Catch per Unit Effort Dynamic of Yellowfin Tuna Related to Sea Surface Temperature and Chlorophyll in Southern Indonesia
Abstract
1. Introduction
2. Results
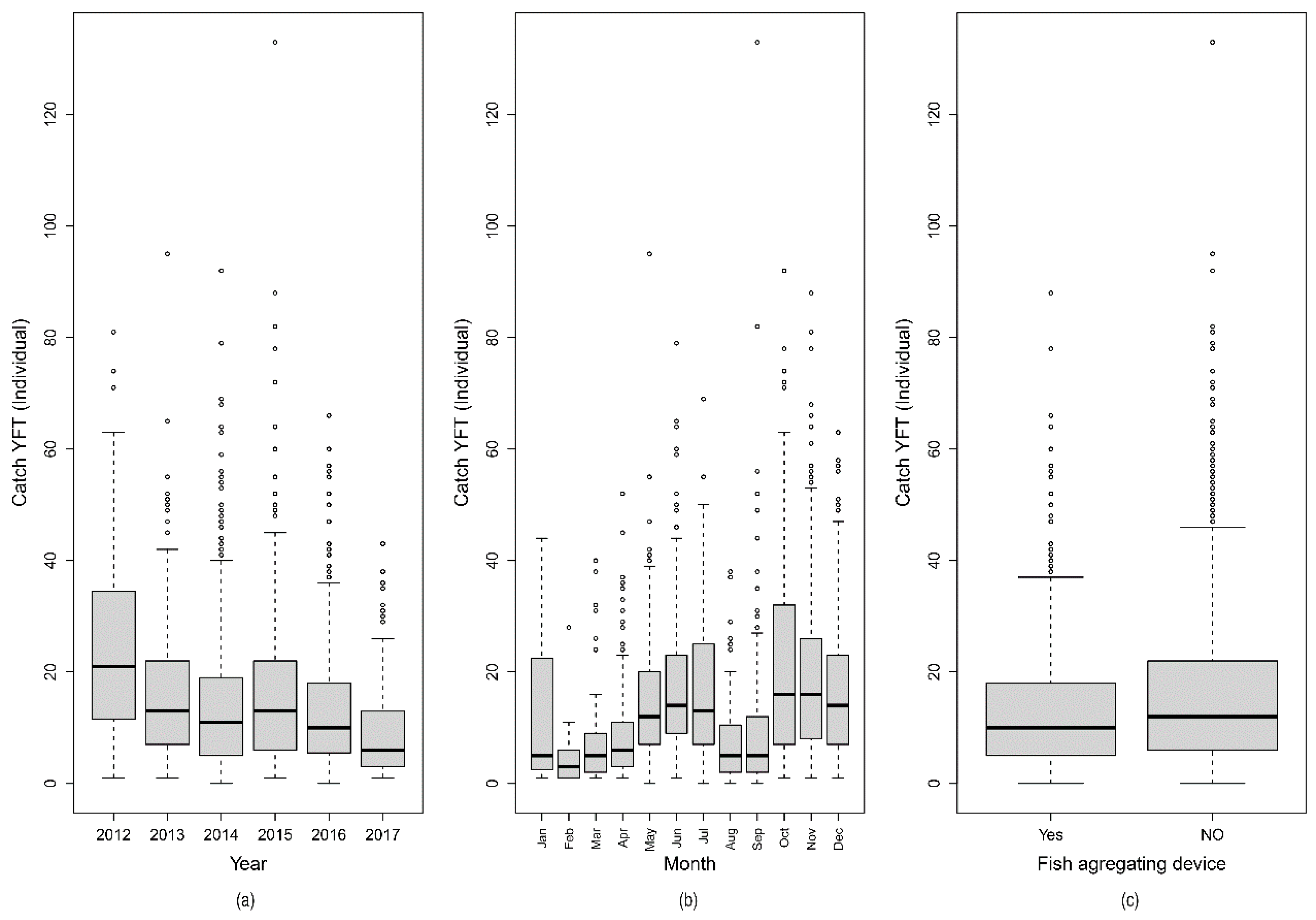
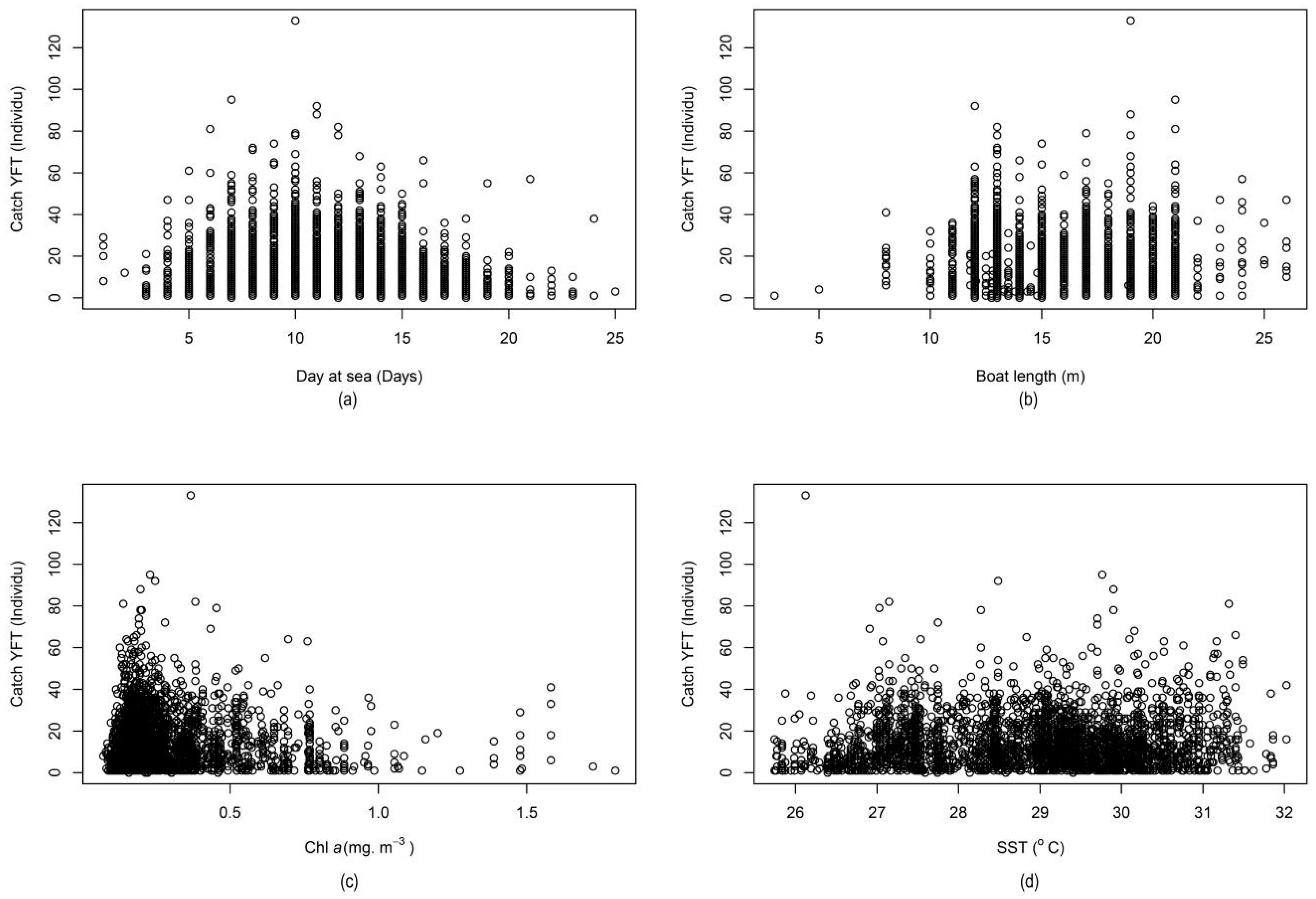
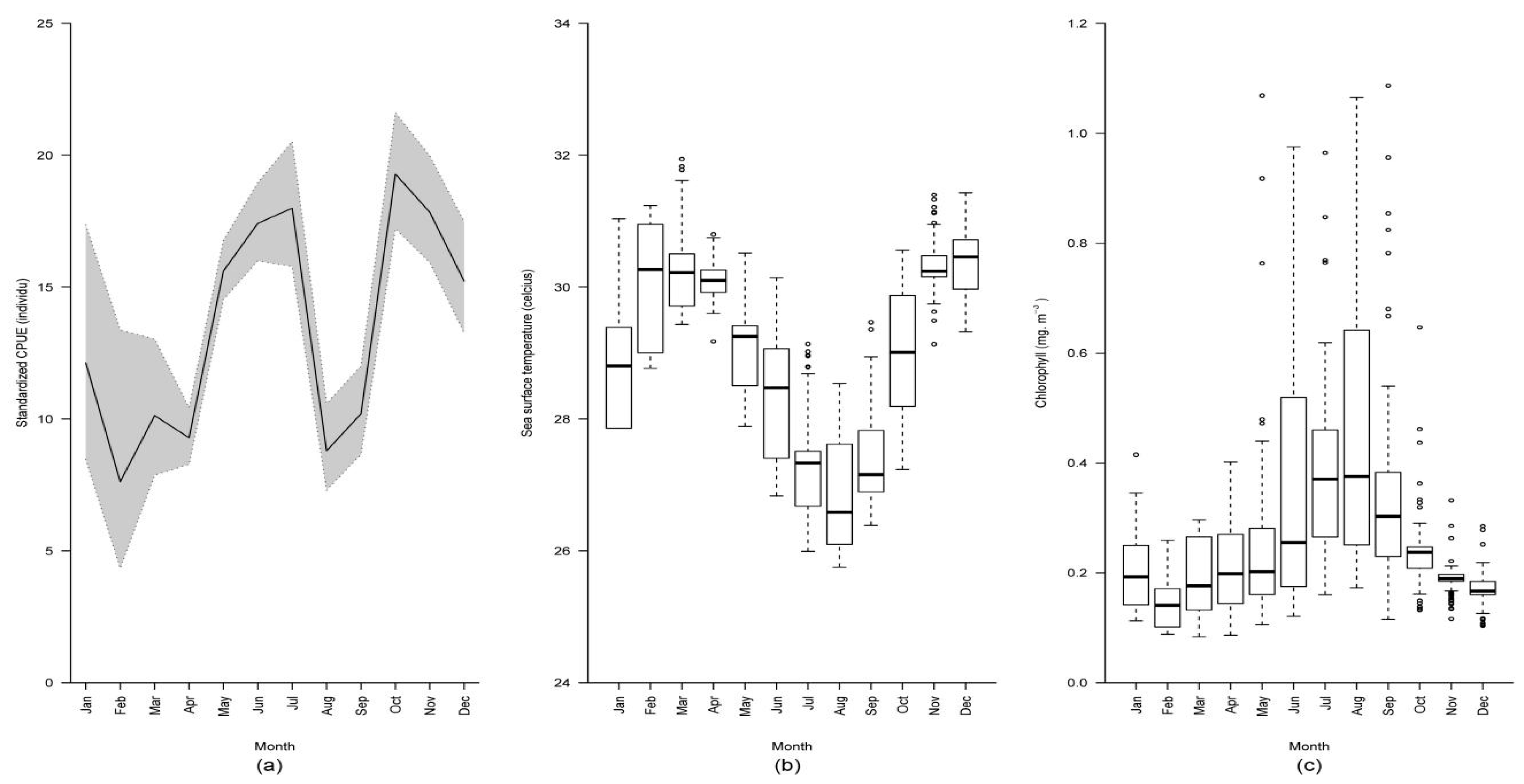
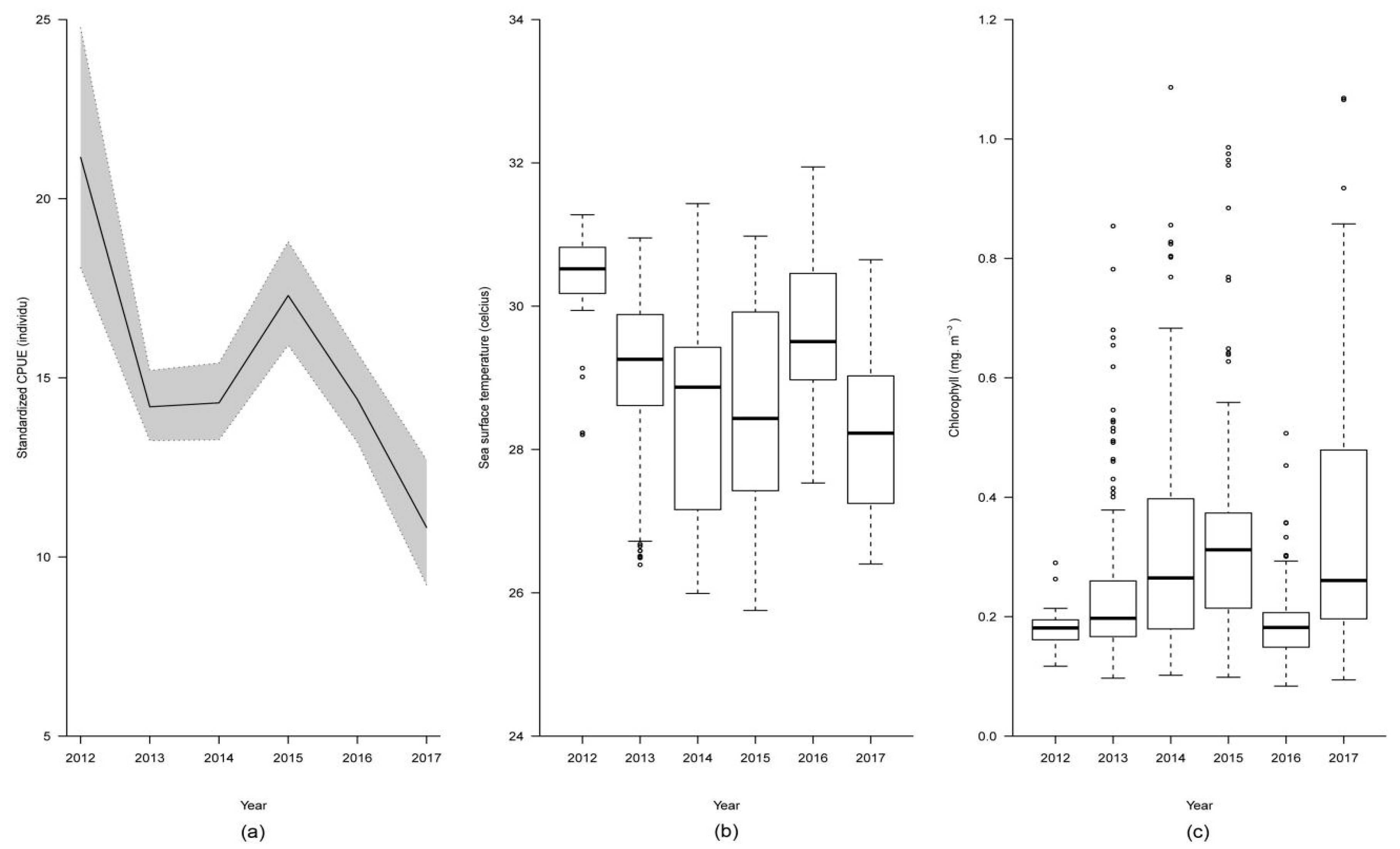
2.1. Distribution and Variation of Nominal Catch per Unit Effort
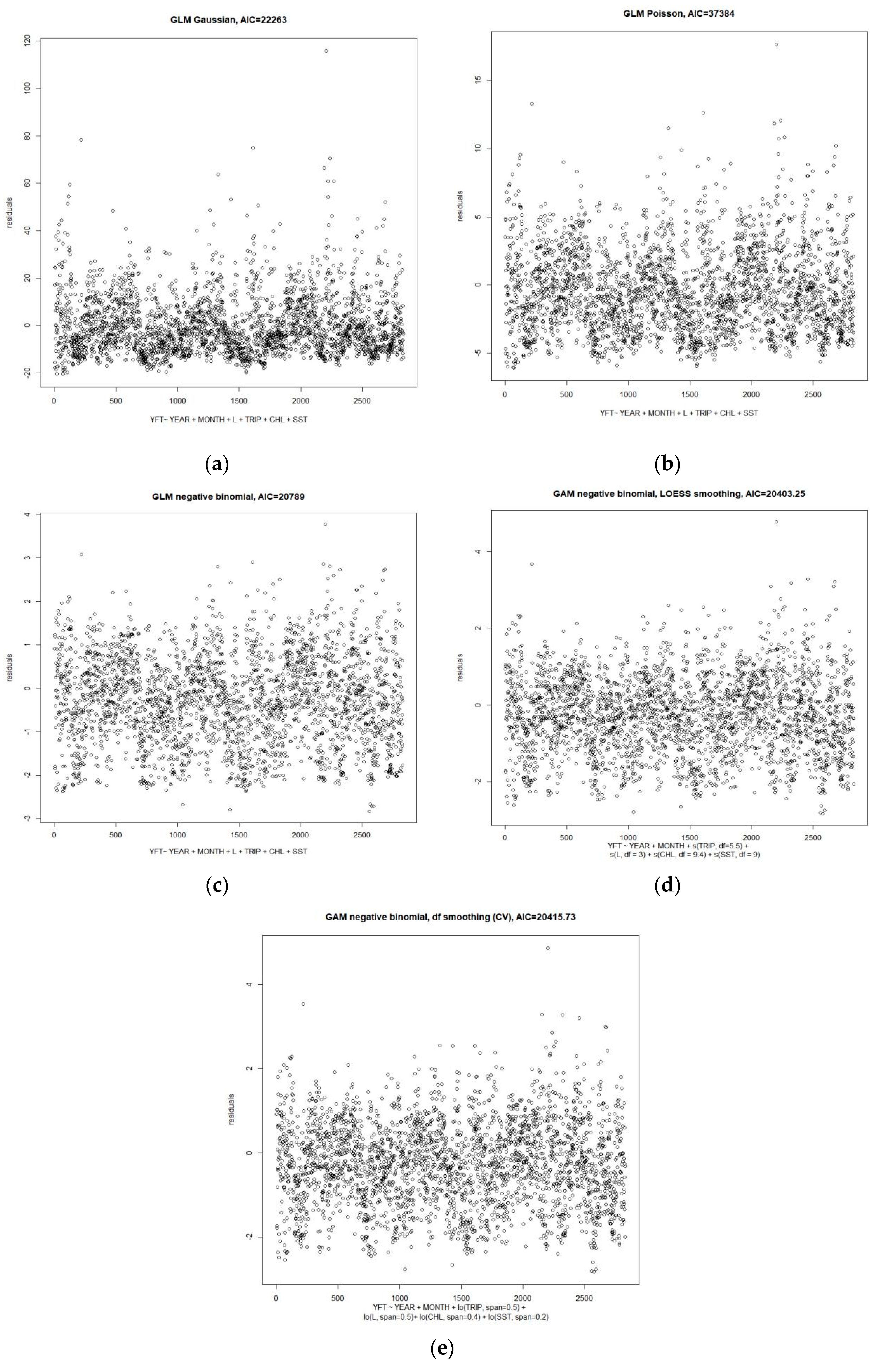
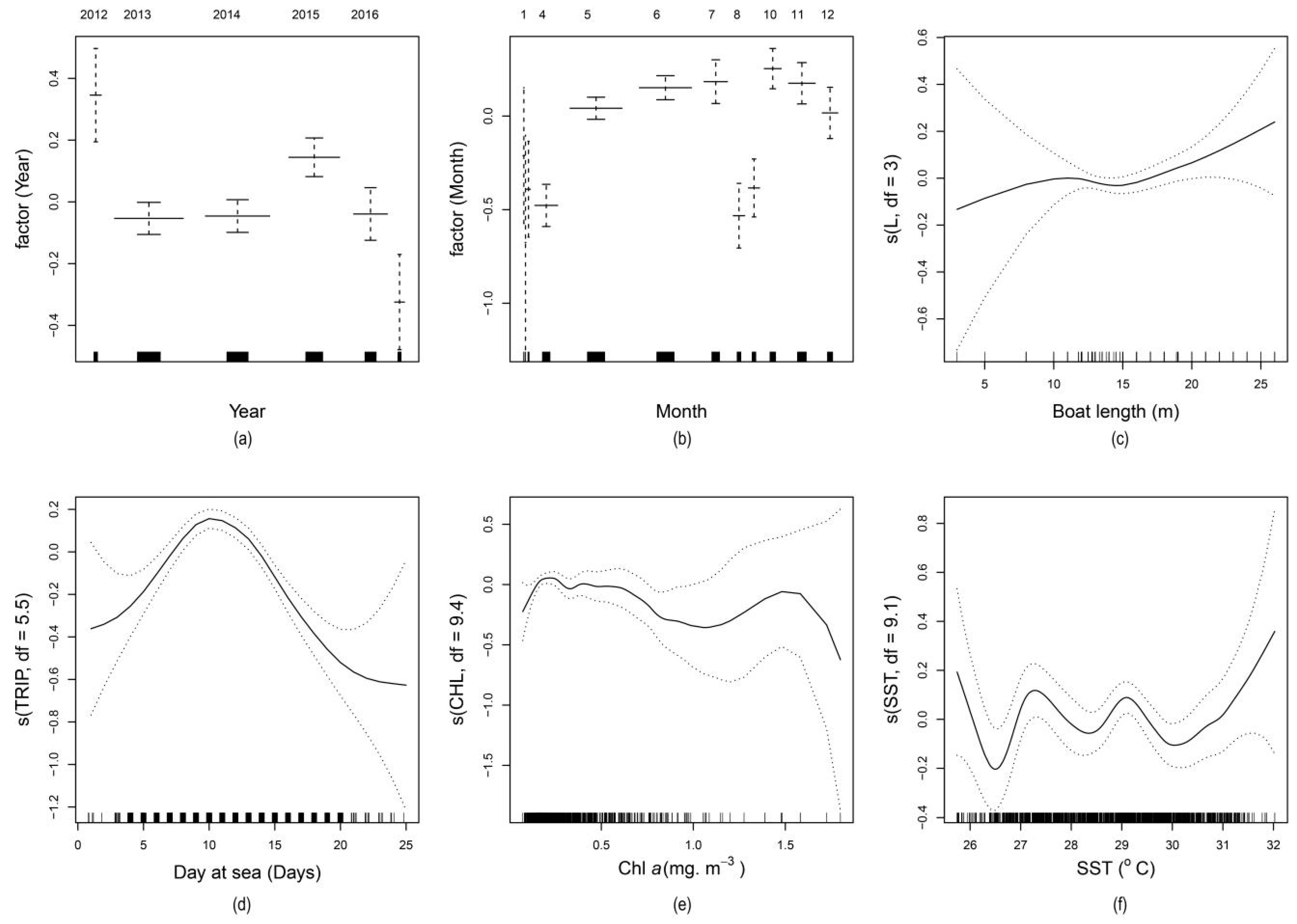
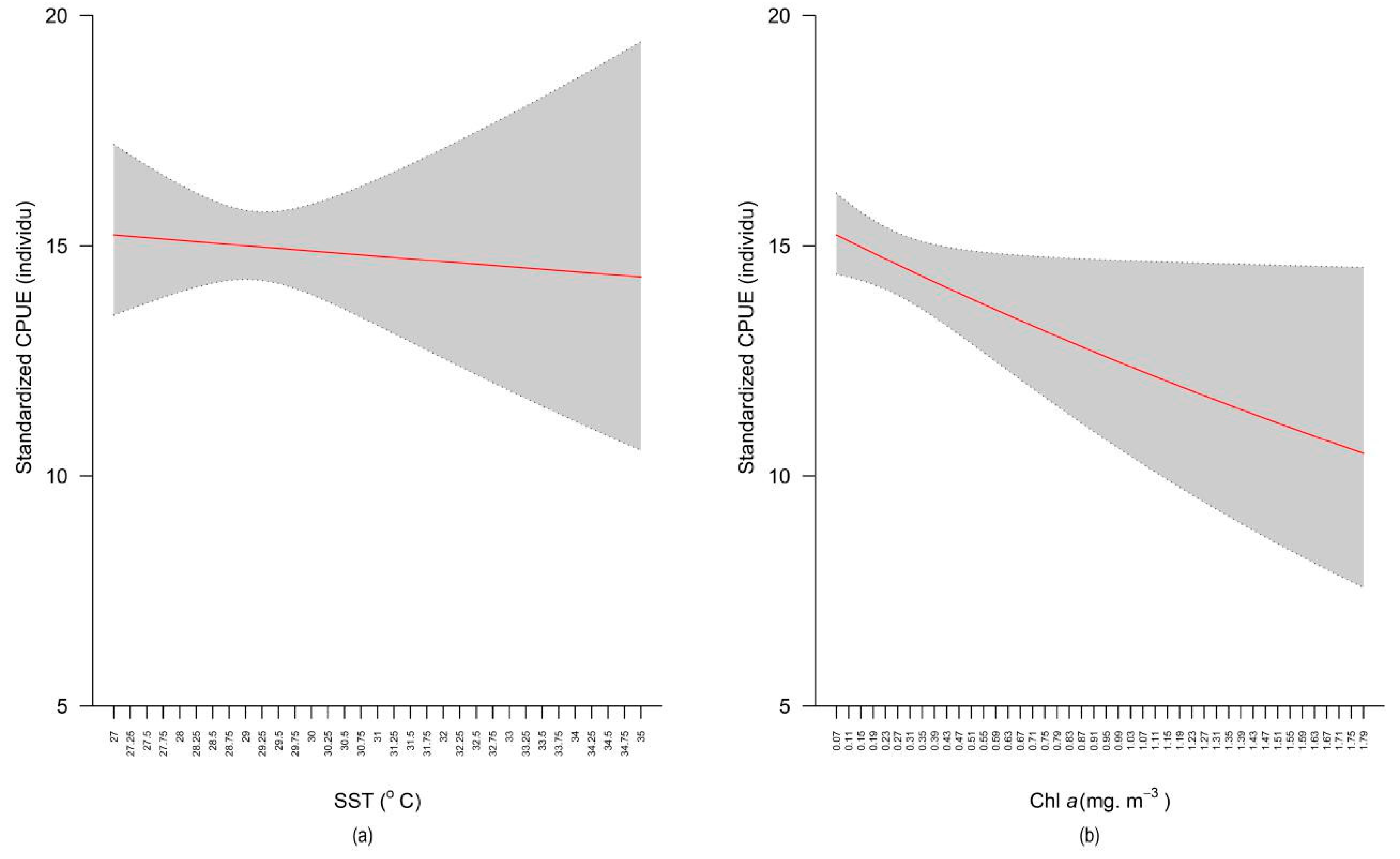
2.2. Distribution and Variation of Standardized Catch per Unit Effort
3. Discussion
4. Materials and Methods
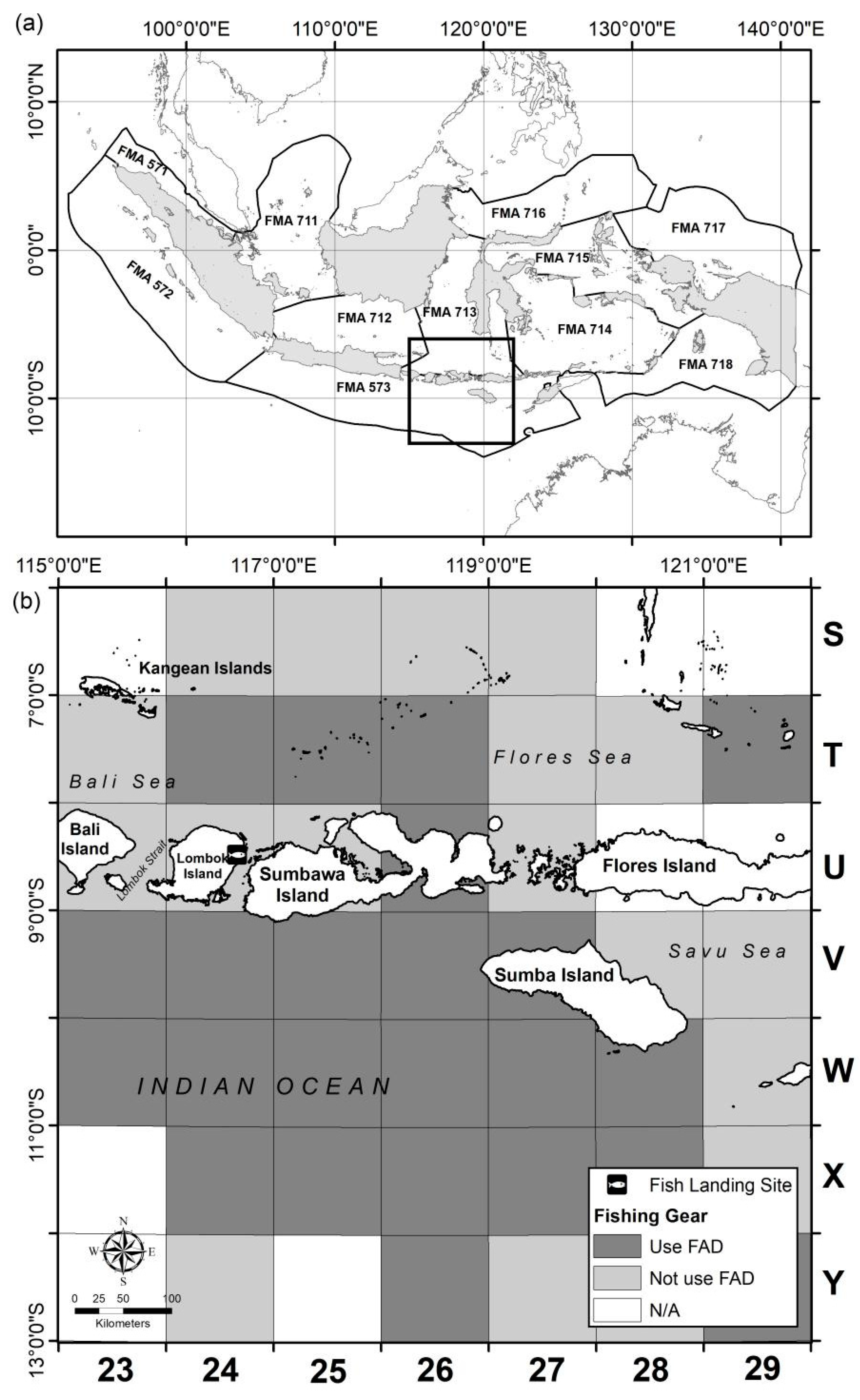
4.1. Data Collection
4.1.1. Fisheries Data
4.1.2. Sea Surface Temperature and Chlorophyll Data
4.2. Data Analyses
- CPUE~Year + month + boat length + days at sea + Chl a + SST… fitted with a simple linear regression or Generalized Linear Model (GLM);
- CPUE~Year + month + lo (boat length) + lo (days at sea) + lo (Chl a) + lo (SST)… fitted with a Generalized Additive Model (GAM) using a loess/local regression, lo [44];
- CPUE~Year + month + s (boat length) + s (days at sea) + s (Chl a) + s(SST)… fitted with a Generalized Additive Model (GAM) using a smoothing function (s(x)) [44].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- FAO. Tuna a Global Perspective; FAO: Rome, Italy, 2018. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture (SOFIA)—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- Tidd, A.; Blanchard, J.L.; Kell, L.; Watson, R.A. Predicting Global Tuna Vulnerabilities with Spatial, Economic, Biological and Climatic Considerations. