Effects of Different Temperatures on the Antibacterial, Immune and Growth Performance of Crucian Carp Epidermal Mucus
Abstract
1. Introduction
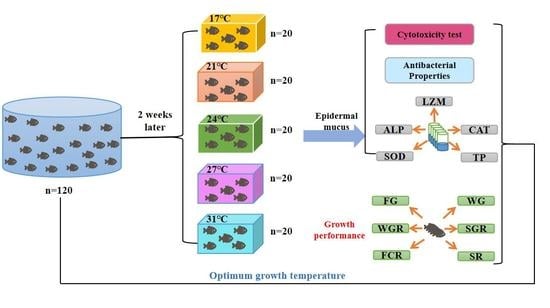
2. Materials and Methods
2.1. Experimental Materials
2.2. Extraction of Epidermal Mucus
2.3. Cytotoxicity Tests
2.4. Antibacterial Activity
2.5. Determination of Enzyme Activity
2.6. Growth Performance
2.7. Statistical Analyses
3. Results
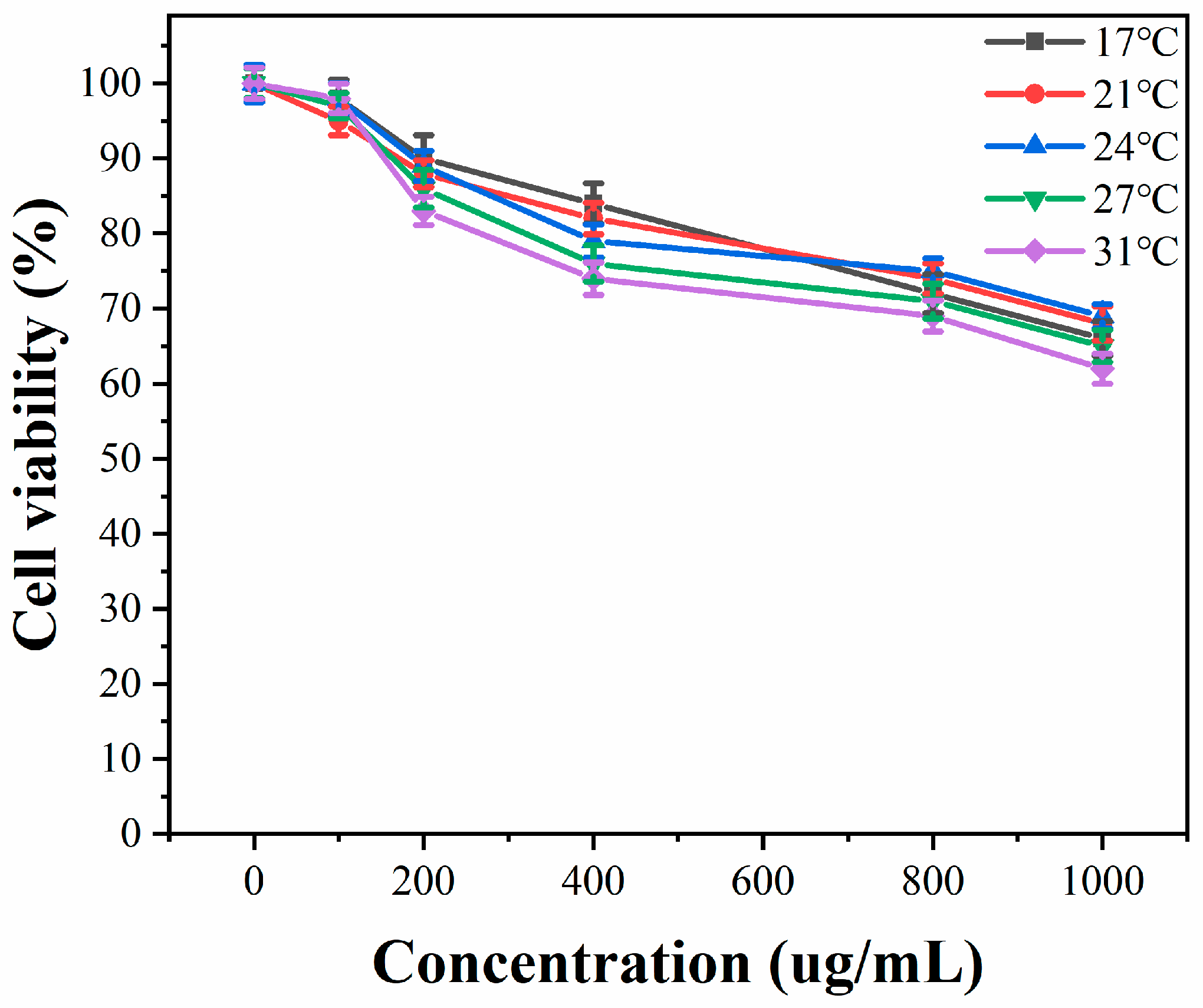
3.1. Cytotoxicity
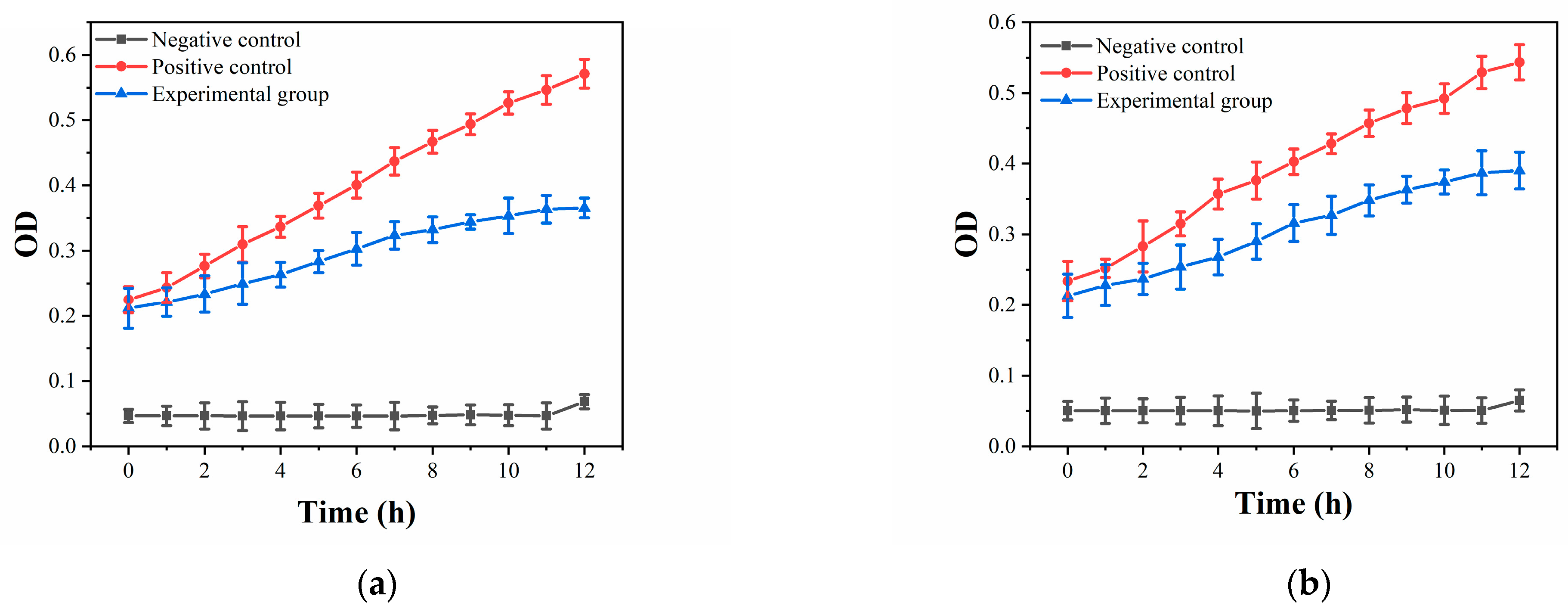
3.2. Antibacterial Properties
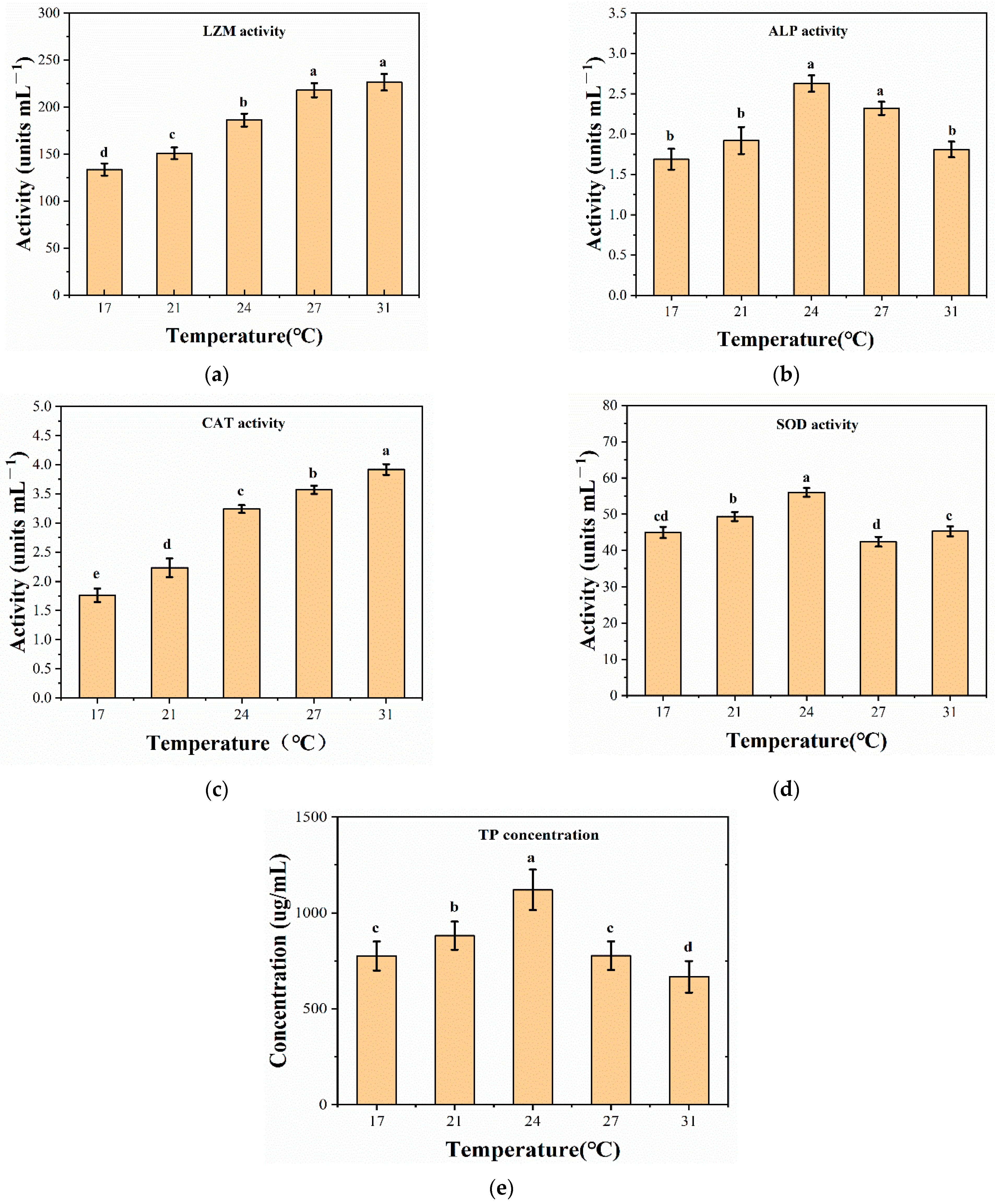
3.3. Enzyme Activity
3.4. Growth Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kapusta, A.; Partyka, K.; Szczepkowska, B.; Jarmołowicz, S.; Hopko, M.; Piotrowska, I.; Kowalska, A.; Zakęś, Z. Impact of diet and culture conditions on the body shape of crucian carp (Carassius carassius L.). J. Appl. Anim. Res. 2013, 41, 462–469. [Google Scholar] [CrossRef][Green Version]
- Sikorska, J.; Kondera, E.; Kamiński, R.; Ługowska, K.; Witeska, M.; Wolnicki, J. Effect of four rearing water temperatures on some performance parameters of larval and juvenile crucian carp, Carassius carassius, under controlled conditions. Aquac. Res. 2018, 49, 3874–3880. [Google Scholar] [CrossRef]
- Wolnicki, J.; Kamiński, R.; Sikorska, J. Combined effects of water temperature and daily food availability period on the growth and survival of tench (Tinca tinca) larvae. Aquac. Res. 2017, 48, 3809–3816. [Google Scholar] [CrossRef]
- Alshammari, E.; Patel, M.; Sachidanandan, M.; Kumar, P.; Adnan, M. Potential Evaluation and Health Fostering Intrinsic Traits of Novel Probiotic Strain Enterococcus durans F3 Isolated from the Gut of Fresh Water Fish Catla catla. Food Sci. Anim. Resour. 2019, 39, 844–861. [Google Scholar] [CrossRef]
- Cabillon, N.A.R.; Lazado, C.C. Mucosal Barrier Functions of Fish under Changing Environmental Conditions. Fishes 2019, 4, 2. [Google Scholar] [CrossRef]
