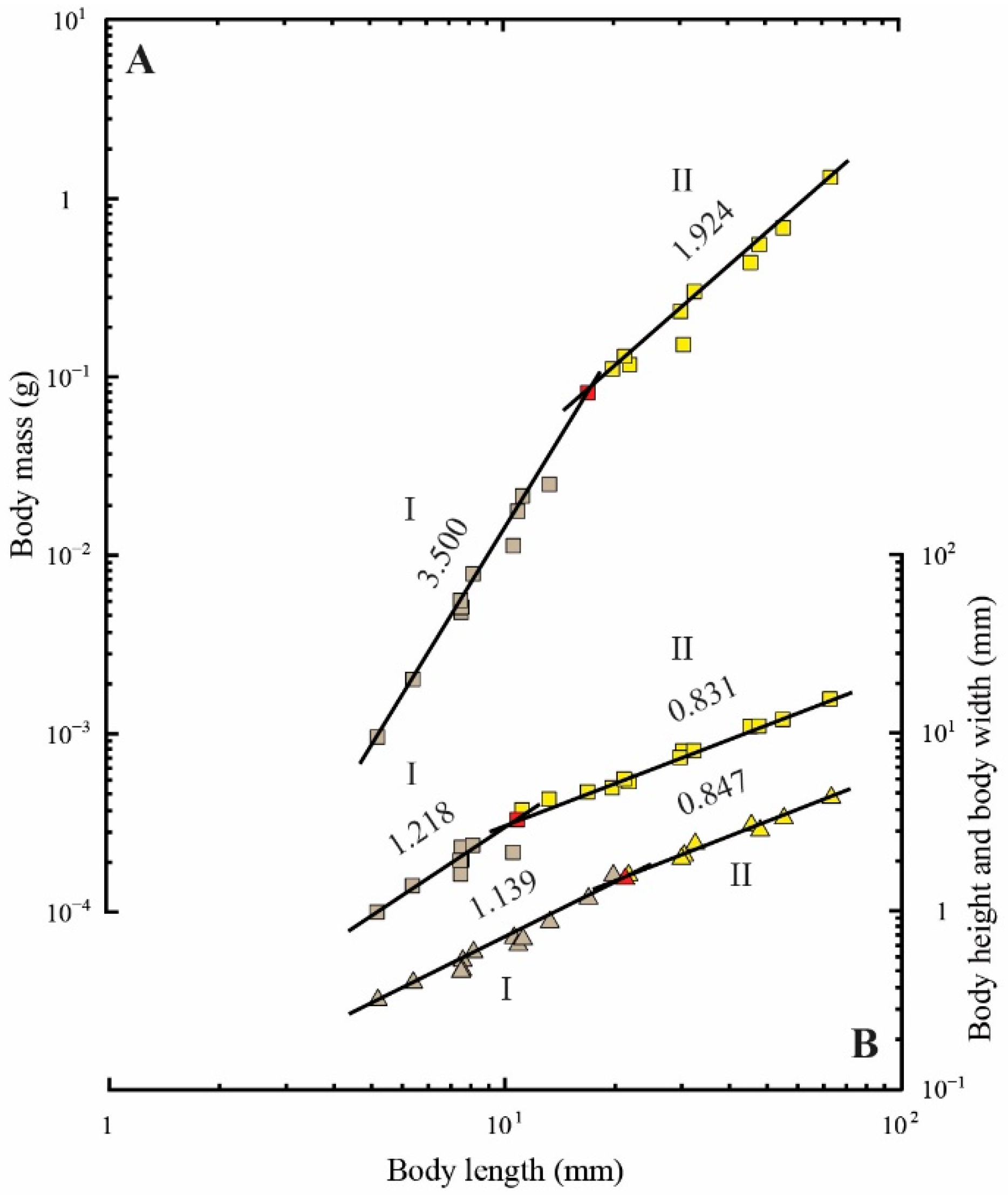
3.4. Regression Analyses of Body Form Measurements
4. Discussion
4.1. Gill Morphometry
4.2. Body Morphometry
4.3. Relationship between Respiratory Area and Metabolism
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozłowski, J.; Teriokhin, A.T. Allocation of energy between growth and reproduction: The Pontryagin Maximum Principle solution for the case of age-and season-dependent mortality. Evol. Ecol. Res. 1999, 1, 423–441. [Google Scholar]
- Czarnołęski, M.; Kozłowski, J.; Stańczykowska, A.; Lewandowski, K. Optimal resource allocation explains growth. Evol. Ecol. Res. 2003, 5, 571–587. [Google Scholar]
- Hou, C.; Zuo, W.; Moses, M.E.; Woodruff, W.H.; Brown, J.H.; West, G.B. Energy uptake and allocation during ontogeny. Science 2008, 322, 736–739. [Google Scholar] [CrossRef] [Green Version]
- Yagi, M.; Kanda, T.; Takeda, T.; Ishimatsu, A.; Oikawa, S. Ontogenetic phase shifts in metabolism: Links to development and anti-predator adaptation. Proc. Royal Soc. B 2010, 277, 2793–2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenfeld, J.; Van Leeuwen, T.; Richards, J.; Allen, D. Relationship between growth and standard metabolic rate: Measurement artefacts and implications for habitat use and life-history adaptation in salmonids. J. Anim. Ecol. 2015, 84, 4–20. [Google Scholar] [CrossRef]
- Glazier, D.S. Is Metabolic Rate a Universal ‘pacemaker’for Biological Processes? Biol. Rev. 2015, 90, 377–407. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.A.; Svendsen, M.B.; Steffensen, J.F. The combined effect of body size and temperature on oxygen consumption rates and the size-dependency of preferred temperature in European perch Perca fluviatilis. J. Fish Biol. 2020, 97, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A. The Respiratory Exchange of Animals and Man; Longmans: London, UK, 1916. [Google Scholar]
- Kleiber, M. Body Size and metabolism. Hilgardia 1932, 6, 315–353. [Google Scholar] [CrossRef] [Green Version]
- Brody, S.; Lardy, H.A. Bioenergetics and growth. J. Phys. Chem. 1946, 50, 168–169. [Google Scholar] [CrossRef]
- Zeuthen, E. Oxygen uptake as related to body size in organisms. Q. Rev. Biol. 1953, 28, 1–12. [Google Scholar] [CrossRef]
- Winberg, G. Rate of metabolism and food requirements of fish. Transl. Fish. Res. Board Can. 1956, 194, 1–253. [Google Scholar]
- Hemmingsen, A. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep. Steno Meml Hosp. 1960, 9, 3–110. [Google Scholar]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Glazier, D.S. The 3/4-power law is not universal: Evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 2006, 56, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Moses, M.E.; Hou, C.; Woodruff, W.H.; West, G.B.; Nekola, J.C.; Zuo, W.; Brown, J.H. Revisiting a model of ontogenetic growth: Estimating model parameters from theory and data. Am. Nat. 