Correlation between Feeding Behaviors and Retinal Photoreceptor Cells of Largemouth Bass, Micropterus salmoides, in Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Site
2.2. Observation of Photoreceptor Cells
2.3. Data Analysis
3. Results
3.1. External Morphology of the Eye
3.2. Retina
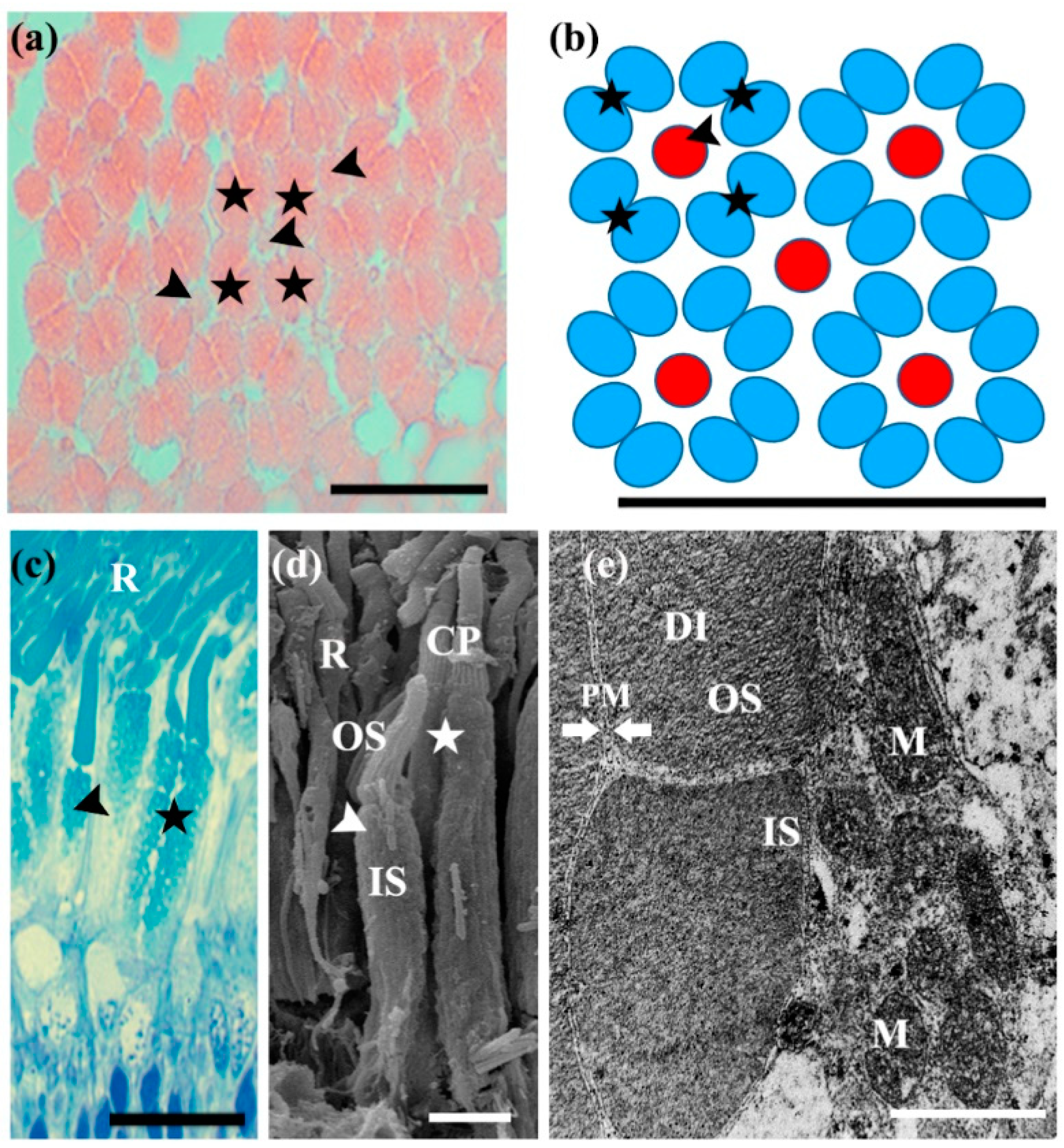
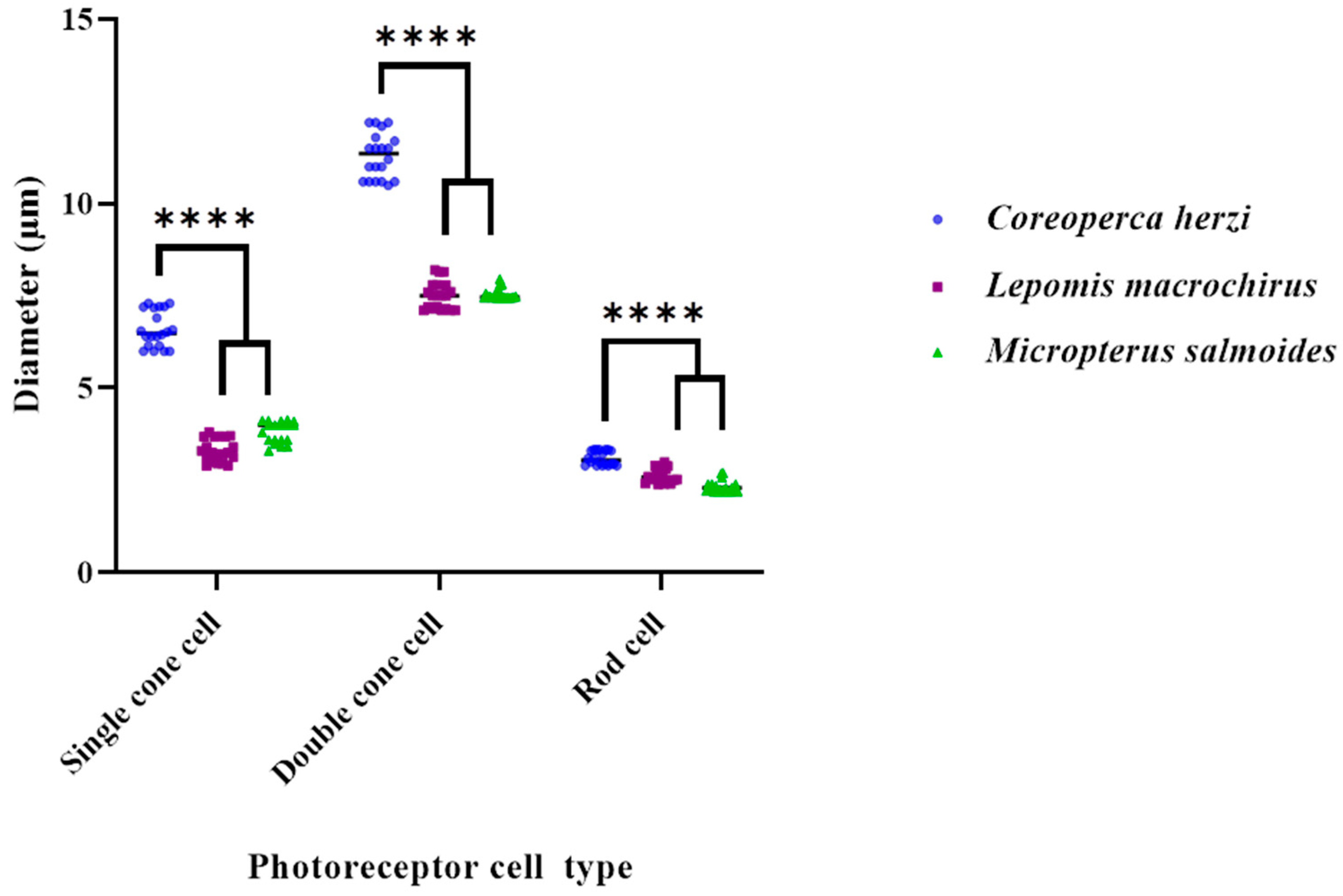
3.3. Photoreceptor Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, I.S.; Park, J.Y. Freshwater Fishes of Korea; Kyo-Hak Publishing Co., Ltd.: Seoul, Korea, 2002. (In Korean) [Google Scholar]
- Ko, M.-H.; Park, J.-Y.; Lee, Y.-J. Feeding habits of an introduced largemouth bass, Micropterus salmoides (Perciformes; Centrachidae), and its influence on ichthyofauna in the lake Okjeong, Korea. Korean J. Ichthyol 2008, 20, 36–44. [Google Scholar]
- Park, J.S.; Kim, S.H.; Kim, H.T.; Kim, J.G.; Park, J.Y.; Kim, H.S. Study on feeding habits of Micropterus salmoides in habitat types from Korea. Korean J. Ichthyol. 2019, 31, 39–53. [Google Scholar] [CrossRef]
- Lindsey, S.; Pauline, F.G.; Peter, M.D.; Jan, H.; Shaun, P.C.; Jennifer, L.K. Functional diversity of the lateral line system among populations of a native Australian freshwater fish. J. Exp. Biol. 2017, 220, 2265–2276. [Google Scholar] [CrossRef]
- Hickley, P.; North, R.; Muchiri, S.M.; Harper, D.M. The diet of largemouth bass, Micropterus salmoides, in Lake Naivasha, Kenya. J. Fish Biol. 1994, 44, 607–619. [Google Scholar] [CrossRef]
- Jang, M.-H.; Lee, J.-W.; Kim, J.-H.; Park, S.-H.; Choi, K.-R.; Lee, H.-J.; Yoon, J.-D. Impact of largemouth bass (Micropterus salmoides) on the population of Korean native fish, crucian carp (Carassius auratus). Korean J. Environ. Biol. 2013, 31, 370–375. [Google Scholar] [CrossRef]
- Kim, I.-T.; Park, J.-R.; Kim, W.-J. Indirect evaluation of aquatic animal diversity in Ilsan Lake through the analysis of the growing condition and stomach contents of largemouth bass, Micropterus salmoides. J. Korean Soc. Environ. Eng. 2013, 35, 953–959. [Google Scholar] [CrossRef]