- Fæste, C.K.; Tartor, H.; Moen, A.; Kristoffersen, A.B.; Dhanasiri, A.K.S.; Anonsen, J.H.; Furmanek, T.; Grove, S. Proteomic profiling of salmon skin mucus for the comparison of sampling methods. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1138, 121965. [Google Scholar] [CrossRef]
- Abolfathi, M.; Akbarzadeh, A.; Hajimoradloo, A.; Joshaghani, H.R. Seasonal changes of hydrolytic enzyme activities in the skin mucus of rainbow trout, Oncorhynchus mykiss at different body sizes. Dev. Comp. Immunol. 2020, 103, 103499. [Google Scholar] [CrossRef]
- Mori, M.; Ito, T.; Washio, R.; Shibasaki, Y.; Namba, A.; Yabu, T.; Iwazaki, D.; Wada, N.; Anzai, H.; Shiba, H.; et al. Enhancement of immune proteins expression in skin mucus of Japanese flounder Paralicthys olivaceus upon feeding a diet supplemented with high concentration of ascorbic acid. Fish Shellfish Immunol. 2021, 114, 20–27. [Google Scholar] [CrossRef]
- Patel, M.; Ashraf, M.S.; Siddiqui, A.J.; Ashraf, S.A.; Sachidanandan, M.; Snoussi, M.; Adnan, M.; Hadi, S. Profiling and Role of Bioactive Molecules from Puntius sophore (Freshwater/Brackish Fish) Skin Mucus with Its Potent Antibacterial, Antiadhesion, and Antibiofilm Activities. Biomolecules 2020, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Grande, B.; Guerreiro, P.M.; Sanahuja, I.; Fernández-Alacid, L.; Ibarz, A. Environmental Salinity Modifies Mucus Exudation and Energy Use in European Sea Bass Juveniles. Animals 2021, 11, 1580. [Google Scholar] [CrossRef]
- Sridhar, A.; Manikandan, D.B.; Palaniyappan, S.; Sekar, R.K.; Ramasamy, T. Correlation Between Three Freshwater Fish Skin Mucus Antiproliferative Effect and Its Elemental Composition Role in Bacterial Growth. Turk. J. Fish. Aquat. Sci. 2021, 21, 233–244. [Google Scholar] [CrossRef]
- Ceballos-Francisco, D.; Cuesta, A.; Esteban, M. Ángeles Effect of Light–Dark Cycle on Skin Mucosal Immune Activities of Gilthead Seabream (Sparus aurata) and European Sea Bass (Dicentrarchus labrax). Fishes 2020, 5, 10. [Google Scholar] [CrossRef]