2008, 171, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Body-mass scaling of metabolic rate: What are the relative roles of cellular versus systemic effects? Biology 2015, 4, 187–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, B.W.; Labecka, A.M.; Gudowska, A.; Antoł, A.; Sikorska, A.; Szabla, N.; Bauchinger, U.; Kozlowski, J.; Czarnoleski, M. Concerted evolution of body mass, cell size and metabolic rate among carabid beetles. J. Insect Physiol. 2021, 132, 104272. [Google Scholar] [CrossRef]
- Darveau, C.; Suarez, R.K.; Andrews, R.D.; Hochachka, P.W. Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 2002, 417, 166–170. [Google Scholar] [CrossRef]
- White, C.R.; Seymour, R.S. Mammalian basal metabolic rate is proportional to body mass2/3. Proc. Natl. Acad. Sci. USA 2003, 100, 4046–4049. [Google Scholar] [CrossRef] [Green Version]
- Kozlowski, J.; Konarzewski, M.; Gawelczyk, A.T. Cell size as a link between noncoding DNA and metabolic rate scaling. Proc. Natl. Acad. Sci. USA 2003, 100, 14080–14085. [Google Scholar] [CrossRef] [Green Version]
- Bokma, F. Evidence against universal metabolic allometry. Funct. Ecol. 2004, 18, 184–187. [Google Scholar] [CrossRef]
- Glazier, D.S. Beyond the ‘3/4-power law’: Variation in the intra-and interspecific scaling of metabolic rate in animals. Biol. 2005, 80, 611–662. [Google Scholar]
- Duncan, R.P.; Forsyth, D.M.; Hone, J. Testing the metabolic theory of ecology: Allometric scaling exponents in mammals. Ecol. 2007, 88, 324–333. [Google Scholar] [CrossRef]
- Glazier, D.S. Effects of metabolic level on the body size scaling of metabolic rate in birds and mammals. Proc. Royal Soc. B 2008, 275, 1405–1410. [Google Scholar] [CrossRef] [Green Version]
- Glazier, D.S. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 2010, 85, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Kolokotrones, T.; Savage, V.; Deeds, E.J.; Fontana, W. Curvature in metabolic scaling. Nature 2010, 464, 753–756. [Google Scholar] [CrossRef]
- White, C.R. There is no single p. Nature 2010, 464, 691–693. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Kearney, M.R. Metabolic scaling in animals: Methods, empirical results, and theoretical explanations. Compr. Physiol. 2011, 4, 231–256. [Google Scholar]
- Kearney, M.R.; White, C.R. Testing metabolic theories. Am. Nat. 2012, 180, 546–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleiber, M. The Fire of Life. An Introduction to Animal Energetics; Wiley: New York, NY, USA, 1961. [Google Scholar]
- Schmidt-Nielsen, K.; Knut, S. Scaling: Why is Animal Size so Important? Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Calder, W.A. Size, Function, and Life History; Harvard University Press: Cambridge, MA, USA, 1996. [Google Scholar]
- West, G.B.; Brown, J.; Enquist, B. Scaling in Biology: Patterns and Processes, Causes and Consequences; Oxford University Press: New York, NY, USA, 2000; Volume 87, p. 112. [Google Scholar]