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McCluney, J.K.; Anderson, C.M.; Anderson, J.L. The Fishery Performance Indicators for Global Tuna Fisheries. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sunoko, R.; Huang, H.W. Indonesia Tuna Fisheries Development and Future Strategy. Mar. Pol. 2014, 43, 174–183. [Google Scholar] [CrossRef]
- Coulter, A.; Cashion, T.; Cisneros-Montemayor, A.M.; Popov, S.; Tsui, G.; Le Manach, F.; Schiller, L.; Palomares, M.L.D.; Zeller, D.; Pauly, D. Using Harmonized Historical Catch Data to Infer the Expansion of Global Tuna Fisheries. Fish. Res. 2020, 221, 105379. [Google Scholar] [CrossRef]
- MMAF. Tuna Fisheries Management Plan; Ministry of Marine Affairs and Fisheries: Jakarta, Indonesia, 2015. [Google Scholar]
- Bordalo-Machado, P. Fishing Effort Analysis and Its Potential to Evaluate Stock Size. Rev. Fish. Sci. 2006, 14, 369–393. [Google Scholar] [CrossRef]
- Kantoussan, J.; Ecoutin, J.M.; Guy Fontenelle, G.; de Morais, L.T.; Lae, R. Catch Per Unit Effort and Yields As Indicators Of Exploited Fish Communities: Application To Two West African Reservoirs. Lake. Reserv. Res. Manag. 2014, 19, 86–97. [Google Scholar] [CrossRef]
- MDPI. Data Collection Protocol for Small Scale Handline Fisheries in Indonesia; MDPI: Denpasar, Indonesia, 2018. [Google Scholar]
- Polovina, J.J.; Howell, E.A. Ecosystem Indicators Derived from Satellite Remotely Sensed Oceanographic Data for the North Pacific. ICES J. Mar. Sci. 2005, 62, 319–327. [Google Scholar] [CrossRef]