- Almroth, B.C.; de Souza, K.B.; Jönsson, E.; Sturve, J. Oxidative stress and biomarker responses in the Atlantic halibut after long term exposure to elevated CO2 and a range of temperatures. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 238, 110321. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Allan, B.J.M.; McQueen, D.E.; Nicol, S.; Parsons, D.M.; Pether, S.M.J.; Pope, S.; Setiawan, A.N.; Smith, N.; Wilson, C.; et al. Ocean warming has a greater effect than acidification on the early life history development and swimming performance of a large circumglobal pelagic fish. Glob. Chang. Biol. 2018, 24, 4368–4385. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Kunzmann, A.; Bögner, M.; Meyer, A.; Thiele, R.; Slater, M.J. Metabolic and molecular stress responses of European seabass, Dicentrarchus labrax at low and high temperature extremes. Ecol. Indic. 2020, 112, 106118. [Google Scholar] [CrossRef]
- Yilmaz, H.A.; Turkmen, S.; Kumlu, M.; Eroldogan, O.T.; Perker, N. Alteration of Growth and Temperature Tolerance of European Sea Bass (Dicentrarchus labrax Linnaeus 1758) in Different Temperature and Salinity Combinations. Turk. J. Fish. Aquat. Sci. 2020, 20, 331–340. [Google Scholar] [CrossRef]
- Nadermann, N.; Seward, R.K.; Volkoff, H. Effects of potential climate change -induced environmental modifications on food intake and the expression of appetite regulators in goldfish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 235, 138–147. [Google Scholar] [CrossRef]
- Masroor, W.; Farcy, E.; Blondeau-Bidet, E.; Venn, A.; Tambutté, E.; Lorin-Nebel, C. Effect of salinity and temperature on the expression of genes involved in branchial ion transport processes in European sea bass. J. Therm. Biol. 2019, 85, 102422. [Google Scholar] [CrossRef]
- Masroor, W.; Farcy, E.; Gros, R.; Lorin-Nebel, C. Effect of combined stress (salinity and temperature) in European sea bass Dicentrarchus labrax osmoregulatory processes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 215, 45–54. [Google Scholar] [CrossRef]