- Savage, V.M.; Gillooly, J.F.; Woodruff, W.H.; West, G.B.; Allen, A.P.; Enquist, B.J.; Brown, J.H. The predominance of quarter-power scaling in biology. Funct. Ecol. 2004, 18, 257–282. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H. The origin of allometric scaling laws in biology from genomes to ecosystems: Towards a quantitative unifying theory of biological structure and organization. J. Exp. Biol. 2005, 208, 1575–1592. [Google Scholar] [CrossRef] [Green Version]
- West, G.B.; Brown, J.H.; Enquist, B.J. The fourth dimension of life: Fractal geometry and allometric scaling of organisms. Science 1999, 284, 1677–1679. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Banavar, J.R.; Moses, M.E.; Brown, J.H.; Damuth, J.; Rinaldo, A.; Sibly, R.M.; Maritan, A. A general basis for quarter-power scaling in animals. Proc. Natl. Acad. Sci. USA 2010, 107, 15816–15820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodds, P.S. Optimal form of branching supply and collection networks. Phys. Rev. Lett. 2010, 104, 048702. [Google Scholar] [CrossRef] [Green Version]
- Kooijman, B.; Kooijman, S. Dynamic Energy Budget Theory for Metabolic Organisation; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Price, C.A.; Weitz, J.S.; Savage, V.M.; Stegen, J.; Clarke, A.; Coomes, D.A.; Dodds, P.S.; Etienne, R.S.; Kerkhoff, A.J.; McCulloh, K. Testing the metabolic theory of ecology. Ecol. Lett. 2012, 15, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.G. Intraspecific scaling of mass to length in pelagic animals: Ontogenetic shape change and its implications. Limnol. Oceanogr. 2012, 57, 1579–1590. [Google Scholar] [CrossRef] [Green Version]
- Glazier, D.S. Metabolic scaling in complex living systems. Systems 2014, 2, 451–540. [Google Scholar] [CrossRef] [Green Version]
- Hirst, A.G.; Glazier, D.S.; Atkinson, D. Body shape shifting during growth permits tests that distinguish between competing geometric theories of metabolic scaling. Ecol. Lett. 2014, 17, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Ivleva, I. The dependence of crustacean respiration rate on body mass and habitat temperature. Int. Rev. Hydrobiol. 1980, 65, 1–47. [Google Scholar] [CrossRef]
- Dodds, P.S.; Rothman, D.H.; Weitz, J.S. Re-examination of the “3/4-law” of metabolism. J. Theor. Biol. 2001, 209, 9–27. [Google Scholar] [CrossRef] [Green Version]
- White, C.R.; Phillips, N.F.; Seymour, R.S. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2006, 2, 125–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.R.; Frappell, P.B.; Chown, S.L. An information-theoretic approach to evaluating the size and temperature dependence of metabolic rate. Proc. Royal Soc. B 2012, 279, 3616–3621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brett, J.; Groves, T. Physiological energetics. Fish Physiol. 1979, 8, 280–352. [Google Scholar]
