Abstract
The aquaculture sector has experienced rapid and important growth with the subsequent increase of feeding and nutritional issues for sustaining this activity, mainly related to the use of high quality, safe and environmentally friendly feed ingredients. The use of additives in aquafeeds has proven to be a suitable option to improve different productive indicators in farmed fish. In the present study, the effect of adding the GHRP-6 peptide, a ghrelin analog, to a commercial diet of gilthead sea bream (Sparus aurata) was studied at two proportions (100 or 500 μg/kg of feed). Both experimental diets show an increase in growth performance, as well as in feed efficiency after 97 days of experiment. The lower inclusion of GHRP-6 (100 μg/kg) results in a better aerobic metabolism, while the higher inclusion significantly increased plasma GH levels in agreement with the GH secretagogue effects of ghrelin. Similar growth outcome and differences between GHRP-6 levels in aerobic metabolism and GH stimulation suggest that improvements in culture performance by this peptide may occur through different mechanisms. Taken together, this compound can be considered as a viable dietary supplement for increasing production efficiency of sea bream aquaculture, although a better understanding of its dose-specific effects is still required.
1. Introduction
Aquaculture is the fastest growing animal food-producing sector in the world [1]. While there are several phenotypic traits that are currently being improved in farmed fish through genetic selection, feed composition, management and farming practices, the improvement of growth rates and feed efficiency remain as the main goal for most species. Accordingly, intense research effort has been made to understand the internal and external factors regulating feed intake [2] and growth [3] in fish. The use of additives such as short- or medium-chain fatty acids [4,5] or nutraceutical compounds from algae [6], among others, have been proven to be suitable options to improve different productive indicators in farmed fish. However, it has been suggested that the use of endogenous feeding and growth regulatory factors as feed additives should be also explored to modulate growth rate and yield in cultured species [2]. In addition, the use of synthetic compounds that stimulate feed intake, feeding efficiency, and physiological pathways related with growth, metabolism or welfare, may open new avenues to increase the competence of this productive activity. Few studies are available in farmed fish in this regard, especially those targeting the regulation of somatic growth.
The growth hormone (GH), mainly produced by the pituitary, is a key regulator of growth, although it is also involved in regulating nutrition, reproduction, physical activity, neuroprotection, immunity, and osmotic pressure [3]. The main action of GH is the stimulation of hepatic insulin-like growth factors (IGFs), which promote growth, protein synthesis, cell proliferation and metabolism [3]. GH releasing hormone (GHRH) is the principal stimulator of GH synthesis and secretion, somatostatin is a potent noncompetitive inhibitor of the release of GH, and ghrelin has a marked growth hormone-stimulating activity, the last linking gastrointestinal-pituitary axis [7]. Ghrelin is a 28 amino acid peptide that was discovered in rat stomach [8] as the endogenous ligand to the previous orphan GHS-receptor (GHS-R), and thus a potent stimulator of pituitary GH release in vertebrates [9], including fish [10]. Ghrelin, often called a “hunger hormone”, plays key roles not only in the control of GH release but also in the regulation of feed intake, energy metabolism, and immune responses in vertebrates [11,12,13,14], even from early stages [15,16]. In gilthead sea bream, ghrelin is highly expressed in the stomach and pyloric caeca [14] as described in other fish [17].
GH secretagogues (GHSs) are a family of synthetic, non-natural peptides, initially termed GH-releasing peptides (GHRPs) [18], which are recognized by the GHS-R. GHSs have no structural homology with GHRH and act via specific receptors present in the pituitary and maybe also at the hypothalamic level [19]. The action of several synthetic GHS on GH secretion has been studied in different animals [20,21], including fish [22]. Among this family, the Growth Hormone-Releasing Peptide 6 (GHRP-6) is a six amino acid synthetic peptide (His-(D-Trp)-Ala-Trp-(D-Phe)-Lys-NH2, MW = 872.44 Da) first described by [23], and it is considered a strong GHS. Few studies have been performed in fish regarding the effects of this synthetic peptide. Moreover, most available information comes from studies in freshwater species. GHRP-6 mimics the orexigenic action of ghrelin in goldfish [24] and increases pituitary Gh secretion both in vitro and in vivo in juvenile tilapia [25,26,27], whereas intraperitoneal administration of GHRP-6 induces Igf-i expression in the liver, and stimulates growth rate when administered by a plastic tube to the pharyngeal cavity of juvenile tilapia [27,28]. In addition, GHRP-6 increased body weight when it was administered by immersion baths to tilapia larvae [26], enhancing their non-specific immunity [27]. In addition, in freshwater fishes (i.e., tilapia and rainbow trout), a related synthetic peptide secretagogue (GHRP-2) increased the levels of Gh after intraperitoneal injections [29,30]. However, to the best of our knowledge, no study has reported similar effects in a marine fish. In the present study, the effect of adding the GHRP-6 peptide to a commercial diet for a marine fish was studied for the first time. The gilthead sea bream (Sparus aurata) was used as the model species, which is one of the most important farmed fish species in Europe [31]. After a feeding trial, different biometric, somatic and feed efficiency indexes were concomitantly analyzed with plasma levels of Gh and Igf-i hormones, as well as several parameters related to the metabolic and welfare status of the animals.
2. Materials and Methods
2.1. Animal Maintenance
Gilthead sea bream (S. aurata) juveniles were provided by a commercial source (PREDOMAR, Carboneras, Almeria) and acclimated to the indoor experimental facilities at the Servicios Centrales de Investigación en Cultivos Marinos (SC-ICM, CASEM, University of Cadiz, Puerto Real, Cadiz, Spain) with seawater in controlled conditions of salinity (36 ppt), temperature (19 °C), and under natural photoperiod at our latitude (36°31′45″ N, 6°11′31″ W, from October 2019 until January 2020). All assay procedures were conducted in their experimental facilities (Spanish Operational Code REGA ES11028000312).
