Effect of MMP/TIMP Balancing of Cynoglossus semilaevis Shell Extracts on Skin Protection
Abstract
:1. Introduction
2. Reagents and Methods
2.1. Materials and Reagents
2.2. Preparation of Cynoglossus semilaevis Shell Extract (CSE)
2.3. Amino Acid Composition Analysis
2.4. Cell Line Selection and Cell Culture
2.5. MTT Assay
2.6. UV-B Irradiation
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Measurement of the Ability to Inhibit Elastase Activity
2.9. Reverse Transcription Polymerase Chain Reaction (RT-PCR) Analysis
2.10. Statistical Analysis
3. Results
3.1. Amino Acid Composition Analysis
3.2. Effect of CSE on HDF Cells Viability
3.3. Effect of CSE on the Type I Pro-Collagen and MMP-1
3.4. Effect of CSE on the Elastase
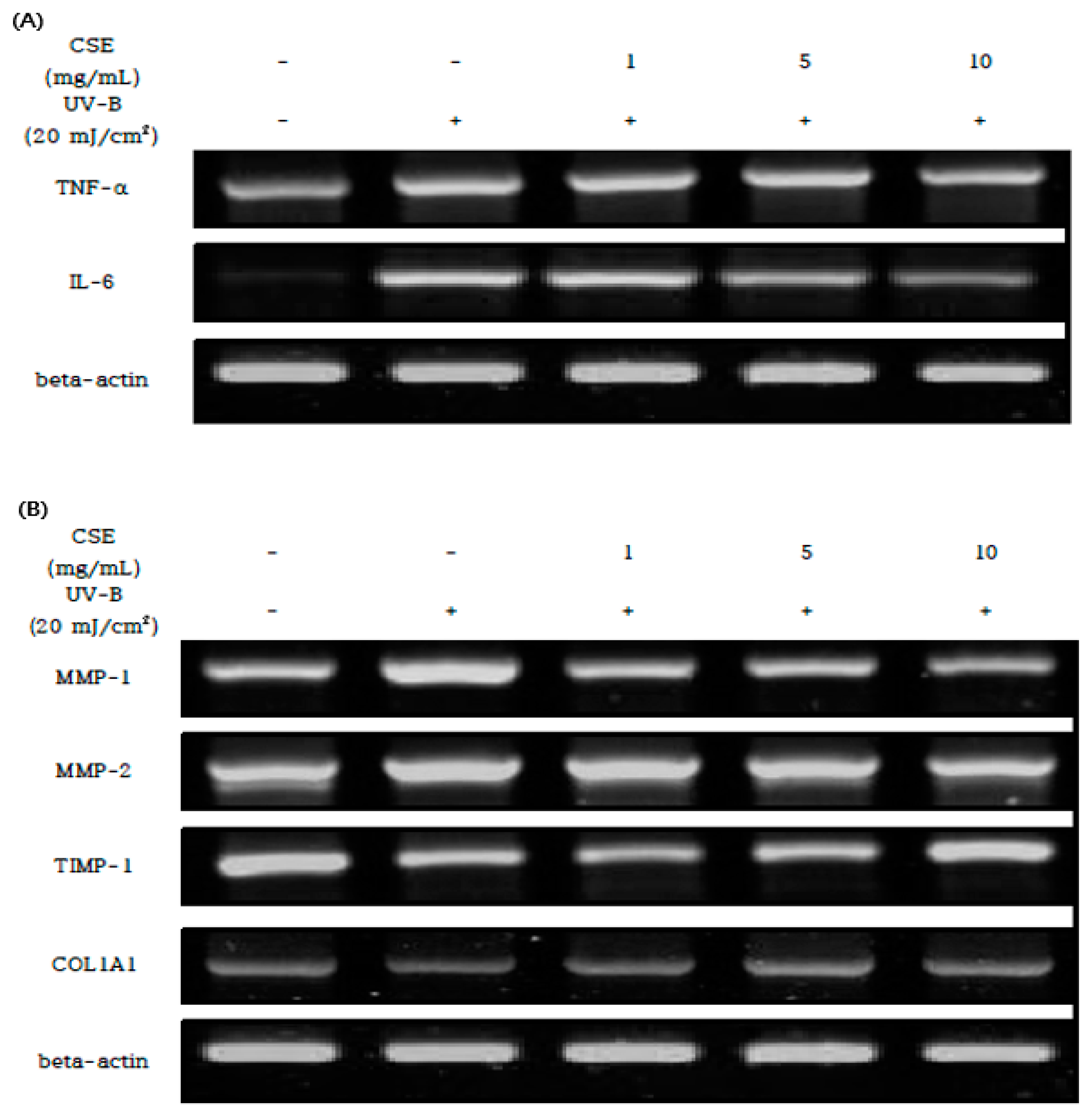
3.5. Effect of CSE on the UV-B Mediated Expression
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kim, J.M.; Jeon, S.W.; Lee, W.G.; Nam, H.J.; Kim, Y.B. Study of Preventing Methods for Skin Aging and Wrinkles. Korean J. Orient. Physiol. Pathol. 2010, 24, 533–542. [Google Scholar]
- Marionnet, C.; Tricaud, C.; Bernerd, F. Exposure to non-extreme solar UV daylight: Spectral characterization, effects on skin and photoprotection. Int. J. Mol. Sci. 2014, 16, 68–90. [Google Scholar] [CrossRef]
- Lee, J.H.; An, H.T.; Chung, J.H.; Kim, K.H.; Eun, H.C.; Cho, K.H. Acute effects of UVB radiation on the proliferation and differentiation of keratinocytes. Photodermatol. Photoimmunol. Photomed. 2002, 18, 253–261. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic aging vs.photoaging; A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Wlaschek, M.; Tantcheva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schuller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Fourniere, M.; Bedoux, G.; Lebonvallet, N.; Leschiera, R.; Goff-Pain, C.L.; Bourgougnon, N.; Latire, T. Poly- and Oligosaccharide Ulva sp. Fractions from Enzyme-Assisted Extraction Modulate the Metabolism of Extracellular Matrix in Human Skin Fibroblasts: Potential in Anti-Aging Dermo-Cosmetic Applications. Mar. Drugs. 2021, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases:multifunctional contributors to tumor progression. Mol. Med. Today 2000, 6, 149–156. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. In Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2017; Volume 147, pp. 1–73. [Google Scholar]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Matalloproteinase inhibitors: Status and scope from marine organisms. Biochem. Res. Int. 2010. [Google Scholar] [CrossRef] [Green Version]