- Glazier, D.S.; Hirst, A.G.; Atkinson, D. Shape shifting predicts ontogenetic changes in metabolic scaling in diverse aquatic invertebrates. Proc. Royal Soc. B 2015, 282, 20142302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubner, M. Ueber Den Einfluss Der Korpergrosse Auf Stoffund Kaftwechsel. Z. Biol. 1883, 19, 535–562. [Google Scholar]
- Okie, J.G. General models for the spectra of surface area scaling strategies of cells and organisms: Fractality, geometric dissimilitude, and internalization. Am. Nat. 2013, 181, 421–439. [Google Scholar] [CrossRef] [PubMed]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for the origin of allometric scaling laws in biology. Science 1997, 276, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.M.; Deeds, E.J.; Fontana, W. Sizing up allometric scaling theory. PLoS Comput. Biol. 2008, 4, e1000171. [Google Scholar] [CrossRef]
- Price, C.A.; Enquist, B.J.; Savage, V.M. A general model for allometric covariation in botanical form and function. Proc. Natl. Acad. Sci. USA 2007, 104, 13204–13209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Hirst, A.G.; Glazier, D.S.; Atkinson, D. Ecological pressures and the contrasting scaling of metabolism and body shape in coexisting taxa: Cephalopods versus teleost fish. Philos. Trans. R. Soc. 2019, 374, 20180543. [Google Scholar] [CrossRef]
- Post, J.R.; Lee, J.A. Metabolic ontogeny of teleost fishes. Can. J. Fish. Aquat. Sci. 1996, 53, 910–923. [Google Scholar] [CrossRef]
- Yagi, M.; Oikawa, S. Ontogenetic phase shifts in metabolism in a flounder Paralichthys olivaceus. Sci. Rep. 2014, 4, 7135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, J.W. Growth and Gill Development in the Small-Mouthed Black Bass, Micropterus Dolomieu Lacépede; Ohio State University Press: Columbus, OH, USA, 1931; pp. 1–46. [Google Scholar]
- Boczkowska-Smyk, W. Development of the respiratory surface in the gills of the rainbow-trout (Salmo irideus Gibb.). Acta Biol. Crac. Ser. Zool. 1961, 4, 89–109. [Google Scholar]
- Ursin, E. A mathematical model of some aspects of fish growth, respiration, and mortality. J. Fish. Res. 1967, 24, 2355–2453. [Google Scholar] [CrossRef]
- Muir, B. Gill dimensions as a function of fish size. J. Fish. Res. 1969, 26, 165–170. [Google Scholar] [CrossRef]
- Muir, B.; Hughes, G. Gill dimensions for three species of tunny. J. Exp. Biol. 1969, 51, 271–285. [Google Scholar] [CrossRef]
- Hughes, G.M. Morphological measurements on the gills of fishes in relation to their respiratory function. Folia. Morphol. 1970, 18, 78–95. [Google Scholar]
- Hughes, G.; Dube, S.; Munshi, J.D. Surface area of the respiratory organs of the Climbing perch, Anabas testudineus (Pisces: Anabantidae). J. Zool. 1973, 170, 227–243. [Google Scholar] [CrossRef]