2.2. Diets
Based on a standard commercial feed for gilthead sea bream (BioMar, Palencia; INICIO Plus 805–Crude protein: 50.0%, Crude fat: 18.0%, Digestible carbohydrates: 16.1%, Crude cellulose: 2.4%, Ash: 8.0%, phosphorous: 1.1%), experimental diets were prepared by its supplementation with the GHRP-6 peptide at the rate of 100 μg GHRP-6/kg of feed (D100) and 500 μg GHRP-6/kg of feed (D500), and a control diet in which only the excipients used for the addition of the peptide were added (see below). GHRP-6 (His-(D-Trp)-Ala-Trp-(D-Phe)-Lys-NH2, MW = 872.44 Da) with a purity >99% was provided by Sigma-Aldrich, USA. Aquafeeds were prepared as described by Adelmann et al. [32] with some modifications. Briefly, peptide reconstituted in PBS was added to a mixture of aluminum hydroxide (Al(OH)3, 10%, Sigma-Aldrich) and polyethylene glycol 1000 (PEG, Sigma-Aldrich) previously melted at 37 °C in a water bath. The pulverized commercial diet was enriched with this suspension containing a final dose of 0, 100 and 500 μg GHRP-6/kg of feed, and then pellets were prepared by cold extrusion using a manual extruder. Subsequently, the feed was dried at 22 °C.
2.3. Experimental Design and Sampling Procedure
Fish with an average initial body mass of 20.6 ± 0.5 g and body length of 10.55 ± 0.08 cm (n = 270) were randomly distributed in nine 400 L tanks (n = 30 fish per tank, 90 fish per experimental diet, three repetitions per treatment) and maintained under constant conditions as described above and fed for 97 days. Experimental diets were offered to visual satiety (ad libitum) two times per day, ensuring that the amount offered in each experimental unit was completely ingested. The feeding test was carried out “blindly”, in such a way that the three feeds were labelled with different colors but with no reference to its composition, eliminating any source of subjectivity when feeding the animals to obtain final results regarding the acceptance and growth performance. Fish were group-weighed and measured after 27 and 57 days of the beginning of the feeding trial. The feed intake was recorded every week, allowing to calculate the feeding efficiency = 100 x (wet weight gain/dry feed intake) for each experimental replicate. No mortalities were registered during the trial.
At the end of the trial (day 97), overnight fasted fish (4 fish per tank, 12 per experimental diet) were randomly selected and deeply anesthetized with 1 mL of 2-phenoxyethanol/L of seawater [6]. After being weighed and measured individually for body mass and length, blood and tissue samples were obtained. Blood was drawn from caudal vein with heparinized syringes, centrifuged at 3000× g for 20 min at 4 °C, and plasma samples were snap-frozen in liquid nitrogen and stored at −80 °C until biochemical and hormone analysis. Before tissue collection, fish were euthanized by cervical section, and livers and perivisceral fat were removed and weighed. Samples of liver and white skeletal muscle were rapidly taken, snap-frozen in liquid nitrogen, and stored at −80 °C until biochemical analyses. Intestine was taken for length measurements.
2.4. Growth Performance and Biometric Parameters
Growth parameters were also evaluated according to the following equations: (i) Condition Factor (K) = 100 × (body mass/fork length); (ii) weight gain (WG, %) = 100 × (body weigh increase/initial body weight); (iii) specific growth rate (SGR, %·day−1) = [100 × (ln final body mass− ln initial body mass)]/days; and (iv) feed efficiency (FE) = weight gain/total feed intake. Survival was calculated by estimating the number of fish at the end of the experiment with respect to the number of fish at the beginning of the experiment.
Biometric indices were estimated in accordance with the following equations: (i) Hepatosomatic index (HSI) = 100 × (liver weight/fish body mass); (ii) Mesenteric fat index (MSI) = 100 × (mesenteric fat/fish body mass); and (iii) Intestine length index (ILI) = 100 × (Li/Lb), where Li and Lb are the intestine and fork body length, respectively.
2.5. Metabolites in Plasma and Tissues
Plasma total protein concentration was determined with a BCA Protein Assay Kit (PIERCE, Thermo Fisher Scientific, Rockford, IL, USA, #23225) using BSA as the standard, whereas plasma glucose (Ref. 1001200), lactate (Ref. 1001330), triglycerides (Ref. 1001311) and cholesterol (Ref. 41021) levels were measured using commercial kits from SPINREACT (Girona, Spain) adapted to 96-well microplates.
Frozen tissues used for the assays of metabolites were homogenized by ultrasonic disruption in 7.5 volumes of ice-cold 0.6 N perchloric acid, neutralized using 1 M KCO3, and centrifuged (30 min, 3220× g at 4 °C). Supernatants were used to measure tissue metabolites. Prior to the centrifugation, an aliquot was removed and frozen at −80 °C for triglyceride determination. Tissue triglycerides and lactate levels were determined spectrophotometrically with commercial kits (SPINREACT, see above). Tissue glycogen concentration was quantified using the method described by [33], where glucose obtained after glycogen breakdown with amyloglucosidase (Sigma-Aldrich A7420) was determined with a commercial kit (SPINREACT, Girona, Spain). All assays were performed using a Bio-Tek Power Wave 340 Microplate spectrophotometer using KCjunior Data Analysis Software (Bio-Tek Instruments, Winooski, VT, USA).
2.6. Hormones
Plasma cortisol levels were measured with a commercial Cortisol Enzyme Immunoassay Kit from ARBORASSAYS (Ref. #K003), whereas plasma Gh and Igf-i were measured through competitive inhibition ELISA using commercial kits (CSB-E12121Fh for GH and CSB-E12122Fh for IGF-I, CUSABIO). All assays were performed following the manufacturer’s protocols.