- Prost-Squarcioni, C.; Fraitag, S.; Heller, M.; Boehm, N. Functional histology of dermis. Ann. Dermatol. Venereol. 2008, 135, 5–20. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, W.S.; Son, E.D.; Ahn, S.M.; Hwang, J.S. In Vitro evalution methods for the development of cosmeceutical ingredients. J. Alternat. Animal Exp. 2007, 1, 33–40. [Google Scholar]
- Talwar, H.S.; Griffiths, C.E.; Fisher, G.J.; Hamilton, T.A.; Voorhees, J.J. Reduced type I and type III pro-collagens in photodamaged adult human skin. J. Investig. Dermatol. 1995, 105, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Gomez, D.E.; Alonso, D.F.; Yoshiji, H.; Thorgeirsson, U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997, 74, 111–122. [Google Scholar] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinase in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Overall, C.M.; Lopez-Otin, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef]
- Baker, A.H.; Edwards, D.R.; Murphy, G. Metalloproteinase inhibitors: Biological actions and therapeutic opportunities. J. Cell Sci. 2002, 115, 3719–3727. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.K.; Kim, Y.A.; Kim, M.M.; Park, J.S.; Kim, J.A.; Kim, S.K.; Lee, B.J.; Nam, T.J.; Seo, Y.W. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol. In Vitro 2008, 22, 1742–1748. [Google Scholar] [CrossRef]
- Yoon, S.O.; Park, S.J.; Yoon, S.Y.; Yun, C.H.; Chung, A.S. Sustained production of H2O2 activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J. Biol. Chem. 2002, 277, 30271–30282. [Google Scholar] [CrossRef] [Green Version]
- Nagase, H.; Woessner, F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inomata, S.; Matsunaga, Y.; Amano, S.; Takada, K.; Kobayashi, K.; Tsunenaga, M.; Nishiyama, T.; Kohno, Y.; Fukuda, M. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investig. Dermatol. 2003, 120, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Matrix metalloproteinases and the development of cancer. Chem. Biol. 1996, 3, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.W.; Lim, H.K.; Kang, K.Y.; Han, H.S.; Do, Y.H.; Park, J.S. Maturation and Spawning of the Female Tongue Sole, Cynoglossus semilaevis in the West Coast of Korea. Dev. Reprod. 2012, 16, 87–93. [Google Scholar]