- Hughes, G.; Singh, B.; Guha, G.; Dube, S.; Munshi, J.D. Respiratory surface areas of an air-breathing siluroid fish Saccobranchus (Heteropneustes) fossilis in relation to body size. J. Zool. 1974, 172, 215–232. [Google Scholar] [CrossRef]
- De Silva, C. Development of the respiratory system in herring and plaice larvae. In The Early Life History of Fish; Spinger: Berlin/Heidelberg, Germany, 1974; pp. 465–485. [Google Scholar]
- Holeton, G. Respiratory morphometrics of white and red blooded antarctic fish. Comp. Biochem. Physiol. 1976, 54, 215–219. [Google Scholar] [CrossRef]
- Hakim, A.; Munshi, J.D.; Hughes, G. Morphometries of the respiratory organs of the Indian green snake-headed fish, Channa Punctata. J. Zool. 1978, 184, 519–553. [Google Scholar] [CrossRef]
- Ellenby, C. Relation between body size and metabolism. Nature 1937, 140, 853. [Google Scholar] [CrossRef]
- Edwards, R. The Relation of Oxygen Consumption to Body Size and to Temperature in the Larvae of Chironomus Riparius Meigen. J. Exp. Biol. 1958, 35, 383–395. [Google Scholar] [CrossRef]
- Ultsch, G.R. Respiratory surface area as a factor controlling the standard rate of O2 consumption of aquatic salamanders. Respir. Physiol. 1976, 26, 357–369. [Google Scholar] [CrossRef]
- Oikawa, S.; Itazawa, Y. Gill and body surface areas of the carp in relation to body mass, with special reference to the metabolism-size relationship. J. Exp. Biol. 1985, 117, 1–14. [Google Scholar] [CrossRef]
- Oikawa, S.; Hirata, M.; Kita, J.; Itazawa, Y. Ontogeny of respiratory area of a marine teleost, porgy, Pagrus Major. Ichthyol. Res. 1999, 46, 233–244. [Google Scholar] [CrossRef]
- Glazier, D.S.; Paul, D.A. Ecology of ontogenetic body-mass scaling of gill surface area in a freshwater crustacean. J. Exp. Biol. 2017, 220, 2120–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Lv, X.; Zhou, J.; Shen, C.; Xia, D.; Xie, H.; Luo, Y. Are the surface areas of the gills and body involved with changing metabolic scaling with temperature? J. Exp. Biol. 2018, 221, jeb174474. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Li, Q.; Zhu, X.; Zhou, J.; Shen, C.; Xia, D.; Djiba, P.K.; Xie, H.; Lv, X.; Jia, J. Ventilation frequency reveals the roles of exchange surface areas in metabolic scaling. Physiol. Biochem. Zool. 2020, 93, 13–22. [Google Scholar] [CrossRef]
- Scheuffele, H.; Jutfelt, F.; Clark, T.D. Investigating the gill-oxygen limitation hypothesis in fishes: Intraspecific scaling relationships of metabolic rate and gill surface area. Conserv. Physiol. 2021, 9, coab040. [Google Scholar] [CrossRef]
- Youson, J.H. 2 First Metamorphosis. Fish Physiol. 1988, 11, 135–196. [Google Scholar]
- Okada, N.; Takagi, Y.; Seikai, T.; Tanaka, M.; Tagawa, M. Asymmetrical development of bones and soft tissues during eye migration of metamorphosing Japanese flounder, Paralichthys Olivaceus. Cell Tissue Res. 2001, 304, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K. Physiological salines for fresh-water teleosts. Prog. Fish-Cult. 1963, 25, 135–140. [Google Scholar] [CrossRef]