2.7. Statistical Analyses
All data were checked for normality and homogeneity of variance using Kolmogorov–Smirnov and Levene’s tests, respectively, with p ≤ 0.05. Differences among treatments (CTRL, D100, D500) were evaluated using the one-way ANOVA (p ≤ 0.05), except for the growth evolution (Figure 1), where significant differences were analyzed using two-way ANOVA followed by Tukey’s test taking (i) diet and (ii) experimental time as the mean factors. In all cases, the Tukey’s test (p ≤ 0.05) was used to determine differences among means. For the values of the somatic index (MSI), the premises of a parametric test were not met, and a non-parametric Kruskal–Wallis test was performed. All results are expressed as the mean ± SEM (standard error of the mean). The software package GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, US) was used for all tests performed and generated figures.
3. Results
3.1. Growth Performance and Biometric Parameters
In general, all fish groups increased their length from 10.5 cm to 14–15 cm and their weight from 20 g to 50–59 g, with an overall weight gain of 144−190% and specific growth rates of 0.92–1.10%·day−1 (Table 1). All the experimental groups presented similar growth trajectory during the first feeding periods (eight weeks), but reached a higher biomass at the end of the experiment in those fish groups that ingested the diets supplemented with the peptide (D100, 56.53 ± 1.56 g and D500, 58.50 ± 0.46 g), compared to the control diet (49.57 ± 1.45 g) (Figure 1). Both the WG and SGR were significantly higher with the diets supplemented with the peptide (Table 1). However, no significant differences were found in final length among the three treatments (Table 1). The analysis of the Condition Factor (K) showed significant differences in the weight–furcal length relationship, with an increase in this index in the experimental groups D100 and D500 with respect to the control group (Table 1).

Table 1.
Growth performance and somatic indexes of juvenile gilthead sea breams fed to visual satiety from October 2019 to January 2020 (14 weeks). Data on body weight, feed intake and growth indexes are the mean ± SEM of triplicate tanks. Data on somatic indexes are the mean ± SEM of 12 fish. Different superscript letters in each row indicate significant differences among dietary treatments based on one-way ANOVA and Tukey’s test (p < 0.05). CTRL: control; D100: 100 µg GHRP-6/kg aquafeed; D500: 500 µg GHRP-6/kg aquafeed.
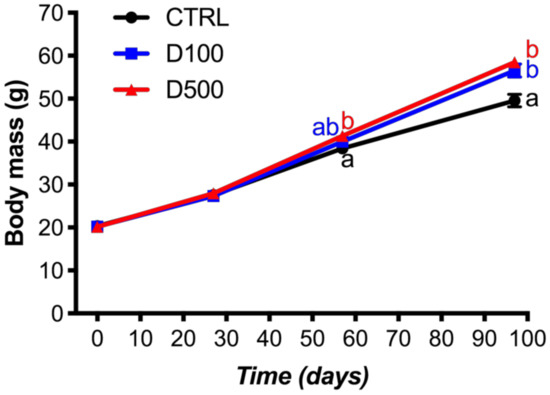
Figure 1.
Weight increase as a function of days per experimental group. The results are expressed as the mean ± SEM of the triplicate tanks for each experimental group. Different letters in each group indicate significant differences among treatments (two-way ANOVA, followed by Tukey’s test; p < 0.05). CTRL: control; D100: 100 µg GHRP-6/kg of feed; D500: 500 μg GHRP-6/kg of feed.
No differences were found in feed intake among the two supplemented diets (D100 and D500). However, fish fed with these two diets exhibited a significantly higher fed intake with respect to the CTRL group. In addition, feed efficiency increased significantly with GHRP-6 peptide supplementation, from 0.63 in fish fed the CTRL diet to 0.77 and 0.79 for D100 and D500 groups, respectively (Table 1). No differences were observed among treatments in the calculated organosomatic indexes HIS, MSI, and ILI (Table 1).
3.2. Metabolites in Plasma and Tissues
No differences were found among the three experimental diets in relation to circulating levels of glucose, triglycerides and proteins (Table 2). However, lactate values showed a significant decrease in fish fed with the D100 diet (2.39 ± 0.22 mM) compared to the CTRL group (3.14 ± 0.24 mM) and to the experimental diet D500 (3.25 ± 0.19 mM) (Table 2). Plasma cholesterol was significantly higher in fish fed both supplemented diets with respect to the CTRL group (Table 2). In the liver, no differences were found on the content of triglycerides and glucose among treatments (Table 2). Nevertheless, a significant improvement in glycogen reserves was detected in fish ingesting the diet with the highest dose of the peptide (D500) (Table 2). No changes were found in the level of glucose, glycogen, triglycerides and lactate in the white skeletal muscle among fish fed the two different experimental diets and the control (Table 2).

Table 2.
Blood and tissue biochemistry of juvenile gilthead sea breams fed to visual satiety from October 2019 to January 2020 (14 weeks). Data are the mean ± SEM of 12 fish. Different superscript letters in each row indicate significant differences among dietary treatments based on one-way ANOVA and Tukey’s test (p < 0.05). CTRL: control; D100: 100 µg GHRP-6/kg aquafeed; D500: 500 µg GHRP-6/kg aquafeed. 1 Values resulting from one-way analysis of variance.
3.3. GH, IGF-I, and Cortisol in Plasma
In general, low amplitude in the response of plasma GH and IGF-I was observed after the supplementation of feed with the peptide, although a positive and significant effect was observed in GH plasma levels in a dose-dependent manner (Figure 2A). IGF-I values on fish fed with D100 diet increased slightly over those observed in controls, while the level of IGF-I was significantly lower in fish ingesting the D500 diet with respect to those who ingested the D100 diet (Figure 2B). This resulted in a statistically significant reduction in IGF-I/GH ratio in animals ingesting the D500 diet with respect to both CTRL and D100 diets (Figure 2C). No differences were found in the circulant levels of cortisol among experimental diets (Table 2).