- Wang, L.; Lee, W.W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.M.; Jeon, Y.J. Protective Effect of Sulfated Polysaccharides from Celluclast-Assisted Extract of Hizikia fusiforme Against Ultraviolet B-Induced Skin Damage by Regulating NF-Κb, ap-1, AND mapkS Signaling Pathways In vitro in Human Dermal Fibroblast. Mar. Drugs. 2018, 16, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Fernando, I.S.; Kim, E.A.; Jeon, Y.J. Soft corals collected from Jeju Island; a potential source of anti-inflammatory phytochemicals. J. Chitin. Chitosan. 2016, 21, 247–254. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Bergamo, B.M.; Vyalil, P.K.; Elmets, C.A. Green tea polyphenols: DNA photodamage and photoimmunology. J. Photochem. Photobiol. 2001, 65, 109–114. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, S.Y.; Kim, E.A.; Lee, J.H.; Kim, Y.S.; Yu, S.K.; Chae, J.B.; Choe, I.H.; Cho, J.H.; Jeon, Y.J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohyd. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, N.; Hua, Y.; Wang, B.; Ling, L.; Xu, B. Metformin Inhibits Angiotensin II-Induced Differentiation of Cardiac Fibroblasts into Myofibroblasts. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.A.; Kim, B.A.; Lee, J.T. Antioxidative and Anti-Aging Effects of Pinus Rigida Mill. Ethyl Acetate Extract on the Human Dermal Fibroblast Cell Line CCD-986sk damaged by Ultraviolet B Radiation. Biomed. J. Sci. Tech. Res. 2019, 12, 1–7. [Google Scholar]
- Song, Y.; Zhu, L.; Li, M. Antifibrotic effects of crocetin in scleroderma fibroblasts and in bleomycin-induced sclerotic mice. Clinics 2013, 10, 1350–1357. [Google Scholar] [CrossRef]
- Choi, J.Y.; Moon, S.H.; Bae, H.M.; Kim, Y.W.; Lee, D.H.; Kim, S.T.; Seo, Y.L.; Wang, H.S.; Choi, Y.W.; Lee, M.W.; et al. Alnus sibirica Extracts Suppress the Expression of Inflammatory Cytokines Induced by Lipopolysaccharides, Tumor Necrosis Factor-α, and Interferon-γ in Human Dermal Fibroblasts. Molecules 2019, 24, 2883. [Google Scholar] [CrossRef] [Green Version]
- Pallela, R.; Na-Young, Y.; Kim, S.K. Anti-photoaging and photoprotective compounds derived from marine organisms. Mar. Drugs. 2010, 8, 1189–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adil, M.D.; Kaiser, P.; Satti, N.K.; Zargar, A.M.; Vishwakarma, R.A.; Tasduq, S.A. Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. J. Ethnopharmacol. 2010, 132, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell Mol. Life Sci. 1999, 55, 639–652. [Google Scholar] [CrossRef] [PubMed]

 : Measures in each group,
: Measures in each group,  : Mean value of untreated group,
: Mean value of untreated group,  : Mean value of CSE treatment group,
: Mean value of CSE treatment group,  : Mean value of positive control.