- Zainuddin, M.; Saitoh, S.I.; Saitoh, K. Detection of Potential Fishing Ground for Albacore Tuna Using Synoptic Measurements of Ocean Color and Thermal Remote Sensing in the Northwestern North Pacific. Geophys. Res. Lett. 2004, 31, 1–4. [Google Scholar] [CrossRef]
- Saitoh, S.; Chassot, E.; Dwivedi, R.; Fonteneau, A.; Kiyofuji, H.; Kumari, B.; Kuno, M.; Matsumura, S.; Platt, T.; Raman; et al. Remote Sensing in Fisheries and Aquaculture; Forget, M.H., Stuart, V., Platt, T., Eds.; The International Ocean-Colour Coordinating Group: Dartmouth, NS, Canada, 2009; Volume 5, pp. 57–76. ISBN 1098-6030. [Google Scholar]
- Lan, K.W.; Lee, M.A.; Lu, H.J.; Shieh, W.J.; Lin, W.K.; Kao, S.C. Ocean Variations Associated with Fishing Conditions for Yellowfin Tuna (Thunnus Albacares) in the Equatorial Atlantic Ocean. ICES J. Mar. Sci. 2011, 68, 1063–1071. [Google Scholar] [CrossRef]
- Song, L.; Zhou, J.; Zhou, Y.; Nishida, T.; Jiang, W.; Wang, J. Environmental Preferences of Bigeye Tuna, Thunnus Obesus, in the Indian Ocean: An Application to a Longline Fishery. Environ. Biol. Fishes. 2009, 85, 153–171. [Google Scholar] [CrossRef]
- Polovina, J.J.; Howell, E.; Kobayashi, D.R.; Seki, M.P. The Transition Zone Chlorophyll Front, a Dynamic Global Feature Defining Migration and Forage Habitat for Marine Resources. Prog. Oceanogr. 2001, 49, 469–483. [Google Scholar] [CrossRef]
- Zainuddin, M.; Farhum, A.; Safruddin, S.; Selamat, M.B.; Sudirman, S.; Nurdin, N.; Syamsuddin, M.; Ridwan, M.; Saitoh, S.I. Detection of Pelagic Habitat Hotspots for Skipjack Tuna in the Gulf of Bone-Flores Sea, Southwestern Coral Triangle Tuna, Indonesia. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Duggan, D.; Wildan; Riza Baroqi, A.; Satria Timur, P.; Juhrin; Nababan, N.; Kochen, M. Simple Economics in Small Scale Tuna Fisheries: Results of an Economic-Survey Conducted in Four MDPI Sites in Eastern Indonesia; MDPI: Denpasar, Indonesia, 2017. [Google Scholar]
- Setyadji, B.; Nugraha, B. Dynamics of Gears, Fleets, Catch and Fishing Season of Smallscale Tuna Fisheries in Labuhan Lombok, West Nusa Tenggara. Indones. Fish. Res. J. 2015, 21, 99–107. [Google Scholar] [CrossRef][Green Version]
- Dunstan, P.K.; Foster, S.D.; King, E.; Risbey, J.; O’Kane, T.J.; Monselesan, D.; Hobday, A.J.; Hartog, J.R.; Thompson, P.A. Global Patterns of Change and Variation in Sea Surface Temperature and Chlorophyll, A. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertignac, M.; Hampton, J.; Lewis, A.; Picaut, J. El Niño Southern Oscillation and tuna in the western Pacific. Nature 1997, 389, 715–718. [Google Scholar] [CrossRef]
- Maunder, M.N.; Sibert, J.R.; Fonteneau, A.; Hampton, J.; Kleiber, P.; Harley, S.J. Interpreting Catch Per Unit Effort Data to Assess the Status of Individual Stocks and Communities. ICES J. Mar. Sci. 2006, 63, 1373–1385. [Google Scholar] [CrossRef]
- BMKG. Data Data El Niño/La Niña; Meteorology and Geophysic Agency of Indonesia: Jakarta, Indonesia, 2019. [Google Scholar]
- Iskandar, I.; Utari, P.A.; Lestari, D.O.; Sari, Q.W.; Setiabudidaya, D.; Khakim, M.Y.N.; Yustian, I.; Dahlan, Z. Evolution of 2015/2016 El Niño and Its Impact on Indonesia. AIP 2017, 080001–080005. [Google Scholar] [CrossRef]