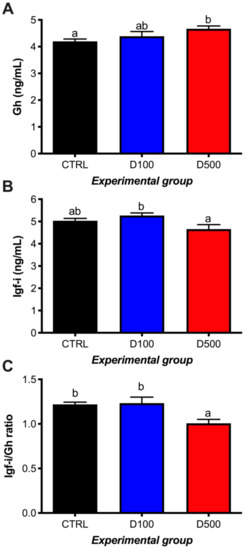
Figure 2.
Plasma Gh (A) and Igf-ilevels (B) in gilthead seabream juveniles fed with GHRP-6 peptide supplementation. Igf-i/Gh ratio (C). The results are expressed as the mean ± SEM of 12 fish for each experimental group. Different letters in each group indicate significant differences among treatments (one-way ANOVA, followed by Tukey’s test; p < 0.05). CTRL: control; D100: 100 µg GHRP-6/kg of feed; D500: 500 μg GHRP-6/kg of feed.
4. Discussion
There are currently numerous studies on the effects of different additives, both natural and synthetic, incorporated into diets to increase growth performance in fish species of interest for aquaculture production. The addition of the peptide GHRP-6 or other ghrelin homologues in commercial feed may represent a suited change in the composition of future diets aimed at maximizing fish growth performance, at least for some developmental stages, which would translate into a greater aquaculture production and efficiency. However, this approach has not been assessed before in a marine fish. In this study, we analyzed for the first time the effect of this peptide on growth performance for a marine fish, the gilthead sea bream (S. aurata). In general, all the growth indices showed an increase with the addition of the peptide to the diets, compared to the non-supplemented control diet (Table 1), whereas survival was not affected during the trial with any of the doses tested (no mortality was observed in any of the experimental tanks).
Even at the lowest inclusion level, this peptide had a positive effect on the final body mass, as well as on the specific growth rate when included in diets and offered in a medium-term feeding trial (Figure 1, Table 1). Similar results were found in tilapia juveniles (Oreochromis sp.), where the groups supplemented with concentrations of 100 μg GHRP-6/kg of feed and 500 μg GHRP-6/kg of feed showed a significant increase in their body weight [27]. Furthermore, in previous studies carried out with other ghrelin homologues, such as the A233 peptide in a proportion of 600 μg A233/kg of feed, groups supplemented with the peptide also increased their body weight compared to the control diet [34]. In this regard, it is important to relate the data obtained on the increase in body weight (WG) with the increase in the size of the animals to know if isometric growth is occurring, evidenced by an increase in biomass proportional to the size, or not [35]. To confirm this relationship, the Condition Factor (K) was used, which represents the relationship between the body mass of each individual versus their length (furcal) [36]. This index is commonly used to compare the condition or wellbeing of fish, whereas its optimum dependents on the species and age/size. Optimum values in the case of the gilthead sea bream vary from 1.5 to 2.5 [37]. Our results reveal that all groups presented a Condition Factor index within the optimal range for this species, although diets supplemented with the peptide GHRP-6 had a higher value (Table 1). Thus, there may be an added benefit of these supplemented feeds if they promote fast muscle growth instead of fat deposition. Indeed, a strong positive correlation is known to occur between K and total lipid content in fish [38]. Other main contributing factors such as gonad weight (i.e., maturation) and gut fill are not issues in our experiment, as fish were immature juveniles, and they were fasted overnight before samplings. It is worthy to note that HSI and MSI indices did not show significant variations, indicating that none of the diets tested caused higher hepatic or mesenteric accumulation of fat than that produced by the control diet. This is also supported by the absence of differences in triglyceride content among dietary treatment in the tissues examined. Thus, the observed increase in K factor can be considered be to results from somatic growth, and indicate that supplemented diets orchestrated a dietary energy partitioning that favors metabolism and growth rather than accumulation, thus not implying undesirable fatter fish. Indeed, this issue is strongly suggested since triglycerides did not show variations in muscle. Whether this effect could be produced by hyperplasia and/or hypertrophy deserves further studies.
Growth enhancement was accompanied in our study by an increase in plasma Gh levels in a dose-dependent manner with respect to the peptide inclusion. This effect was expected, as the GHRP-6 is considered a strong GHS. Previous studies showed that the GHRP-6 is able to stimulate Gh secretion in tilapia primary cultures of pituitary cells [25,26]. The GHS dependent increase in serum Gh in tilapia juveniles also increased transcription of Igf-i in the liver, when the peptide was administered intraperitoneally and orally [27]. However, although we did not measure gene expression in liver, we found no evidence of this effect. Indeed, in our study plasma Igf-i levels decreased with the D500 diet (Figure 2B). This agrees with the previously reported inverse correlation between circulating Gh and Igf-i in sea bream, likely due to an Igf-i feedback inhibition on pituitary Gh synthesis and secretion [39]. In spite of this, the improved growth response was somewhat unexpected given that plasma Igf-i reflects differences in growth potentiality between S. aurata strains [40] and genetic families [41], as occurs in other fish because of its widely known stimulating effect on myogenic cell proliferation, differentiation, and protein synthesis. Yet, it has been suggested that the correlation between Igf-i and growth can reflect scarce variations in fish [14], and it can be variable across fish species and physiological contexts due to the actions of a wide range of endogenous and exogenous factors [14,42]. For instance, a certain seasonal lag has been found among circulating Igf levels and growth rates in the production cycle of gilthead sea bream [43]. In our study, the inclusion of the peptide induced the secretion of Gh but also increased feed intake (see below), and this may promote different physiological responses depending on the doses of the peptide. For instance, higher ingestion may lead to higher energy availability and storage, but concomitantly an increase in Gh levels may favor a flux of lipids from adipose tissue toward the skeletal muscle to fuel growth, because of its lipolytic action [39]. Even so, more studies are required on the mode of action of GHRP-6 in marine fish, which may differ to some extent from freshwater fish.