: Mean value of positive control.
 : Measures in each group,
: Measures in each group,  : Mean value of untreated group,
: Mean value of untreated group,  : Mean value of CSE treatment group,
: Mean value of CSE treatment group,  : Mean value of positive control.
: Mean value of positive control.
 : Measures in each group,
: Measures in each group,  : Mean value of untreated group,
: Mean value of untreated group,  : Mean value of CSE treatment group,
: Mean value of CSE treatment group,  : Mean value of positive control.
: Mean value of positive control.
 : Measures in each group,
: Measures in each group,  : Mean value of untreated group,
: Mean value of untreated group,  : Mean value of CSE treatment group,
: Mean value of CSE treatment group,  : Mean value of positive control.
: Mean value of positive control.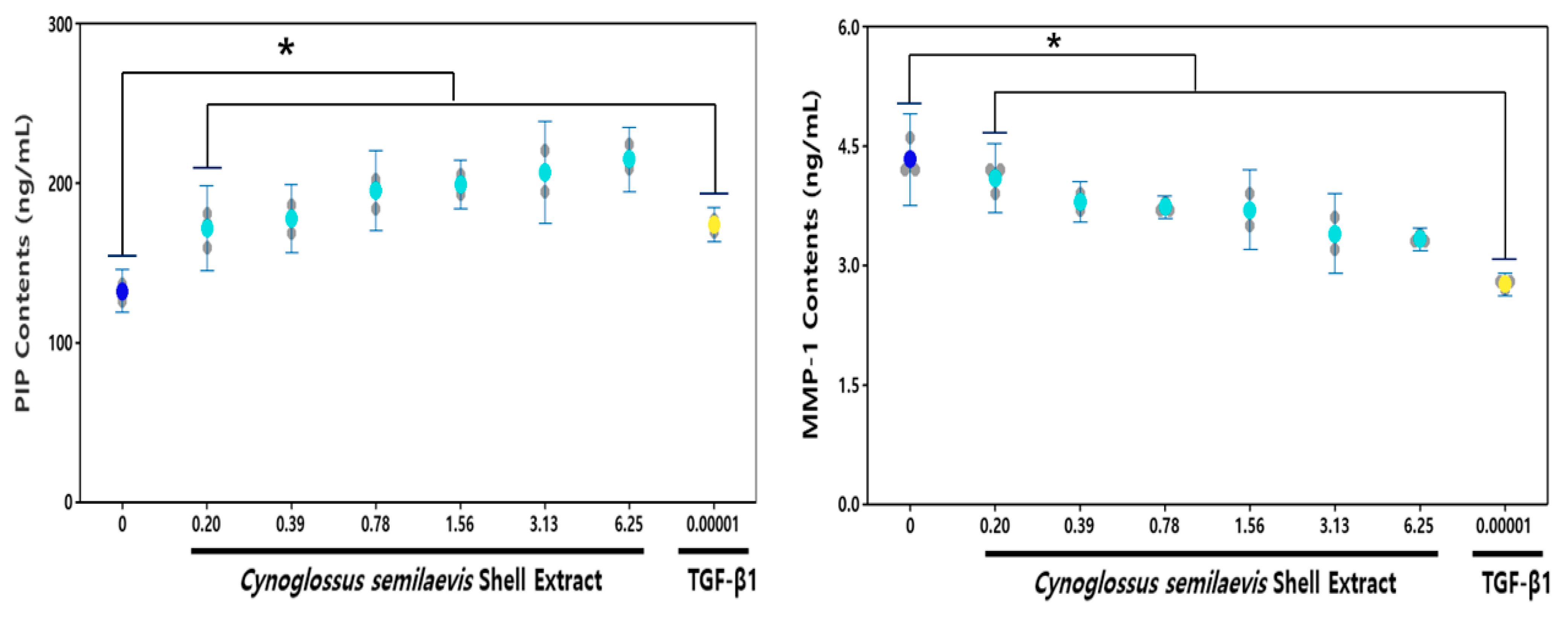
 : Measures in each group,
: Measures in each group,  : Mean value of untreated group,
: Mean value of untreated group,  : Mean value of CSE treatment group,
: Mean value of CSE treatment group,  : Mean value of positive control.