- Gamperl, A.K.; Ajiboye, O.O.; Zanuzzo, F.S.; Sandrelli, R.M.; Peroni, E.D.F.C.; Beemelmanns, A. The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic Salmon (Salmo salar). Aquaculture 2020, 519, 734874. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Chatzigiannidou, I.; Feidantsis, K.; Kounna, C.; Chatzifotis, S. Effect of water temperature on cellular stress responses in meagre (Argyrosomus regius). Fish Physiol. Biochem. 2020, 46, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.F.; Braz-Mota, S.; Val, A.L.; Almeida-Val, V.M.F. Predicting thermal sensitivity of three Amazon fishes exposed to climate change scenarios. Ecol. Indic. 2019, 101, 533–540. [Google Scholar] [CrossRef]
- Islam, M.A.; Uddin, M.H.; Uddin, M.J.; Shahjahan, M. Temperature changes influenced the growth performance and physiological functions of Thai pangas Pangasianodon hypophthalmus. Aquacult. Rep. 2019, 13, 100179. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Thiele, R.; Slater, M.J. Effects of extreme ambient temperature in European seabass, Dicentrarchus labrax acclimated at different salinities: Growth performance, metabolic and molecular stress responses. Sci. Total. Environ. 2020, 735, 139371. [Google Scholar] [CrossRef]
- Islam, M.J.; Slater, M.J.; Bögner, M.; Zeytin, S.; Kunzmann, A. Extreme ambient temperature effects in European seabass, Dicentrarchus labrax: Growth performance and hemato-biochemical parameters. Aquaculture 2020, 522, 735093. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Hoseinifar, S.H.; Tongsiri, S.; Chitmanat, C.; Musthafa, M.S.; El-Haroun, E.; Ringo, E. Modulation of growth, innate immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) culture under biofloc system by supplementing pineapple peel powder and Lactobacillus plantarum. Fish Shellfish Immunol. 2021, 115, 212–220. [Google Scholar] [CrossRef]
- Rogatsky, E. Pandora box of BCA assay. Investigation of the accuracy and linearity of the microplate bicinchoninic protein assay: Analytical challenges and method modifications to minimize systematic errors. Anal. Biochem. 2021, 631, 114321. [Google Scholar] [CrossRef]