- Null, J. El Niño and La Niña Years and Intensities. Available online: https://ggweather.com/enso/oni.htm (accessed on 11 August 2019).
- Susanto, R.D.; Gordon, A.L.; Zheng, Q. Upwelling within the Indonesian Seas and its relation to Monsoon and ENSO. Geophys. Res. Lett. 2001, 28, 1599–1602. [Google Scholar] [CrossRef]
- Hendiarti, N.; Siegel, H.; Ohde, T. Investigation of Different Coastal Processes in Indonesian Waters Using SeaWiFS Data. Deep-Sea Res. Pt. II 2004, 51, 85–97. [Google Scholar] [CrossRef]
- Roger, C. Relationships among Yellowfin and Skipjack Tuna, Their Prey-fish and Plankton in the Tropical Western Indian Ocean. Fish. Oceanogr. 1994, 3, 133–141. [Google Scholar] [CrossRef]
- Suárez-Sánchez, J.; Ritter-Ortiz, W.; Gay-García, C.; Torres-Jácome, J. ENSO-Tuna Relations in the Eastern Pacific Ocean and Its Prediction as a Non-Linear Dynamic System. Atmosfera 2004, 17, 245–258. [Google Scholar]
- Blackburn, M. Conditions Related to Upwelling Which Determine Distribution of Tropical Tunas off Western Baja California. Fish. Bull. 1969, 68, 147–176. [Google Scholar]
- Kumar, P.S.; Pillai, G.N.; Manjush, U. El Nino Southern Oscillation (ENSO) Impact on Tuna Fisheries in Indian Ocean. SpringerPlus 2014, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ningsih, N.S.; Rakhmaputeri, N.; Harto, A.B. Upwelling Variability along the Southern Coast of Bali and in Nusa Tenggara Waters. Ocean Sci. J. 2013, 48, 49–57. [Google Scholar] [CrossRef]
- Lehodey, P.; Andre, J.M.; Bertignac, M.; Hampton, J.; Stoens, A.; Menkes, C.; Memery, L.; Grima, N. Predicting Skipjack Tuna Forage Distributions in the Equatorial Pacific Using a Coupled Dynamical Bio-Geochemical Model. Fish. Oceanogr. 1998, 7, 317–325. [Google Scholar] [CrossRef]