On the other hand, both experimental diets (D100 and D500) showed higher feeding efficiency (FE), which translates into better feed conversion. The control group showed 62% conversion, while the experimental groups presented higher values with 79% and 80% conversion for groups D100 and D500, respectively, which represents an improvement of 17–18%. These results are interesting from the aquaculture industry perspective, given that the efficiencies shown here at a constant temperature of 19 ºC are close to those reported for this species of fish grown in summer conditions, with temperature between 23 ºC and 27 ºC, and with higher metabolic rates and feed intake [5,40,41]. However, the peptide produced an increment in feed intake at 19 ºC with respect to that of control fish, which can be understood as a likely promoted metabolism and growth at low temperature. Indeed, plasma cholesterol and hepatic glycogen reflected the variations observed in feed intake. In line with this, ghrelin is involved in the regulation of feed intake and is known as the hunger hormone [14]. Previous studies in other species such as golden carp (Carassius auratus) or tilapia using ghrelin analog peptides through different procedures (e.g., oral administration or intraperitoneal injection) produce increases in the feed intake [27,28,34,44,45]. These previous results are in accordance with the observations of this work. The positive effect of GHRP-6 on feed intake of the gilthead sea bream can be considered as a good feature in terms of production together with the higher FE registered.
Finally, the ILI did not show differences among the three treatment groups. It is known that there is a strong correlation between diet and ILI, where animals that are naturally fed diets based mainly on plant compounds (herbivores) have longer intestines than animals naturally fed higher levels of animal protein (piscivores) [46]. However, some carnivore fish exhibit certain flexibility, and when exposed to changes in their usual diet toward more vegetable proteins, their intestines undergo morphological adaptations for a better use of the new diet. This feature has been previously shown in the gilthead sea bream [6,41]. In our study, we used a specific diet for this species with a high content of animal protein, which was only supplemented with the studied peptide and without introducing major changes in its formulation. Therefore, it was expected that the animals did not have to adapt their absorption surface (i.e., intestinal length). However, more studies are required to know if the improvement on FE index may be influenced by other changes at the intestine, such as changes in the conformation of the enterocytes (e.g., increased surface area or length of the microvilli), changes in paracellular routes that improve the absorption of nutrients, or even changes in the trans-epithelial selectivity of the intestine [4].
In addition, no significant differences were found in glucose values in the tissues analyzed (Table 2). It has been proposed that glycolysis in fish is more important as a supplier of biosynthetic products than as a way of producing pyruvate for its subsequent oxidation [47]. The glucose values obtained, being similar for the three diets, show that the experimental peptide GHRP-6 incorporated by ingestion does not alter the metabolic pathways related to glucose utilization. This result matches with previous observations in studies focused on the incorporation of other additives in the teleost diet, such as tryptophan, heptanoate or compounds extracted from medicinal herbs and microalgae [5,48,49], where the animals under non-stressful conditions presented a balance in glucose values similar to those observed in this experiment. Lactate, on the other hand, presents lower values for the D100 diet than in the CTRL and D500 diets. This result suggests that the D100 diet may favor oxidative over anaerobic metabolism in white skeletal muscle, or that lactate uptake and elimination by the liver or other tissues is encouraged. Thus, lactate production or accumulation after high metabolic demand caused by stress, physical exercise or oxygen concentrations below that needed to sustain mitochondrial aerobic activity has been previously demonstrated in this fish species [50,51]. Although, our results were obtained in free-swimming and resting fish, and low plasma cortisol levels demonstrated that all groups maintain a homeostatic state. The positive effect of the D100 diet on aerobic metabolism may be the result of more metabolites being metabolized in the mitochondria through aerobic processes, such as fatty acids or glucose. This fact could suggest a greater energy production without the need to require anaerobic routes more than its production and withdrawal for extra energy supply, thus presenting a homeostatic load with similar levels in the other energy substrates analyzed (glucose, TAG and proteins) with the control.
5. Conclusions and Open Issues
In the present study, we demonstrated for the first time that GHRP-6 stimulates growth performance in juveniles of a marine fish, the gilthead sea bream, when administered as a feed additive. In general, growth is favored by diets supplemented with both concentrations of the peptide, even under a temperature condition (19 °C) far from the optimal for this species growth. On the other hand, no physiological or metabolic alterations were detected in studied individuals, finding even better aerobic food management by the specimens fed the D100 diet. The higher inclusion of GHRP-6 (500 μg/kg) significantly increased plasma Gh levels with respect to controls, in agreement with the GH secretagogue effects of ghrelin. However, similar growth outcome and differences between GHRP-6 inclusion levels in aerobic metabolism (D100), and Gh and Igf-i plasma levels, suggest that improvements in culture performance may have occurred through different mechanisms. Thus, this peptide can be considered as a viable supplement for increasing the production efficiency of gilthead sea bream although more studies are required to better understand its mode of action at different inclusion levels, including to delve into the biological effects of GHS on marine fish growth and feed efficiency. On the other hand, it must be considered that the use of this peptide as an additive during the processing of commercial foods can be developed to benefit animal health and nutrition [52], and though steam cooking and extrusion at high temperatures can lead to its partial denaturation, results reported in this work strongly suggest the stability of the compound during manipulation. Future studies that address the appropriate way to incorporate this peptide in commercial diets are necessary. For example, a recent study in rainbow trout tested the inclusion of the neuropeptide PACAP (pituitary adenylate cyclase activating peptide) in the diet through an oil-based preformulation of the peptide using water-in-oil (W/O) emulsions from a dispersed aqueous phase and a continuous oily phase, with good results [53]. This work opens a new path for deeper investigations of the effects and possible applications of the GHRP-6 peptide as an additive in diets for farmed marine fish.