- Liao, Y.H.; Brown, M.B.; Martin, G.P. Turbidimetric and HPLC assays for the determination of formulated lysozyme activity. J. Pharm. Pharmacol. 2001, 53, 549–554. [Google Scholar] [CrossRef]
- Li, N.; Huang, H.-Q.; Zhang, G.-S.; Cui, W. Effect of 5-AZn-2′-deoxycytidine on proliferation of human lung adenocarcinoma cell line A549 in vitro. Asian Pac. J. Trop. Med. 2013, 6, 982–985. [Google Scholar] [CrossRef]
- Wu, T.; Shen, X. Response of Wumeng Semi-Fine Wool Sheep to Copper-Contaminated Environment. Pol. J. Environ. Stud. 2020, 29, 2917–2924. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Peng, H.; Arabi, M.; Li, J.; Xiong, H.; Choo, J.; Chen, L. Ratiometric fluorescence and colorimetry dual-mode assay based on manganese dioxide nanosheets for visual detection of alkaline phosphatase activity. Sensors Actuators B Chem. 2020, 302, 127176. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, G.; Du, X.; Liu, Y.; Wang, B.; Xu, G.; Mao, H. Effects of Nutrient Solution Irrigation Quantity and Downy Mildew Infection on Growth and Physiological Traits of Greenhouse Cucumber. Agronomy 2020, 10, 1921. [Google Scholar] [CrossRef]
- Cai, H.Z.; Chen, Y.X.; Xu, L.S.; Zou, Y.P.; Zhou, X.L.; Liang, G.X.; Wang, D.Q.; Tao, Z.M. Differently PEGylated Polymer Nanoparticles for Pancreatic Cancer Delivery: Using a Novel Near-Infrared Emissive and Biodegradable Polymer as the Fluorescence Tracer. Front. Bioeng. Biotechnol. 2021, 9, 699610. [Google Scholar] [CrossRef]
- Dominguez, M.; Takemura, A.; Tsuchiya, M.; Nakamura, S. Impact of different environmental factors on the circulating immunoglobulin levels in the Nile tilapia, Oreochromis niloticus. Aquaculture 2004, 241, 491–500. [Google Scholar] [CrossRef]
- Firmino, J.P.; Fernández-Alacid, L.; Vallejos-Vidal, E.; Salomón, R.; Sanahuja, I.; Tort, L.; Ibarz, A.; Reyes-López, F.E.; Gisbert, E. Carvacrol, Thymol, and Garlic Essential Oil Promote Skin Innate Immunity in Gilthead Seabream (Sparus aurata) Through the Multifactorial Modulation of the Secretory Pathway and Enhancement of Mucus Protective Capacity. Front. Immunol. 2021, 12, 633621. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Abellán, E.; Meseguer, J.; Esteban, M.A. Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immunol. 2014, 40, 24–31. [Google Scholar] [CrossRef]