- Mitchem, L.D.; Stanis, S.; Zhou, M.; Loew, E.; Epifanio, J.M.; Fuller, R.C. Seeing red: Color vision in the largemouth bass. Curr. Zool. 2018, 65, 43–52. [Google Scholar] [CrossRef]
- Lyall, A.H. Cone arrangements in teleost retinae. J. Cell Sci. 1957, 3, 189–201. [Google Scholar] [CrossRef]
- Collins, B.A.; MacNichol, E.F., Jr. Triple cones found in retinas of 3 fish species. Experientia 1979, 35, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Fernald, R.D. Cone mosaics in a teleost retina: No difference between light and dark adapted states. Experientia 1982, 38, 1337–1339. [Google Scholar] [CrossRef]
- McFarland, W.N. The visual world of coral reef fishes. In The ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Academic Press: New York, NY, USA, 1991; pp. 16–38. [Google Scholar]
- Nag, T.C.; Bhattacharjee, J. Retinal vascularisation in the loach Noemacheilus rupicola rupicola: Coexistence of falciform process and vitreal vessels. Environ. Biol. Fishes 1993, 36, 385–388. [Google Scholar] [CrossRef]
- Kim, J.G.; Park, J.Y.; Kim, C.H. Visual cells in the retina of the aucha perch Coreoperca herzi Herzenstein, 1896 (Pisces; Centropomidae) of Korea. J. Appl. Ichthyol. 2013, 30, 172–174. [Google Scholar] [CrossRef]
- Pankhurst, N.W. The relationship of ocular morphology to feeding modes and activity periods in shallow marine teleosts from New Zealand. Environ. Biol. Fishes 1989, 26, 201–211. [Google Scholar] [CrossRef]
- Myrberg, A.A., Jr.; Fuiman, L.A. The sensory world of reef fishes. In Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem; Sale, P.F., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 123–148. [Google Scholar]
- Ali, M.A.; Anctil, M. Retinas of Fishes: An Atlas; Springer: New York, NY, USA, 1976; p. 284. [Google Scholar] [CrossRef]
- Strykowski, J.L.; Schech, J.M. Effectiveness of recommended euthanasia methods in larval zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 81–84. [Google Scholar] [PubMed]
