Effects of Continuous Light (LD24:0) Modulate the Expression of Lysozyme, Mucin and Peripheral Blood Cells in Rainbow Trout
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Preparation of Reduced Mucins
2.3. Lysozyme Concentration
2.4. Mucin Concentration
2.5. Blood Samples
2.6. Statistical Analysis
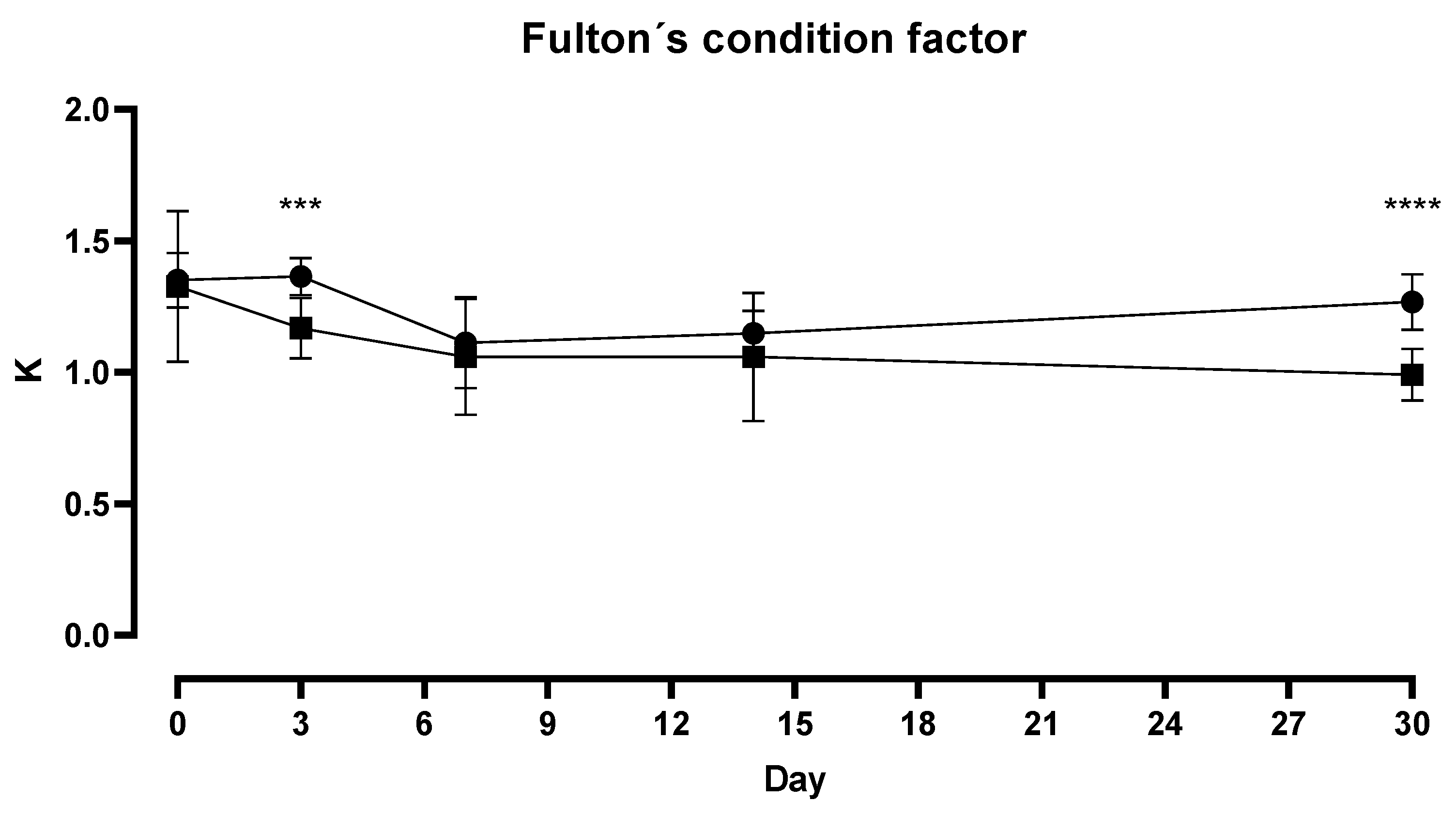
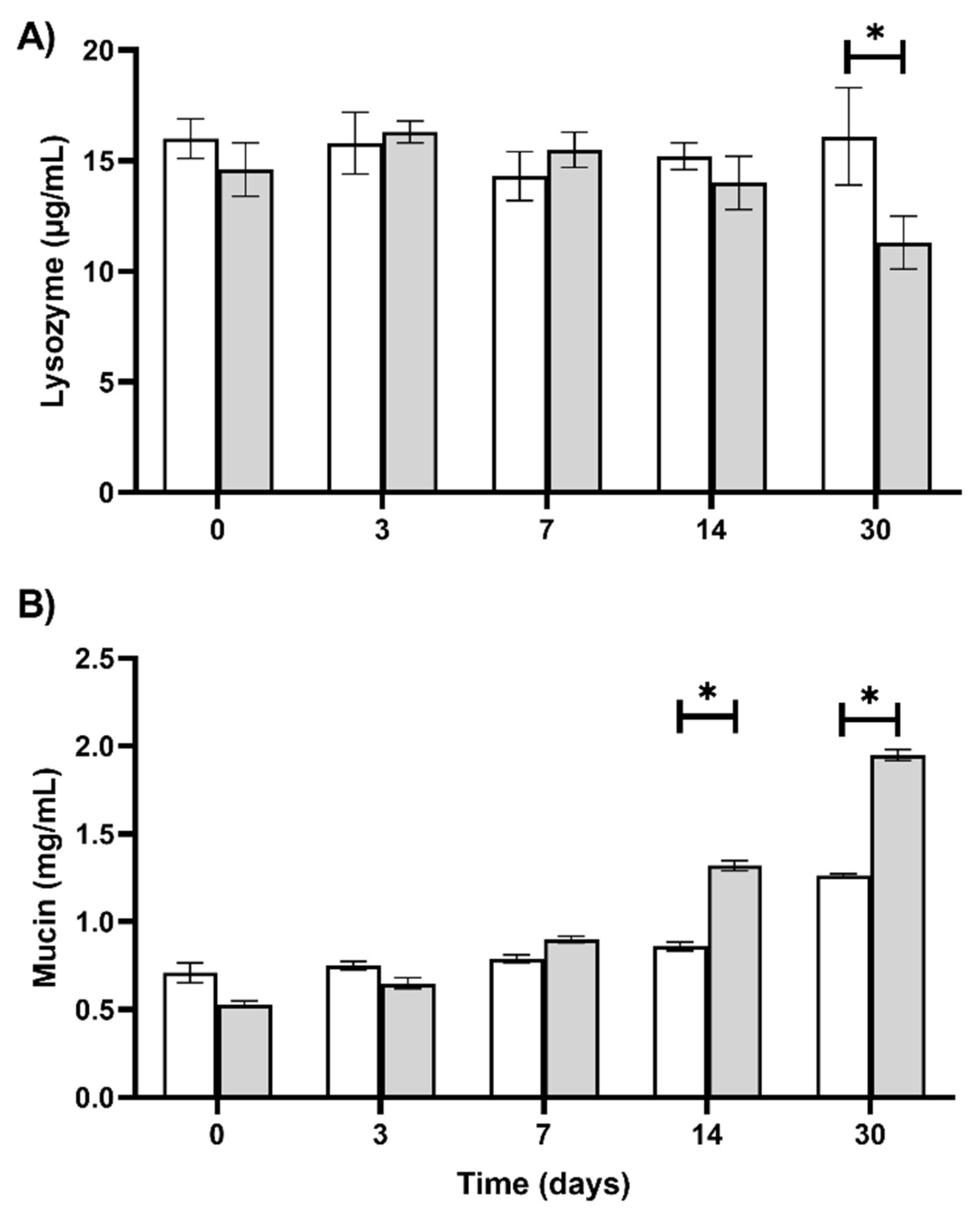
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteban, M.A.; Cuesta, A.; Rodríguez, A.; Meseguer, J. Effect of photoperiod on the fish innate immune system: A link between fish pineal gland and the immune system. J. Pineal Res. 2006, 41, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Morro, B.; Balseiro, P.; Albalat, A.; Pedrosa, C.; Mackenzie, S.; Nakamura, S.; Shimizu, M.; Nilsen, T.O.; Sveier, H.; Ebbesson, L.O.; et al. Effects of different photoperiod regimes on the smoltification and seawater adaptation of seawater-farmed rainbow trout (Oncorhynchus mykiss): Insights from Na+, K+–ATPase activity and transcription of osmoregulation and growth regulation genes. Aquaculture 2019, 30, 282–292. [Google Scholar] [CrossRef]
- Karlsen, Ø.; Norberg, B.; Kjesbu, O.S.; Taranger, G.L. Effects of photoperiod and exercise on growth, liver size, and age at puberty in farmed Atlantic cod (Gadus morhua L.). ICES Mar. Sci. Symp. 2006, 63, 355–364. [Google Scholar] [CrossRef]
- Iversen, M.; Mulugeta, T.; West, A.C.; Jørgensen, E.H.; Samuel, A.M.; Simen Rød Sandve, M.; Hazlerigg, D. Photoperiod-dependent developmental reprogramming of the transcriptional response to seawater entry in Atlantic salmon (Salmo salar). G3 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.J.; Longland, R.; Woolcott, H.; Porter, J.R. Effect of elevated winter–spring water temperature on sexual maturation in photoperiod manipulated stocks of rainbow trout (Oncorhynchus mykiss). Aquaculture 2010, 22, 236–244. [Google Scholar] [CrossRef]
- Taranger, G.L.; Carrillo, M.; Schulz, R.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.A.; Dufourf, S.; Karlseng, Ø.; Norbergg, B.; et al. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010, 3, 483–515. [Google Scholar] [CrossRef]
- Pino Martinez, E.; Balseiro, P.; Pedrosa, C.; Haugen, T.S.; Mitchell, F.S.; Handeland, S.O. The effect of photoperiod manipulation on Atlantic salmon growth, smoltification and sexual maturation: A case study of a commercial RAS. Aquac. Res. 2020, 52, 2593–2608. [Google Scholar] [CrossRef]