- Palaksha, K.; Shin, G.-W.; Kim, Y.-R.; Jung, T.S. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2008, 24, 479–488. [Google Scholar] [CrossRef]
- Valero, Y.; García-Alcázar, A.; Esteban, M.Á.; Cuesta, A.; Chaves-Pozo, E. Seasonal variations of the humoral immune parameters of European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2014, 39, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.J.; Clark, B.E.; Van Eenennaam, J.P.; Schreier, A.D.; Todgham, A.E. The effects of warm temperature acclimation on constitutive stress, immunity, and metabolism in white sturgeon (Acipenser transmontanus) of different ploidies. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 224, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Guo, Z.-X.; Ye, C.-X.; Wang, A.-L. Effect of dietary astaxanthin on the growth performance, non-specific immunity, and antioxidant capacity of pufferfish (Takifugu obscurus) under high temperature stress. Fish Physiol. Biochem. 2018, 44, 209–218. [Google Scholar] [CrossRef]
- Mougin, J.; Roquigny, R.; Flahaut, C.; Bonnin-Jusserand, M.; Grard, T.; Le Bris, C. Abundance and spatial patterns over time of Vibrionaceae and Vibrio harveyi in water and biofilm from a seabass aquaculture facility. Aquaculture 2021, 542, 736862. [Google Scholar] [CrossRef]
- Ndong, D.; Chen, Y.Y.; Lin, Y.H.; Vaseeharan, B.; Chen, J.C. The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol. 2007, 22, 686–694. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Ma, A.-J.; Wang, X.-A. The immune response of turbot, Scophthalmus maximus (L.), skin to high water temperature. J. Fish Dis. 2011, 34, 619–627. [Google Scholar] [CrossRef]