- Gurr, G.T. A Practical Manual of Medical and Biological Staining Techniques; Interscience Publishers: New York, NY, USA, 1956. [Google Scholar] [CrossRef][Green Version]
- Donatii, L.; Fanta, E. Morphology of the retina in the freshwater fish Metynnis roosevelti Eigenmann (Characidae, Serrasalminae) and the effects of monochromatic red light. Rev. Bras. Zool. 1999, 16, 151–173. [Google Scholar] [CrossRef][Green Version]
- Kusmic, C.; Gualtieri, P. Morphology and spectral sensitivities of retinal and extraretinal photoreceptors in freshwater teleosts. Micron 2000, 31, 183–200. [Google Scholar] [CrossRef]
- Kunz, Y.W. Cone mosaics in a teleost retina: Changes during light and dark adaptation. Experientia 1980, 36, 1371–1374. [Google Scholar] [CrossRef]
- Fernald, R.D. Aquatic adaptations in fish eyes. In Sensory Biology of Aquatic Animals; Atema, J., Fay, R.R., Popper, A.N., Tavolga, W.N., Eds.; Springer: New York, NY, USA, 1988; pp. 435–485. [Google Scholar] [CrossRef]
- Van der Meer, H.J. Constructional morphology of photoreceptor patterns in percomorph fish. Acta Biotheor. 1992, 40, 51–85. [Google Scholar] [CrossRef]
- Salem, M.A. Structure and function of the retinal pigment epithelium, photoreceptors and cornea in the eye of Sardinella aurita (Clupeidae, Teleostei). J. Basic. Appl. Zool. 2016, 75, 1–12. [Google Scholar] [CrossRef]
- Cameron, D.A.; Pugh, E.N., Jr. Double cones as a basis for a new type of polarization vision in vertebrates. Nature 1991, 353, 161–164. [Google Scholar] [CrossRef]
- Engström, K. Cone types and cone arrangements in teleost retinae. Acta Zool. 1963, 44, 179–243. [Google Scholar] [CrossRef]
- Nicol, J.A.C. The Eyes of Fishes; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Hunt, D.E.; Rawlinson, N.J.F.; Thomas, G.A.; Cobcroft, J.M. Investigating photoreceptor densities, potential visual acuity, and cone mosaics of shallow water, temperate fish species. Vis. Res. 2015, 111, 13–21. [Google Scholar] [CrossRef] [PubMed]

| Site | N | Total Length (mm) | Weight (g) | Head Length (mm) | Eye Diameter (%) |
|---|---|---|---|---|---|