- Hughes, G. The dimensions of fish gills in relation to their function. J. Exp. Biol. 1966, 45, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G. Measurement of gill area in fishes: Practices and problems. J. Mar. Biol. Assoc. UK 1984, 64, 637–655. [Google Scholar] [CrossRef]
- Duthie, G.; Hughes, G. The effects of reduced gill Area and hyperoxia on the oxygen consumption and swimming speed of rainbow trout. J. Exp. Biol. 1987, 127, 349–354. [Google Scholar] [CrossRef]
- Hughes, G.; Al-Kadhomiy, N. Changes in scaling of respiratory systems during the development of fishes. J. Mar. Biol. Assoc. UK 1988, 68, 489–498. [Google Scholar] [CrossRef]
- Sadler, J.; Pankhurst, P.; King, H. High prevalence of skeletal deformity and reduced gill surface area in triploid Atlantic salmon (Salmo salar L.). Aquaculture 2001, 198, 369–386. [Google Scholar] [CrossRef]
- Saunders, R.L. The irrigation of the gills in fishes: I. Studies of the mechanism of branchial irrigation. Can. J. Zool. 1961, 39, 637–653. [Google Scholar] [CrossRef]
- Hughes, G.; Morgan, M. The structure of fish gills in relation to their respiratory function. Biol. Rev. 1973, 48, 419–475. [Google Scholar] [CrossRef]
- Iwai, T.; GM, H. Preliminary morphometric study on gill development in black sea bream (Acanthopagrus schlegeli). Bull. Jap. Soc. Sci. Fish. 1977, 43, 929–934. [Google Scholar] [CrossRef]
- Ricker, W.E. Linear regressions in fishery research. J. Fish. Res. 1973, 30, 409–434. [Google Scholar] [CrossRef]
- Wieser, W. Developmental and Metabolic Constraints of the Scope for Activity in Young Rainbow Trout (Salmo Gairdneri). J. Exp. Biol. 1985, 118, 133–142. [Google Scholar] [CrossRef]
- Oikawa, S.; Itazawa, Y. Relationship between metabolic rate in vitro and body mass in a marine teleost, porgy Pagrus major. Fish Physiol. Biochem. 1992, 10, 177–182. [Google Scholar] [CrossRef]
- Crețu, M.; Guriencu, R.; Dediu, L.; Stroe, M. Comparison of Metabolic Rates of Young of the Year Beluga (Huso huso), Sterlet (Acipenser ruthenus) and Bester Hybrid Reared in a Recirculating Aquaculture System. Fishes 2021, 6, 46. [Google Scholar] [CrossRef]
- Hughes, G. Dimensions and the respiration of lower vertebrates. In Scale Effects in Animal Locomotion; Academic Press: New York, NY, USA, 1977; pp. 57–81. [Google Scholar]
- Glazier, D.S.; Borrelli, J.J.; Hoffman, C.L. Effects of fish predators on the mass-related energetics of a keystone freshwater crustacean. Biology 2020, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Fukuhara, O. Morphological and functional development of Japanese flounder in early life stage. Bull. Jap. Soc. Scient. Fish. 1986, 52, 81–91. [Google Scholar] [CrossRef]
- Brett, J. The Relation of Size to Rate of Oxygen Consumption and Sustained Swimming Speed of Sockeye Salmon (Oncorhynchus nerka). J. Fish. Res. 1965, 22, 1491–1501. [Google Scholar] [CrossRef]