- Souza, D.C.D.M.; Dos Santos, M.C.; Chagas, E.C. Immune response of teleost fish to helminth parasite infection. Rev. Bras. Parasitol. Vet. 2019, 28, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; El-Haroun, E.R.; Hassaan, M.S.; Bowyer, P.H. A Solid-State Fermentation (SSF) supplement improved performance, digestive function and gut ultrastructure of rainbow trout (Oncorhynchus mykiss) fed plant protein diets containing yellow lupin meal. Aquaculture 2021, 545, 737177. [Google Scholar] [CrossRef]
- Kumari, U.; Verma, N.; Nigam, A.K.; Mittal, S.; Mittal, A.K. Wound-healing potential of curcumin in the carp, Labeo rohita. Aquac. Res. 2016, 48, 2411–2427. [Google Scholar] [CrossRef]
- Esteban, M.A.; Cuesta, A.; Rodriguez, A.; Meseguer, J. Effect of photoperiod on the fish innate immune system: A link between fish pineal gland and the immune system. J. Pineal Res. 2006, 41, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, C.; Ytteborg, E.; Timmerhaus, G.; Høst, V.; Handeland, S.; Jørgensen, S.M.; Krasnov, A. Atlantic salmon skin barrier functions gradually enhance after seawater transfer. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Espinosa-Ruíz, C.; Esteban, M.Á. Wound-Induced Changes in Antioxidant Enzyme Activities in Skin Mucus and in Gene Expression in the Skin of Gilthead Seabream (Sparus aurata L.). Fishes 2021, 6, 15. [Google Scholar] [CrossRef]
- Ai-Jun, M.; Zhi-Hui, H.; Xin-An, W. Changes in protein composition of epidermal mucus in turbot Scophthalmus maximus (L.) under high water temperature. Fish Physiol. Biochem. 2013, 39, 1411–1418. [Google Scholar] [CrossRef] [PubMed]

| Crude Protein (%) ≥ | Crude Fiber (%) ≤ | Crude Fat (%) ≥ | Crude Ash (%) ≤ | Total Phosphors (%) ≥ | Moisture (%) ≤ | Lysine (%) ≥ |
|---|---|---|---|---|---|---|
| 35.0 | 12.0 | 5.0 | 15.0 | 1.0 | 12.5 | 1.6 |
| 17 °C | 21 °C | 24 °C | 27 °C | 31 °C | |
|---|---|---|---|---|---|
| IW (g) | 9.19 ± 1.09 | 9.07 ± 0.81 | 9.11 ± 1.14 | 9.49 ± 0.99 | 8.93 ± 1.40 |
| FG (g) | |||||
| 3 weeks | 11.99 ± 0.96 bc | 12.73 ± 0.81 ab | 13.33 ± 1.06 a | 12.16 ± 0.94 bc | 11.17 ± 1.33 c |
| 6 weeks | 13.96 ± 0.87 b | 14.92 ± 1.60 b | 16.36 ± 1.03 a | 14.25 ± 0.99 b | 12.73 ± 1.33 c |