- Wang, K.; Li, K.; Liu, L.; Tanase, C.; Mols, R.; van der Meer, M. Effects of light intensity and photoperiod on the growth and stress response of juvenile Nile tilapia (Oreochromis niloticus) in a recirculating aquaculture system. Aquac. Fish. 2020. [Google Scholar] [CrossRef]
- Cabillon, N.A.R.; Lazado, C.C. Mucosal barrier functions of fish under changing environmental conditions. Fishes 2019, 4, 2. [Google Scholar] [CrossRef]
- Valenzuela, A.; Campos, V.; Yañez, F.; Alveal, K.; Gutiérrez, P.; Rivas, M.; Contreras, N.; Klempau, A.; Fernandez, I.; Oyarzún, C. Application of artificial photoperiod in fish: A factor that increases susceptibility to infectious diseases? Fish. Physiol. 2012, 38, 943–950. [Google Scholar] [CrossRef]
- Leonardi, M.; Klempau, A. Artificial photoperiod influence on the immune system of juvenile rainbow trout Oncorhynchus mykiss. Aquaculture 2003, 221, 581–591. [Google Scholar] [CrossRef]
- Valenzuela, A.E.; Silva, V.M.; Klempau, A.E. Effects of constant light on haematological parameters of cultured rainbow trout (Oncorhynchus mykiss) in the Southern Hemisphere. Fish. Physiol. Biochem. 2006, 32, 113–120. [Google Scholar] [CrossRef]
- Biswas, A.K.; Seoka, M.; Tanaka, Y.; Takii, K.; Kumai, H. Effect of photoperiod manipulation on the growth performance and stress response of juvenile red sea bream (Pagrus major). Aquaculture 2006, 258, 350–356. [Google Scholar] [CrossRef]
- Lazado, C.C.; Vilhelm Skov, P. Secretory proteins in the skin mucus of Nile Tilapia (Oreochromis niloticus) are modulated temporally by photoperiod and bacterial endotoxin cues. Fishes 2019, 4, 57. [Google Scholar] [CrossRef]
- Valenzuela, A.E.; Silva, V.M.; Klempau, A.E. Some changes in the haematological parameters of rainbow trout (Oncorhynchus mykiss) exposed to three artificial photoperiod regimes. Fish. Physiol. Biochem. 2008, 33, 45–48. [Google Scholar] [CrossRef]
- Valenzuela, A.E.; Silva, V.M.; Klempau, A.E. Effects of different artificial photoperiods and temperatures on haematological parameters of rainbow trout (Oncorhynchus mykiss). Fish. Physiol. Biochem. 2008, 34, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Xua, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.A.; Jørgensend, L.V.G.; Heinecked, R.D.; Buchmannd, K.; LaPatrae, S.; Sunyera, J.O. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef]