- Ortega-García, S.; Lluch-Cota, S.E. Distribución de la abundancia de atún y su relación con la concentración de pigmentos fotosintéticos derivados de satélite en aguas al sur de México. Invest. Geogr. México 1996, 4, 85–93. [Google Scholar]
- Singh, A.A.; Sakuramoto, K.; Suzuki, N. Impact of Climatic Factors on Albacore Tuna Thunnus alalung in the South Pacific Ocean. Am. J. Clim. Chang. 2015, 4, 295–312. [Google Scholar] [CrossRef]
- Zagaglia, C.R.; Lorenzzetti, J.A.; Stech, J.L. Remote Sensing Data and Longline Catches of Yellowfin Tuna (Thunnus Albacares) in the Equatorial Atlantic. Remote. Sens. 2004, 93, 267–281. [Google Scholar] [CrossRef]
- Goujon, M.; Labaisse-Bodilis, C. Effets Des Plans de Protection Des Thonidés de l’Atlantique Depuis 1997 d’après Les Observations Faites Sur Les Thoniers Senneurs Gérés Par Les Armements Français. Collect. Vol. Sci. Pap. ICCAT 2001, 52, 575–589. [Google Scholar]
- Satrioajie, W.N.; Suyadi; Syahailatua, A.; Wouthuyzen, S. The Importance of the Banda Sea for Tuna Conservation Area: A Review of Studies on the Biology and the Ecology of Tuna. IOP Conf. Ser. Earth Environ. Sci. 2018, 184. [Google Scholar] [CrossRef]
- Ministry of Agriculture. Potensi Sumber Daya Ikan dan Jumlah Tangkapan yang Diperbolehkan (JTB); Ministry of Agriculture: Jakarta, Indonesia, 1999. [Google Scholar]