| WG (g) | |||||
| 3 weeks | 2.80 ± 0.96 bc | 3.66 ± 0.81 ab | 4.22 ± 1.06 a | 2.67 ± 0.94 bc | 2.24 ± 1.33 c |
| 6 weeks | 4.77 ± 0.87 b | 5.85 ± 1.60 b | 7.25 ± 1.03 a | 4.76 ± 0.99 b | 3.80 ± 1.33 c |
| WGR (%) | |||||
| 3 weeks | 32.16 ± 2.02 b | 40.69 ± 4.75 ab | 47.49 ± 1.29 a | 29.87 ± 2.03 b | 25.81 ± 6.13 c |
| 6 weeks | 53.27 ± 1.37 b | 65.34 ± 3.08 b | 81.28 ± 5.61 a | 50.70 ± 6.17 bc | 43.79 ± 5.22 c |
| SGR (%) | |||||
| 3 weeks | 1.27 ± 0.03 b | 1.61 ± 0.02 b | 1.81 ± 0.01 a | 1.18 ± 0.02 b | 1.07 ± 0.01 c |
| 6 weeks | 0.97 ± 0.01 b | 1.19 ± 0.03 b | 1.39 ± 0.01 a | 1.01 ± 0.01 b | 0.84 ± 0.02 c |
| FCR (%) | |||||
| 3 weeks | 1.01 ± 0.02 b | 1.03 ± 0.02 b | 1.04 ± 0.01 a | 1.03 ± 0.01 b | 1.01 ± 0.01 b |
| 6 weeks | 1.07 ± 0.01 b | 1.08 ± 0.01 b | 1.12 ± 0.02 a | 1.06 ± 0.02 b | 1.04 ± 0.02 b |
| SR (%) | |||||
| 3 weeks | 100 | 100 | 100 | 100 | 95.60 |
| 6 weeks | 95.00 ± 0.82 | 93.35 ± 1.25 | 93.35 ± 1.25 | 86.65 ± 1.25 | 84.21 ± 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Ma, G.; Liu, Y.; Wang, Y.; Du, X.; Shi, Q.; Mao, H. Effects of Different Temperatures on the Antibacterial, Immune and Growth Performance of Crucian Carp Epidermal Mucus. Fishes 2021, 6, 66. https://doi.org/10.3390/fishes6040066
Wang B, Ma G, Liu Y, Wang Y, Du X, Shi Q, Mao H. Effects of Different Temperatures on the Antibacterial, Immune and Growth Performance of Crucian Carp Epidermal Mucus. Fishes. 2021; 6(4):66. https://doi.org/10.3390/fishes6040066
Chicago/Turabian StyleWang, Bin, Guoxin Ma, Yong Liu, Yafei Wang, Xiaoxue Du, Qiang Shi, and Hanping Mao. 2021. "Effects of Different Temperatures on the Antibacterial, Immune and Growth Performance of Crucian Carp Epidermal Mucus" Fishes 6, no. 4: 66. https://doi.org/10.3390/fishes6040066
APA StyleWang, B., Ma, G., Liu, Y., Wang, Y., Du, X., Shi, Q., & Mao, H. (2021). Effects of Different Temperatures on the Antibacterial, Immune and Growth Performance of Crucian Carp Epidermal Mucus. Fishes, 6(4), 66. https://doi.org/10.3390/fishes6040066