- Hughes, G.; Gaymer, R.; MOORE, M.; Woakes, A. Respiratory exchange and body size in the Aldabra giant tortoise. J. Exp. Biol. 1971, 55, 651–665. [Google Scholar] [CrossRef]
- Brett, J. The metabolic demand for oxygen in fish, particularly salmonids, and a comparison with other vertebrates. Respir. Physiol. 1972, 14, 151–170. [Google Scholar] [CrossRef]
- Brett, J.; Glass, N. Metabolic Rates and Critical Swimming Speeds of Sockeye Salmon (Oncorhynchus nerka) in Relation to Size and Temperature. J. Fish. Res. 1973, 30, 379–387. [Google Scholar] [CrossRef]
- Hughes, G. Scaling of respiratory areas in relation to oxygen consumption of vertebrates. Experientia 1984, 40, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Priede, I.G. Metabolic scope in fishes. In Fish Energetics New Perspectives; Springer: Dordrecht, The Netherlands, 1985; pp. 33–64. [Google Scholar]
- Wilson, R.W.; Bergman, H.L.; Wood, C.M. Metabolic Costs and Physiological Consequences of Acclimation to Aluminum in Juvenile Rainbow Trout (Oncorhynchus mykiss). 2: Gill Morphology, Swimming Performance, and Aerobic Scope. Can. J. Fish. Aquat. Sci. 1994, 51, 536–544. [Google Scholar] [CrossRef]
- Rombough, P.; Moroz, B. The scaling and potential importance of cutaneous and branchial surfaces in respiratory gas exchange in larval and juvenile walleye. J. Exp. Biol. 1997, 200, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, J.F. Respiratory Systems and Metabolic Rates. Fish Physiol. 2005, 22, 203–238. [Google Scholar]
- Killen, S.S.; Glazier, D.S.; Rezende, E.L.; Clark, T.D.; Atkinson, D.; Willener, A.S.; Halsey, L.G. Ecological influences and morphological correlates of resting and maximal metabolic rates across teleost fish species. Am. Nat. 2016, 187, 592–606. [Google Scholar] [CrossRef] [Green Version]
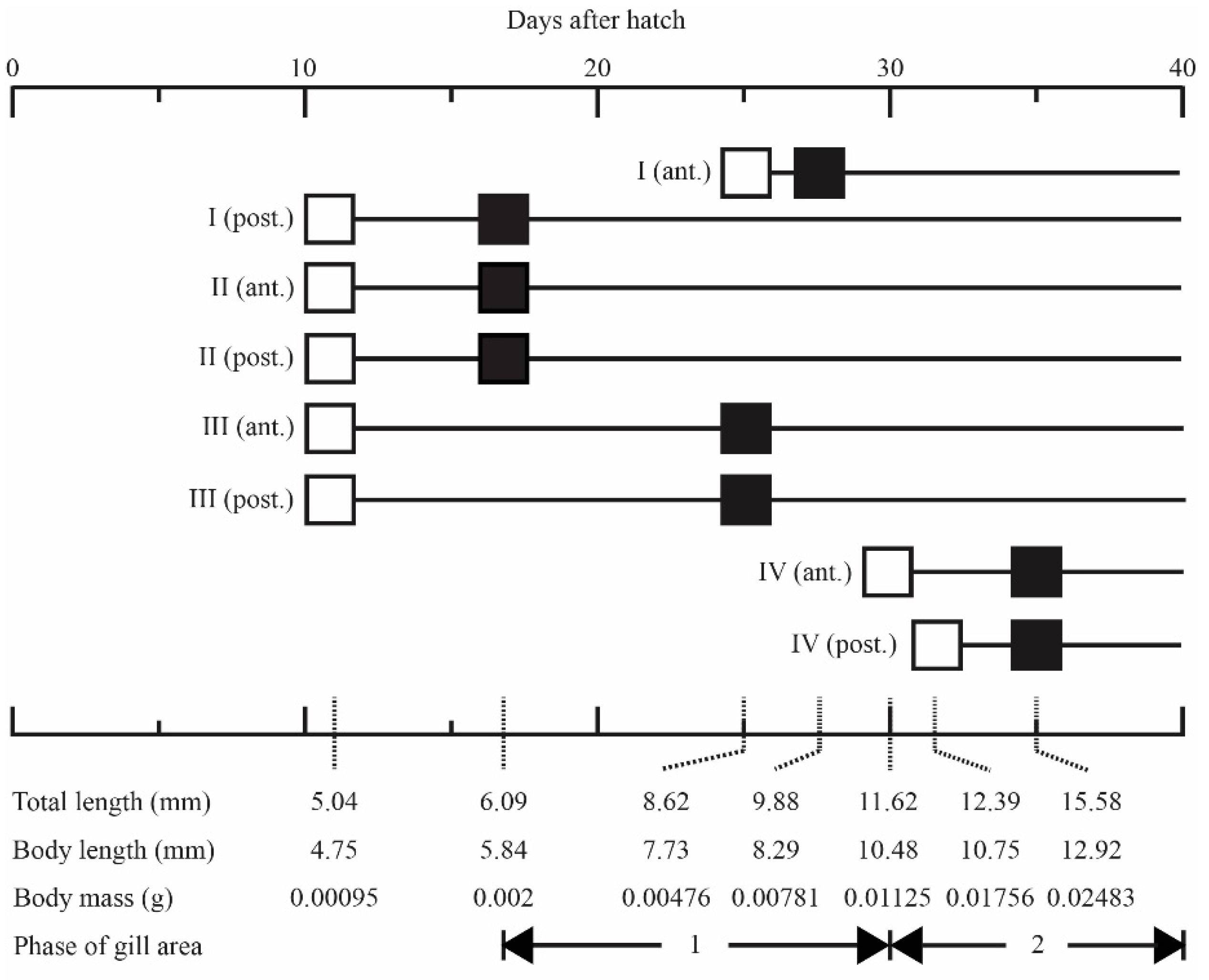

| Y | Range of Body Mass (g) | n | α | β ( ± SE) | r2 |
|---|---|---|---|---|---|
| Gill area (mm2) | 0.00200–0.01125 | 7 | 8.6 × 104 | 2.197 ± 0.287 ** | 0.921 |
| 0.01125–0.11665 | 7 | 1340.21 | 1.354 ± 0.079 ** | 0.983 | |
| 0.11665–1.30738 | 9 | 833.61 | 1.114 ± 0.074 | 0.970 | |
| Total filament number | 0.00095–0.00555 | 6 | 1031.72 | 0.406 ± 0.136 * | 0.689 |
| 0.00555–0.08122 | 7 | 2994.11 | 0.578 ± 0.061 *** | 0.947 | |
| 0.08122–1.30738 | 11 | 1189.48 | 0.265 ± 0.030 *** | 0.896 | |
| Total filament length (L, mm) | 0.00095–0.00555 | 6 | 7100.39 | 1.165 ± 0.137 | 0.948 |
| 0.00555–0.15100 | 11 | 1339.96 | 0.834 ± 0.035 ** | 0.984 | |
| 0.15100–1.30738 | 7 | 987.77 | 0.632 ± 0.064 ** | 0.951 | |
| Spacing of the secondary lamellae (1/d’, mm−1) | 0.00200–0.00781 | 6 | 152.88 | 0.348 ± 0.137 ** | 0.618 |
| 0.00781–0.11035 | 7 | 20.27 | −0.058 ± 0.039 *** | 0.308 | |
| 0.11035–1.30738 | 10 | 24.92 | −0.033 ± 0.068 *** | 0.045 | |
| Average bilateral area of the secondary lamellae (bl, mm2) | 0.00200–0.00555 | 5 | 1000.76 | 2.107 ± 0.229 * | 0.966 |