Author Contributions
Conceived and designed the experiment, L.R.-V., R.M., J.A.M.-S., J.M.M. and M.P.E.; performed the experiments, L.R.-V., I.M., J.A.M.-S. and E.P.; analyzed the data, L.R.-V., J.A.M.-S., I.M., E.P. and R.M.; interpreted the results, L.R.-V., J.A.M.-S., R.M., E.P. and J.M.M.; wrote the manuscript, L.R.-V., J.A.M.-S., R.M. and E.P.; writing—review and editing, L.R.-V., J.A.M.-S., R.M., E.P., J.M.M. and M.P.E.; project administration and funding acquisition, J.A.M.-S. and J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported under the Fellowship for short stays to prestigious researchers awarded by the University of Cadiz (reference EST2019-021) to L.R.-V. Additional funding was obtained by the projects AGL2016-76069-C2-1-R of MICINN (Spain) to J.M.M, and co-financed by the European Union under the 2014–2020 ERDF Operational Programme and by the Department of Economic Transformation, Industry, Knowledge, and Universities of the Regional Government of Andalusia (Project reference: FEDER-UCA18-107182) to J.A.M.-S. J.M.M. and J.A.M.-S. belong to the Fish Welfare and Stress Network (AGL2016-81808-REDT) supported by the Spanish Ministry of Economy, Industry and Competitiveness.
Institutional Review Board Statement
The study was conducted following the guidelines for experimental procedures in animal research from the Ethics and Animal Welfare Committee of the University of Cadiz, according to the Spanish (RD53/2013) and European Union (2010/63/UE) legislation. The Ethical Committee from the Autonomous Andalusian Government approved the experiments (Junta de Andalucía reference number 04/04/2019/056).
Data Availability Statement
The data that support the findings of this study are all presented in the figures and tables, as well as available from the authors upon reasonable request.
Acknowledgments
The authors wish to thank Servicios Centrales de Investigación en Cultivos Marinos (SCI-CM, CASEM, University of Cádiz, Puerto Real, Cádiz, Spain) for providing experimental fish and for their excellent technical assistance. In addition, we acknowledge the support of the University of Almería (Experimental Feeds Service, https://www.ual.es/universidad/serviciosgenerales/stecnicos/perifericos-convenio/piensos-experimentales; accessed on 10 January 2022) on aquafeed elaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Meeting the sustainable development goals. In The State of World Fisheries and Aquaculture 2018; FAO: Rome, Italy, 2018; Available online: https://www.fao.org/documents/card/es/c/I9540EN (accessed on 26 January 2022).
- Bertucci, J.I.; Blanco, A.M.; Sundarrajan, L.; Rajeswari, J.J.; Velasco, C.; Unniappan, S. Nutrient Regulation of Endocrine Factors Influencing Feeding and Growth in Fish. Front. Endocrinol. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Unniappan, S. A Comparative Update on the Neuroendocrine Regulation of Growth Hormone in Vertebrates. Front. Endocrinol. 2021, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Estensoro, I.; Ballester-Lozano, G.; Benedito-Palos, L.; Grammes, F.; Martos-Sitcha, J.A.; Mydland, L.T.; Calduch-Giner, J.A.; Fuentes, J.; Karalazos, V.; Ortiz, A.; et al. Dietary butyrate helps to restore the intestinal status of a marine teleost (Sparus aurata) fed extreme diets low in fish meal and fish oil. PLoS ONE 2016, 11, e0166564. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Simó-Mirabet, P.; Piazzon, M.C.; de las Heras, V.; Calduch-Giner, J.A.; Puyalto, M.; Tinsley, J.; Makol, A.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Dietary sodium heptanoate helps to improve feed efficiency, growth hormone status and swimming performance in gilthead sea bream (Sparus aurata). Aquac. Nutr. 2018, 24, 1638–1651. [Google Scholar] [CrossRef]
- Perera, E.; Sánchez-Ruiz, D.; Sáez, M.I.; Galafat, A.; Barany, A.; Fernández-Castro, M.; Antonio Jesús Vizcaíno, A.J.; Fuentes, J.; Martínez, T.F.; Mancera, J.M.; et al. Low dietary inclusion of nutraceuticals from microalgae improves feed efficiency and modifies intermediary metabolisms in gilthead sea bream (Sparus aurata). Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Khatib, N.; Gaidhane, S.; Gaidhane, A.M.; Khatib, M.; Simkhada, P.; Gode, D.; Zahiruddin, Q.S. Ghrelin: Ghrelin as a regulatory Peptide in growth hormone secretion. J. Clin. Diagn Res. 2014, 8, MC13–MC17. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Kaiya, H.; Kangawa, K.; Miyazato, M. What is the general action of ghrelin for vertebrates? Comparisons of ghrelin’s effects across vertebrates. Gen. Comp. Endocrinol. 2013, 181, 187–191. [Google Scholar] [CrossRef]
- Riley, L.G.; Hirano, T.; Grau, E.G. Rat ghrelin stimulates growth hormone and prolactin release in the tilapia, O. mossambicus. Zool. Sci. 2002, 19, 797–800. [Google Scholar] [CrossRef]
- Kaiya, H.; Miyazato, M.; Kangawa, K.; Peter, R.E.; Unniappan, S. Ghrelin: Amultifunctional hormone in non-mammalian vertebrates. Comp. Biochem. Physiol. A 2008, 149, 109–128. [Google Scholar] [CrossRef]
- Kaiya, H.; Miyazato, M.; Kangawa, K. Recent advances in the phylogenetic study of ghrelin. Peptides 2011, 32, 2155–2174. [Google Scholar] [CrossRef]