: Mean value of positive control.
 : Measures in each group,
: Measures in each group,  : Mean value of untreated group,
: Mean value of untreated group,  : Mean value of CSE treatment group,
: Mean value of CSE treatment group,  : Mean value of positive control.
: Mean value of positive control.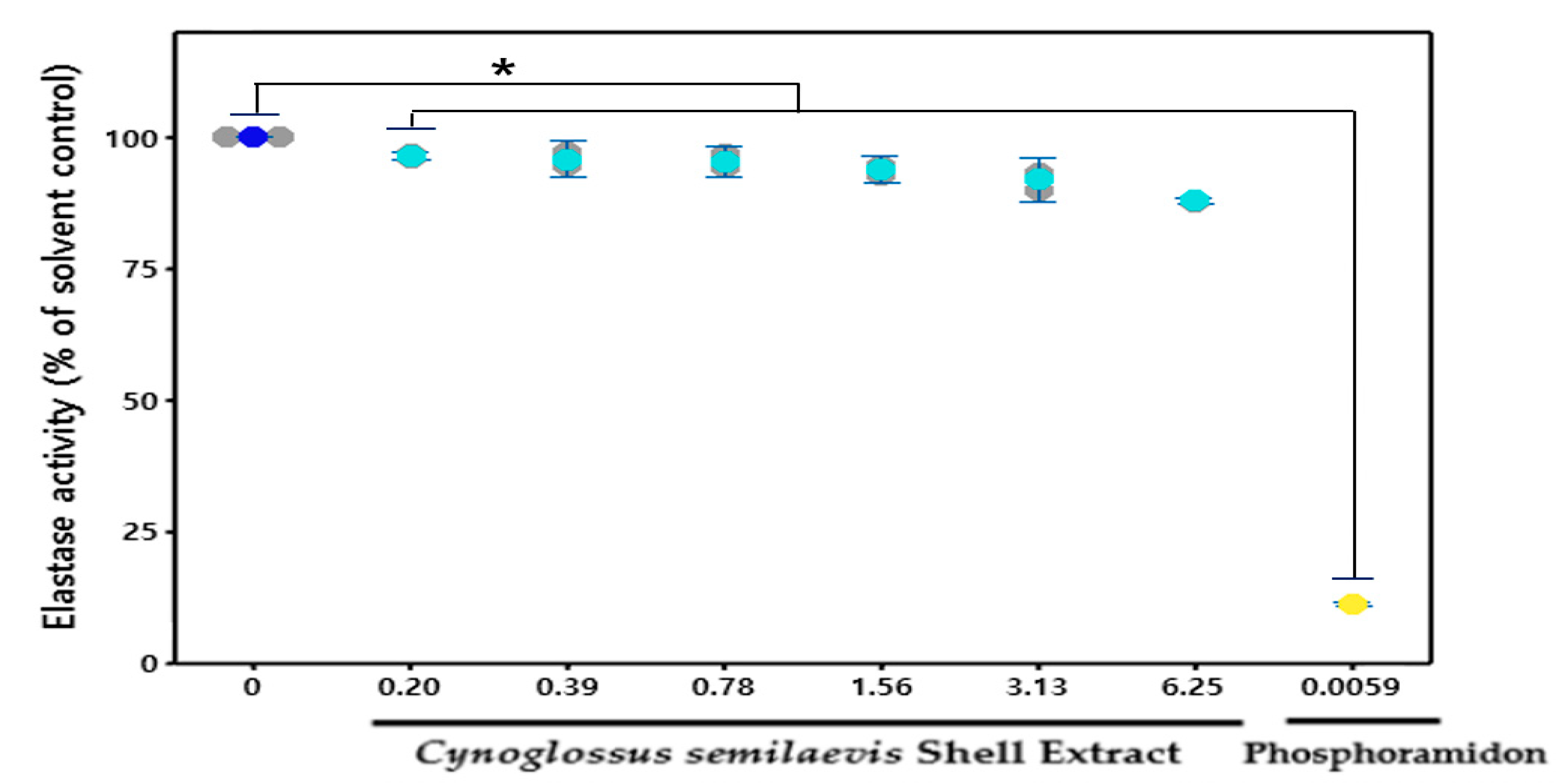
| Cynoglossus semilaevis Shell Extract | |||
|---|---|---|---|
| Amino Acid | Physiological (Free) Amino Acid | ||
| Kind | Content (μg/mL) | Kind | Content (μg/mL) |
| Aspartic acid (Asp) | 1217 | o-Phosphoserine | 4 |
| Threonine (Thr) | 447 | Taurine | 33 |
| Serine (Ser) | 807 | o-Phosphoethanolamine | 1 |
| Glutamic acid (Glu) | 1980 | Sarcosine | 0 |
| Glycine (Gly) | 3905 | L-2-Aminoadipic Acid | 2 |
| Cysteine (Cys) | 4537 | L-Citruline | 8 |
| Alanine (Als) | 30 | DL-2-Aminobutyric | 0 |
| Valine (Val) | 582 | LCystathionine | 10 |
| Methionine (Met) | 417 | β-Alanine | 8 |
| Isoleucine (Ile) | 225 | DL-3-Aminoisobutyric Acid | 7 |
| Leucine (Leu) | 621 | 4-Aminobutyric acid | 1 |
| Tyrosine (Tyr) | 177 | 2-Aminoethanol | 133 |