| 0.00555–0.11665 | 9 | 1.35 | 0.873 ± 0.067 | 0.960 | |
| 0.11665–1.30738 | 9 | 1.41 | 0.864 ± 0.079 | 0.945 |
| Y | Range of Body Mass (g) | n | α | β ( ± SE) | r2 |
|---|---|---|---|---|---|
| Total cutaneous area (mm2) | 0.00095–0.00555 | 6 | 3784.48 | 0.815 ± 0.064 * | 0.976 |
| 0.00555–0.11035 | 8 | 1383.61 | 0.625 ± 0.041 *** | 0.974 | |
| 0.11035–1.30738 | 10 | 2598.44 | 0.873 ± 0.059 | 0.964 | |
| Body surface area (mm2) | 0.00095–0.00555 | 6 | 1135.94 | 0.676 ± 0.047 ** | 0.981 |
| 0.00555–0.11665 | 9 | 850.97 | 0.623 ± 0.041 *** | 0.971 | |
| 0.11665–1.30738 | 9 | 1743.07 | 0.893 ± 0.089 | 0.936 | |
| Bilateral fin area (mm2) | 0.00095–0.00555 | 6 | 395.15 | 0.574 ± 0.139 * | 0.810 |
| 0.00555–0.11035 | 8 | 557.18 | 0.643 ± 0.064 ** | 0.944 | |
| 0.11035–1.30738 | 10 | 826.98 | 0.790 ± 0.062 ** | 0.953 | |
| Total length (mm) | 0.00095–0.00555 | 6 | 65.32 | 0.373 ± 0.029 *** | 0.976 |
| 0.00555–0.08122 | 7 | 39.33 | 0.276 ± 0.025 *** | 0.961 | |
| 0.08122–1.30738 | 11 | 69.08 | 0.488 ± 0.033 *** | 0.961 |
| Y | Range of Body Length (mm) | n | α | β ( ± SE) | r2 |
|---|---|---|---|---|---|
| Body mass (g) | 4.75–16.17 | 12 | 4.03 × 10−6 | 3.500 ± 0.139 *** | 0.984 |
| 16.17–66.30 | 11 | 3.67 × 10−4 | 1.924 ± 0.113 *** | 0.970 | |
| Mean body height (mm) | 4.75–10.75 | 9 | 1.57 × 10−1 | 1.218 ± 0.210 ** | 0.827 |
| 10.75–66.30 | 14 | 4.65 × 10−1 | 0.831 ± 0.025 *** | 0.989 | |
| Mean body width (mm) | 4.75–20.02 | 14 | 4.90 × 10−2 | 1.139 ± 0.057 * | 0.971 |
| 20.02–66.30 | 9 | 1.22 × 10−1 | 0.847 ± 0.041 ** | 0.984 |
| Species | Mass-Specific Areas of Gill Surface (mm2) | Mass-Specific Areas of Body Surface (mm2) | Ratio | Ref. |
|---|---|---|---|---|
| Red porgy | 362.74 | 9156.83 | 25.24 | [75] |
| Japanese flounder | 50.56 | 8507.87 | 168.26 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.I. Ontogeny of the Respiratory Area in Relation to Body Mass with Reference to Resting Metabolism in the Japanese Flounder, Paralichthys olivaceus (Temminck & Schlegel, 1846). Fishes 2022, 7, 39. https://doi.org/10.3390/fishes7010039
Kim DI. Ontogeny of the Respiratory Area in Relation to Body Mass with Reference to Resting Metabolism in the Japanese Flounder, Paralichthys olivaceus (Temminck & Schlegel, 1846). Fishes. 2022; 7(1):39. https://doi.org/10.3390/fishes7010039
Chicago/Turabian StyleKim, Dong In. 2022. "Ontogeny of the Respiratory Area in Relation to Body Mass with Reference to Resting Metabolism in the Japanese Flounder, Paralichthys olivaceus (Temminck & Schlegel, 1846)" Fishes 7, no. 1: 39. https://doi.org/10.3390/fishes7010039
APA StyleKim, D. I. (2022). Ontogeny of the Respiratory Area in Relation to Body Mass with Reference to Resting Metabolism in the Japanese Flounder, Paralichthys olivaceus (Temminck & Schlegel, 1846). Fishes, 7(1), 39. https://doi.org/10.3390/fishes7010039