- Compean-Jimenez, G.A.; Dreyfus-Leon, M.J. Interaction between vessels fishing for yellowfin tuna (Thunnus albacares) in the northeastern and southeastern Pacific. In Status of Interaction of Pacific Tuna Fisheries in 1995, Proceeding of the Second FAO Expert Consultation on Interaction of Pacific Tuna Fisheries, Shimizu, Japan, 23–31 January 1995; Shomura, R.S., Majkowski, J., Harman, R.F., Eds.; FAO: Rome, Italy, 1996; pp. 339–349. [Google Scholar]
- Hu, C.; Lee, Z.; Franz, B. Chlorophyll a Algorithms For Oligotrophic Oceans: A Novel Approach Based On Three-Band Reflectance Difference. J. Geophys. Res. 2012, 117, 1–25. [Google Scholar] [CrossRef]
- Johnston, F.R.; Boyland, J.E.; Meadows, M.; Shale, E. Some Properties of a Simple Moving Average When Applied to Forecasting a Time Series. J. Oper. Res. Soc. 1999, 50, 1267–1271. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.E.; Heiberger, R.M. Statistical Models in S.; Routledge: New York, NY, USA, 2017; pp. 145–193. ISBN 9780203738535. [Google Scholar]
- Hastie, T. gam: Generalized Additive Models. Available online: https://cran.r-project.org/package=gam (accessed on 15 January 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. Available online: https://cran.r-project.org/package=MuMIn (accessed on 15 January 2020).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology With R.; Gail, A., Krickeberg, K., Samet, J., Tsiatis, A., Wong, W., Eds.; Springer Science & Business Media, LCC: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
| Year | Catch of YFT (Individual) | Trip Duration (Days) | Length of Boat (m) | SST (°C) | Chl a (mg m−3) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| 2012 (n = 131) | 25 ± 18 | 10.45 ± 3.11 | 17.30 ± 3.63 | 30.48 ± 0.72 | 0.17 ± 0.03 |