| Phenylalanine (Phe) | 398 | DL-plusallo-δ-Hydroxylysine | 0 |
| Lysine (Lys) | 709 | L-Ornithine | 3 |
| Histidine (His) | 235 | L-1-Methylhistidine | 0 |
| Arginine (Arg) | 1562 | L-3-Methylhistidine | 0 |
| Proline (Pro) | 1432 | L-Anserine | 0 |
| L-Carnosine | 0 | ||
| hydroxyl-L-proline | 1554 | ||
| Total | 19,281 | Total | 1764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.-C.; Lee, I.-A. Effect of MMP/TIMP Balancing of Cynoglossus semilaevis Shell Extracts on Skin Protection. Fishes 2021, 6, 34. https://doi.org/10.3390/fishes6030034
Choi S-C, Lee I-A. Effect of MMP/TIMP Balancing of Cynoglossus semilaevis Shell Extracts on Skin Protection. Fishes. 2021; 6(3):34. https://doi.org/10.3390/fishes6030034
Chicago/Turabian StyleChoi, Soo-Cheol, and In-Ah Lee. 2021. "Effect of MMP/TIMP Balancing of Cynoglossus semilaevis Shell Extracts on Skin Protection" Fishes 6, no. 3: 34. https://doi.org/10.3390/fishes6030034
APA StyleChoi, S.-C., & Lee, I.-A. (2021). Effect of MMP/TIMP Balancing of Cynoglossus semilaevis Shell Extracts on Skin Protection. Fishes, 6(3), 34. https://doi.org/10.3390/fishes6030034