- Ellis, A. E Immunity to bacteria in fish. Fish. Shell Fish Immun. 1999, 9, 291–308. [Google Scholar] [CrossRef]
- Datta, S.; Datta, S.C. Purification and characterization of fish surface mucin. Ital. J. Biochem. 1987, 36, 143–152. [Google Scholar]
- Urbina, M.A.; Glover, C.N. Should I stay or should I go?: Physiological, metabolic and biochemical consequences of voluntary emersion upon aquatic hypoxia in the scaleless fish Galaxias maculatus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2012, 182, 1057–1067. [Google Scholar] [CrossRef]
- Campbell, T.W.; Murru, F. An introduction to fish hematology. Comp. Cont. Ed. Vet. Sci 1990, 12, 525–533. [Google Scholar]
- Klontz, G.W. Fish hematology. In Techniques in Fish Immunology, 3rd ed.; Stolen, J.D., Rowley, A.F., Zelikoff, J.T., Kaatari, S.L., Smith, S.A., Eds.; SOS Publications: Fair Haven, NJ, USA, 1994; pp. 121–131. [Google Scholar]
- Jones, R.; Petrell, R.; Pauly, D. Using modified length-weight relationships to assess the condition of fish. Aquac. Eng. 1999, 20, 261–276. [Google Scholar] [CrossRef]
- Angelidis, P.; Baudin-Laurencin, F.; Youinou, P. Stress in rainbow trout, Salmo gairdneri: Effects upon phagocyte chemiluminescence, circulating leucocytes and susceptibility to Aeromonas salmonicida. J. Fish. Biol. 1987, 31, 113–122. [Google Scholar] [CrossRef]
- Pickering, A.D.; Griffiths, R.; Pottinger, T.G. A comparison of the effects of overhead cover on the growth, survival and haematology of juvenile Atlantic salmon, Salmo salar L.; brown trout, Salmo trutta L.; and rainbow trout, Salmo gairdneri Richardson. Aquaculture 1987, 66, 109–124. [Google Scholar] [CrossRef]
- Melingen, G.O.; Pettersen, E.F.; Wergeland, H.I. Leucocyte populations and response to immunization and photoperiod manipulation in Atlantic salmon (Salmo salar L.) 0 + smolt. Aquaculture 2002, 214, 381–396. [Google Scholar] [CrossRef]
- Grzelak, A.K.; Davis, D.J.; Caraker, S.M.; Crim, M.J.; Spitsbergen, J.M.; Wiedmeyer, C.E. Stress Leukogram Induced by Acute and Chronic Stress in Zebrafish (Danio rerio). Comp. Med. 2017, 67, 263–269. [Google Scholar]
- Maule, A.G.; Schreck, C.B. Changes in numbers of leukocytes in immune organs of juvenile coho salmon after acute stress or cortisol treatment. J. Aquat. Animal Health 1990, 2, 298–304. [Google Scholar] [CrossRef]