- Jönsson, E. The role of ghrelin in energy balance regulation in fish. Gen. Comp. Endocrinol 2013, 187, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Perelló-Amorós, M.; Vélez, E.J.; Vela-Albesa, J.; Sánchez-Moya, A.; Riera-Heredia, N.; Hedén, I.; Fernández-Borràs, J.; Blasco, J.; Calduch-Giner, J.A.; Navarro, I.; et al. Ghrelin and Its Receptors in Gilthead Sea Bream: Nutritional Regulation. Front. Endocrinol. 2018, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Guillén, C.; Dias, J.; Rocha, F.; Castanheira, M.F.; Martins, C.I.; Laizé, V.; Gavaiaa, P.J.; Engrola, S. Does a ghrelin stimulus during zebrafish embryonic stage modulate its performance on the long-term? Comp. Biochem. Physiol. A 2019, 228, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Guillén, C.; Yufera, M.; Engrola, S. Ghrelin in Senegalese sole (Solea senegalensis) post-larvae: Paracrine effects on food intake. Comp. Biochem. Physiol. A 2017, 204, 85–92. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Bernardini, G.; Gornati, R.; Saroglia, M. Sea bass ghrelin: Molecular cloning and mRNA quantification during fasting and refeeding. Gen. Comp. Endocrinol. 2008, 155, 341–351. [Google Scholar] [CrossRef]
- Momany, F.A.; Bowers, C.Y.; Reynolds, G.A.; Chang, D.; Hong, A.; Newlander, K. Design, synthesis and biological activity of peptides which release growth hormone, in vitro. Endocrinology 1981, 108, 31–39. [Google Scholar] [CrossRef]
- Camanni, F.; Ghigo, E.; Arvat, E. Growth Hormone-Releasing Peptides and Their Analogs. Front. Neuroendocrinol. 1998, 19, 47–72. [Google Scholar] [CrossRef]
- Hashizume, T.; Sasaki, K.; Sakai, M.; Tauchi, S.; Masuda, H. The effect of new growth hormone-releasing peptide (KP 102) on the release of growth hormone in goats. Anim. Sci. Technol. 1997, 68, 247–256. [Google Scholar]
- Roh, S.G.; Lee, H.G.; Phung, L.T.; Hidari, H. Characterization of Growth Hormone Secretion to Growth Hormone releasing Peptide-2 in Domestic Animals-A Review. Asian-Australas J. Anim. Sci. 2002, 15, 757–766. [Google Scholar] [CrossRef]
- Chan, C.B.; Fung, C.K.; Fung, W.; Margaret, C.L.; Cheng, C.H. Stimulation of growth hormone secretion from seabream pituitary cells in primary culture by growth hormone secretagogues is independent of growth hormone transcription. Comp. Biochem. Physiol. C 2004, 139, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bowers, C.Y.; Momany, F.A.; Reynolds, G.A.; Hong, A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology 1984, 114, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Yahashia, S.; Kang, K.S.; Kaiya, H.; Matsudaa, K. GHRP-6 mimics ghrelin-induced stimulation of food intake and suppression of locomotor activity in goldfish. Peptides 2012, 34, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.M.; Rodríguez, A.; Helguera, Y.; Morales, R.; González, O.; Acosta, J.; Besada, V.; Sánchez, A.; Estrada, M.P. Recombinant novel pituitary adenylate cyclase activating polypeptide (PACAP) from African catfish (Clarias gariepinus) authenticates its biological function as a growth promoting factor in lower vertebrates. J. Endocrinol. 2008, 197, 583–597. [Google Scholar] [CrossRef]
- Martinez, R.; Ubieta, K.; Herrera, F.; Forellat, A.; Morales, R.; de la Nuez, A.; Rodriguez, R.; Reyes, O.; Oliva, A.; Estrada, M.P. A novel GH secretagogue, A233, exhibits enhanced growth activity and innate immune system stimulation in teleosts fish. J. Endocrinol. 2012, 214, 409–419. [Google Scholar] [CrossRef]
- Martinez, R.; Carpio, Y.; Morales, A.; Lugo, J.M.; Herrera, F.; Zaldívar, C.; Carrillo, O.; Arenal, A.; Pimentel, E.; Estrada, M.P. Oral administration of the growth hormone secretagogue-6 (GHRP-6) enhances growth and non-specific immune responses in tilapia (Oreochromis sp.). Aquaculture 2016, 452, 304–310. [Google Scholar] [CrossRef]
- Lugo, J.M.; Oliva, A.; Morales, A.; Reyes, O.; Garay, H.E.; Herrera, F.; Cabrales, R.; Perez, E.; Estrada, M.P. The biological role of pituitary adenylate cyclase-activating polypeptide (PACAP) in growth and feeding behavior in juvenile fish. J. Pept. Sci. 2010, 16, 633–643. [Google Scholar] [CrossRef]
- Shepherd, B.S.; Eckert, S.M.; Parhar, I.S.; Vijayan, M.M.; Wakabayashi, I.; Hirano, T. The hexapeptide KP-102 (D-ala-D-beta-Nal-ala-trp-D-phe-lys-NH(2) stimulates growth hormone release in a cichlid fish (Oreochromis mossambicus). J. Endocrinol. 2000, 167, 7–10. [Google Scholar] [CrossRef]
- Shepherd, B.S.; Johnson, J.K.; Silverstein, J.T.; Parhar, I.S.; Vijayan, M.M.; McGuire, A.; Weber, G.M. Endocrine and orexigenic actions of growth hormone secretagogues in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. A 2007, 146, 390–399. [Google Scholar] [CrossRef]
- Rigos, G.; Kogiannou, D.; Padrós, F.; Cristofol, C.; Florio, D.; Fioravanti, M.; Zarza, C. Best therapeutic practices for the use of antibacterial agents in finfish aquaculture: A particular view on European seabass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata) in Mediterranean aquaculture. Rev. Aquac. 2021, 13, 1285–1323. [Google Scholar] [CrossRef]
- Adelmann, M.; Kollner, B.; Bergmann, S.M.; Fischer, U.; Lange, B.; Weitschies, W.; Enzmann, P.J.; Fichtner, D. Development of an oral vaccine for immunisation of rainbow trout (Oncorhynchus mykiss) against viral haemorrhagic septicaemia. Vaccine 2008, 26, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Decker, K. Glycogen. Determination with amyloglucosidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 1127–1131. [Google Scholar]