| 1 | 1 | 200 | 150 | 65.4 | 24.8 |
| 2 | 210 | 155 | 70.5 | 25.0 | |
| 3 | 240 | 195 | 73.4 | 25.5 | |
| 4 | 220 | 175 | 75.2 | 25.2 | |
| 5 | 210 | 150 | 72.5 | 25.1 | |
| 2 | 1 | 260 | 200 | 80.2 | 25.5 |
| 2 | 220 | 180 | 74.8 | 25.1 | |
| 3 | 250 | 190 | 82.5 | 25.5 | |
| 4 | 200 | 170 | 65.0 | 25.0 | |
| 5 | 210 | 155 | 66.5 | 25.0 |
| Cell Types | (µm) | Coreoperca herzi | Lepomis macrochirus | Micropterus salmoides | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | ||
| Rods | Length | 75.3 ± 6.2 | 69.3–83. 8 | 74.7 ± 5.7 | 69.6–83.3 | 75.6 ± 6.3 | 69.6–83.3 |
| Diameter | 3.1 ± 0.24 | 2.9–3.33 | 2.7 ± 0.2 | 2.52–2.9 | 2.3 ± 0.2 | 2.2–2.7 | |
| Single Cones | Length | 24.0 ± 0.04 | 23.6–24.9 | 31.4 ± 3.0 | 25.0–32.4 | 27.8 ± 1.6 | 25.0–32.4 |
| Diameter | 6.6 ± 0.5 | 6.0–7.3 | 3.3 ± 0.3 | 2.9–3.7 | 3.8 ± 0.2 | 3.3–4.1 | |
| Double Cones | Length | 21.8 ± 1.3 | 20.0–25.1 | 34.3 ± 2.1 | 38.7–40.6 | 39.3 ± 0.8 | 38.7–40.6 |
| Diameter | 11.3 ± 0.4 | 10.6–12.2 | 7.5 ± 0.4 | 7.1–8.2 | 7.5 ± 0.2 | 7.46–7.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.G.; Kim, S.H.; Park, J.Y.; Yoo, S.-H. Correlation between Feeding Behaviors and Retinal Photoreceptor Cells of Largemouth Bass, Micropterus salmoides, in Korea. Fishes 2022, 7, 25. https://doi.org/10.3390/fishes7010025
Kim JG, Kim SH, Park JY, Yoo S-H. Correlation between Feeding Behaviors and Retinal Photoreceptor Cells of Largemouth Bass, Micropterus salmoides, in Korea. Fishes. 2022; 7(1):25. https://doi.org/10.3390/fishes7010025
Chicago/Turabian StyleKim, Jae Goo, Su Hwan Kim, Jong Young Park, and Su-Hyang Yoo. 2022. "Correlation between Feeding Behaviors and Retinal Photoreceptor Cells of Largemouth Bass, Micropterus salmoides, in Korea" Fishes 7, no. 1: 25. https://doi.org/10.3390/fishes7010025
APA StyleKim, J. G., Kim, S. H., Park, J. Y., & Yoo, S.-H. (2022). Correlation between Feeding Behaviors and Retinal Photoreceptor Cells of Largemouth Bass, Micropterus salmoides, in Korea. Fishes, 7(1), 25. https://doi.org/10.3390/fishes7010025