- Heinzel, K.; Benz, C.; Bleu, C.C. A silent chemokine recptor regulates steady-state leukocyte homing in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8421–8426. [Google Scholar] [CrossRef]
- Lie, O.; Evensen, O.; Sorensen, A.; Froysadal, E. Study of lysozyme activity in some fish species. Dis. Aquat. Org. 1989, 6, 1–5. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish. Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Abellan, E.; Meseguer, J.; Esteban, M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish. Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ingram, G.A. Substances involved in the natural resistance of fish to infection a review. J. Fish. Biol. 1980, 16, 23–60. [Google Scholar] [CrossRef]
- Fletcher, T. Non-antibody molecules and the defense mechanisms of fish. In Stress and Fish; Pickering, A.D., Ed.; Academy Press: Washington, DC, USA, 1981; pp. 171–183. [Google Scholar]
- Esteban, M.A. An overview of the immunological defences in fish skin. Int. Sch. Res. Not. 2012, 2012, 853470. [Google Scholar]
- Shepard, K.L. Functions for fish mucus. Rev. Fish Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Urbina, M.A.; Meredith, A.S.; Forster, M.E.; Glover, C.N. The importance of cutaneous gas exchange in aquatic and aerial mediums in galaxiid fishes. J. Fish. Biol. 2014, 84, 759–773. [Google Scholar] [CrossRef]
- Urbina, M.A.; Glover, C.N. Effect of salinity on osmoregulation, metabolism and nitrogen excretion in the amphidromous fish, inanga (Galaxias maculatus). J. Exp. Mar. Bio. Ecol. 2015, 473, 7–15. [Google Scholar] [CrossRef]
- Tiralongo, F.; Messina, G.; Lombardo, B.M.; Longhitano, L.; Li Volti, G.; Tibullo, D. Skin mucus of marine fish as a source for the development of antimicrobial agents. Front. Mar. Sci. 2020, 7, 760. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H.N. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar]
- Minniti, G.; Rød Sandve, S.; Padra, J.T.; Heldal Hagen, L.; Lindén, S.; Pope, P.B.; Arntzen, M.Ø.; Vaaje-Kolstad, G. The farmed Atlantic Salmon (Salmo salar) skin-mucus proteome and its nutrient potential for the resident bacterial community. Genes 2019, 10, 515. [Google Scholar] [CrossRef]
- Holloway, J.; Shoemaker, C.A.; Ottinger, C.A. Serum lysozyme levels in paddle Fish walleye. J. Aquat Anim Health 1993, 5, 324–326. [Google Scholar] [CrossRef]