- Martínez, R.; Morales, C.; Arenal, A.; Morales, A.; Herrera, F.; González, V.; Estrada, M.P. Growth Hormone Secretagogue (A233) Improves Growth and Changes the Tissue Fatty Acid Profile in Juvenile Tilapia (Oreochromis niloticus). Lipids 2018, 53, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, R.; González, J.; Montoya, G.; Jara, A.; Ortíz, N.; Piedra, P.; Habit, E. Relación longitud-peso y factor de condición de los peces nativos del río San Pedro (cuenca del río Valdivia, Chile). Gayana (Concepción) 2012, 76, 86–100. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Ortega, A. Cultivo de Dorada (Sparus aurata); Fundación Observatorio Español de Acuicultura, Consejo Superior de Investigaciones Científicas, Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2008; pp. 1–44. [Google Scholar]
- Herbinger, C.M.; Friars, G.W. Correlation between condition factor and total lipid content in Atlantic salmon, Salmo salar L., parr. Aqua Res. 1991, 22, 527–529. [Google Scholar] [CrossRef]
- Pérez-Sánchez, J. The involvement of growth hormone in growth regulation, energy homeostasis and immune function in the gilthead sea bream (Sparus aurata): A short review. Fish Physiol Biochem. 2000, 22, 135–144. [Google Scholar] [CrossRef]
- Simó-Mirabet, P.; Felip, A.; Estensoro, I.; Martos-Sitcha, J.A.; de las Heras, V.; Calduch-Giner, J.; Puyalto, M.; Karalazos, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Impact of low fish meal and fish oil diets on the performance, sex steroid profile and male-female sex reversal of gilthead sea bream (Sparus aurata) over a three-year production cycle. Aquaculture 2018, 490, 64–74. [Google Scholar] [CrossRef]
- Perera, E.; Simó-Mirabet, P.; Shin, H.S.; Rosell-Moll, E.; Naya-Catalá, F.; de las Heras, V.; Martos-Sitcha, J.A.; Karalazos, V.; Armero, E.; Pérez-Sánchez, J. Selection for growth is associated in gilthead sea bream (Sparus aurata) with diet flexibility, changes in growth patterns and higher intestine plasticity. Aquaculture 2019, 507, 349–360. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Cartas, D.; Miliou, H. Factors influencing GH and IGF-I gene expression on growth in teleost fish: How can aquaculture industry benefit? Rev. Aquac. 2020, 12, 1637–1662. [Google Scholar] [CrossRef]
- Mingarro, M.; de Celis, S.V.R.; Astola, A.; Pendón, C.; Valdivia, M.M.; Pérez-Sánchez, J. Endocrine mediators of seasonal growth in gilthead sea bream (Sparus aurata): The growth hormone and somatolactin paradigm. Gen. Comp. Endocrinol. 2002, 128, 102–111. [Google Scholar] [CrossRef]
- Unniappan, S.; Lin, X.; Cervini, L.; Rivier, J.; Kaiya, H.; Kangawa, K.; Peter, R.E. Goldfish ghrelin: Molecular characterization of the complementary deoxyribonucleic acid, partial gene structure and evidence for its stimulatory role in food intake. Endocrinology 2002, 143, 4143–4146. [Google Scholar] [CrossRef] [PubMed]
- Unniappan, S.; Peter, R.E. In vitro and in vivo effects of ghrelin on luteinizing hormone and growth hormone release in goldfish. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2004, 286, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.E.; McIntyre, P.B.; Buels, K.S.; Gilbert, D.M.; Michel, E. Diet predicts intestine length in Lake Tanganyika’s cichlid fishes. Funct. Ecol. 2009, 23, 1122–1131. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Saccol, E.M.H.; Parrado-Sanabria, Y.A.; Gagliardi, L.; Jerez-Cepa, I.; Mourão, R.H.V.; Heinzmann, B.M.; Baldisserotto, B.; Pavanato, M.A.; Mancera, J.M.; Martos-Sitcha, J.A. Myrcia sylvatica essential oil in the diet of gilthead sea bream (Sparus aurata L.) attenuates the stress response induced by high stocking density. Aquac. Nutr. 2018, 24, 1381–1392. [Google Scholar] [CrossRef]
- Azeredo, R.; Machado, M.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Moura, J.; Peres, H.; Oliva-Teles, A.; Afonso, A.; Mancera, J.M.; Costas, B. Dietary tryptophan induces opposite health-related responses in the Senegalese sole (Solea senegalensis) reared at low or high stocking densities with implications in disease resistance. Front. Physiol. 2019, 10, 508. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Martos-Sitcha, J.A.; Queiroz, A.; Calduch-Giner, J.A.; Gonçalves, J.F.M.; Rocha, C.M.; Abreu, H.T.; Scharma, J.W.; Ozorio, R.O.A.; Pérez-Sánchez, J. Dietary supplementation of heat-treated Gracilaria and Ulva seaweeds enhanced acute hypoxia tolerance in gilthead sea bream (Sparus aurata). Biol. Open 2017, 6, 897–908. [Google Scholar] [CrossRef]
- Naya-Català, F.; Martos-Sitcha, J.A.; de las Heras, V.; Simó-Mirabet, P.; Calduch-Giner, J.À.; Pérez-Sánchez, J. Targeting the Mild-Hypoxia Driving Force for Metabolic and Muscle Transcriptional Reprogramming of Gilthead Sea Bream (Sparus aurata) Juveniles. Biology 2021, 10, 416. [Google Scholar] [CrossRef]
- Poudel, P.; Levesque, C.L.; Samuel, R.; St-Pierre, B. Dietary inclusion of Peptiva, a peptide-based feed additive, can accelerate the maturation of the fecal bacterial microbiome in weaned pigs. BMC Vet. Res. 2020, 16, 1–13. [Google Scholar] [CrossRef]
- Herrera, F.; Velazquez, J.; Lugo, J.M.; Orellana, P.; Ruiz, J.; Vega, M.; Romero, A.; Santos, N.; Ramses, G.; Rodríguez-Ramos, T.; et al. Oral Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) formulation modified muscle fatty acid profile and cytokines transcription in head kidney in rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Rep. 2021, 20, 100772. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).