- Shailesh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar]
- Moeck, A.; Peters, G. Lysozyme activity in rainbow trout, Oncorhynchus mykiss (Walbaum), stressed by handling, transport and water pollution. J. Fish. Biol. 1990, 37, 873–885. [Google Scholar] [CrossRef]
- Røed, K.; Larsen, H.; Linder, R.; Refstie, T. Genetic variation in lysozyme activity in rainbow trout (Oncorhynchus mykiss). Aquaculture 1993, 109, 237–244. [Google Scholar] [CrossRef]
- Demers, N.E.; Bayne, C.J. The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev. Comp. Immunol. 1997, 21, 363–373. [Google Scholar] [CrossRef]
- Cortés, R.; Teles, M.; Trídico, R.; Acerete, L.; Tort, L. Effects of cortisol administered through slow-release implants on innate immune responses in rainbow trout (Oncorhynchus mykiss). Int. J. Genom. 2013, 2013, 619714. [Google Scholar] [CrossRef]
- Sunyer, J.O.; Gomez, E.; Navarro, V.; Quesada, J.; Tort, L. Depression of humoral components of the immune system and physiological responses in gilthead sea bream Sparus aurata after daily acute stress. Can. J. Fish. Aquat. 1995, 52, 2339–2346. [Google Scholar] [CrossRef]
- Ortuño, J.; Esteban, M.A.; Meseguer, J. Effects of short-term crowding stress on the gilthead seabream (Sparus aurata L.) innate immune response. Fish. Shellfish Immunol. 2001, 11, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Burgos, A.; Valenzuela, A.; Gonzalez, M.; Klempau, A. Non-specific defence mechanisms of rainbow trout (Oncorhynchus mykiss) during artificial photoperiod. Bull. Eur. Ass. Fish. Phatol. 2004, 24, 240–245. [Google Scholar]
- Redondo, M.J.; Alvarez-Pellitero, P. Carbohydrate patterns in the digestive tract of Sparus aurata (L.) and Psetta maxima (L.) (Teleostei) parasitized by Enteromyxum leei and E. scophthalmi (Myxozoa). Parasitol. Int. 2010, 59, 445–453. [Google Scholar] [CrossRef][Green Version]
- Estensoro, I.; Jung-Schroers, V.; Alvarez-Pellitero, P.; Steinhagen, D.; Sitja-Bobadilla, A. Effects of Enteromyxum leei (Myxozoa) infection on gilthead sea bream (Sparus aurata) (Teleostei) intestinal mucus: Glycoprotein profile and bacterial adhesion. Parasitol. Res. 2013, 112, 567e76. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Wang, H.; Ghia, J.E.; Haq, N.; Deng, Y.; Velcich, A.; Grencis, R.K.; Thornton, D.J.; Khan, W.I. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology 2010, 138, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Padra, J.T.; Murugan, A.V.M.; Sundell, K.; Sundh, H.; Benktander, J. Fish pathogen binding to mucins from Atlantic salmon and Arctic char differs in avidity and specificity and is modulated by fluid velocity. PLoS ONE 2019, 24, e0215583. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L. Mucosal immunity. Pediatrics 2003, 111, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H. Adaptive physiological processes in the host during gastrointestinal parasitism. Int. J. Parasitol. 2001, 31, 231–244. [Google Scholar] [CrossRef]
- Busch, L.; Borda, E. Mucinas salivales: Estructura química, mecanismos de liberación y participación en la defensa no inmunológica de la cavidad bucal. Revista de la Facultad de Odontología 2009, 24, 171–176. [Google Scholar]
- Zuchelkowski, E.; Pinkstaff, C.; Hinton, D. Mucosubstance histochemistry in control and acid-stressed epidermis of brown bullhead catfish Ictalurus nebulosus (LeSueur). Anat. Rec. 1985, 212, 327–335. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, A.; Rodríguez, I.; Schulz, B.; Cortés, R.; Acosta, J.; Campos, V.; Escobar-Aguirre, S. Effects of Continuous Light (LD24:0) Modulate the Expression of Lysozyme, Mucin and Peripheral Blood Cells in Rainbow Trout. Fishes 2022, 7, 28. https://doi.org/10.3390/fishes7010028
Valenzuela A, Rodríguez I, Schulz B, Cortés R, Acosta J, Campos V, Escobar-Aguirre S. Effects of Continuous Light (LD24:0) Modulate the Expression of Lysozyme, Mucin and Peripheral Blood Cells in Rainbow Trout. Fishes. 2022; 7(1):28. https://doi.org/10.3390/fishes7010028
Chicago/Turabian StyleValenzuela, Ariel, Ignacia Rodríguez, Berta Schulz, Raúl Cortés, Jannel Acosta, Víctor Campos, and Sebastián Escobar-Aguirre. 2022. "Effects of Continuous Light (LD24:0) Modulate the Expression of Lysozyme, Mucin and Peripheral Blood Cells in Rainbow Trout" Fishes 7, no. 1: 28. https://doi.org/10.3390/fishes7010028
APA StyleValenzuela, A., Rodríguez, I., Schulz, B., Cortés, R., Acosta, J., Campos, V., & Escobar-Aguirre, S. (2022). Effects of Continuous Light (LD24:0) Modulate the Expression of Lysozyme, Mucin and Peripheral Blood Cells in Rainbow Trout. Fishes, 7(1), 28. https://doi.org/10.3390/fishes7010028