| 2013 (n = 816) | 15 ± 11 | 10.73 ± 3.60 | 16.03 ± 2.97 | 29.07 ± 1.05 | 0.24 ± 0.12 |
| 2014 (n = 796) | 14 ± 12 | 11.84 ± 3.69 | 14.34 ± 2.65 | 28.48 ± 1.35 | 0.33 ± 0.25 |
| 2015 (n = 603) | 16 ± 14 | 12.16 ± 3.36 | 14.58 ± 2.81 | 28.50 ± 1.42 | 0.33 ± 0.20 |
| 2016 (n = 400) | 14 ± 12 | 11.61 ± 3.73 | 13.62 ± 2.33 | 29.55 ± 1.11 | 0.18 ± 0.06 |
| 2017 (n = 117) | 10 ± 9 | 11.65 ± 3.77 | 13.23 ± 1.75 | 28.29 ± 1.13 | 0.42 ± 0.31 |
| 2012–2017 (n = 2831) | 15 ± 13 | 11.48 ± 3.63 | 14.87 ± 2.95 | 28.89 ± 1.33 | 0.28 ± 0.19 |
| Parameter | Degree of Freedom | Sum of Square | Mean of Square | F Value | p-Value |
|---|---|---|---|---|---|
| Year | 5 | 18,740 | 3748 | 24.57 | <2 × 10−16 |
| Residuals | 2825 | 430,887 | 153 | ||
| Month | 11 | 38,558 | 3505 | 24.04 | <2 × 10−16 |
| Residuals | 2819 | 411,069 | 146 | ||
| FAD | 1 | 1788 | 1787.9 | 11.29 | 0.000788 |
| Residuals | 2829 | 447,839 | 158.3 |
| No | Full Model | Over-Dispersion | AIC |
|---|---|---|---|
| Generalized Linear Models | |||
| 1 | CPUE ~ Year + month + boat length + days at sea + Chl a + SST; family = Gaussian | NA | 22,263.00 |
| 2 | CPUE ~ Year + month + boat length + days at sea + Chl a + SST; family = Poisson | 9.8 | 37,384.00 |
| 3 | CPUE ~ Year + month + boat length + days at sea + chl a + SST; family = negative-binomial | 9.8 | 20,789.00 |
| Generalized Additive Models | |||
| 4 | CPUE~ Year + month + lo(boat length, span = 0.5) + lo(days at sea, span = 0.5) + lo(Chl a, span = 0.4) + lo(Chl a, span = 0.2); family = negative binomial (θ = 1.75) | NA | 20,415.73 |
| 5 | CPUE ~ Year + month + s(boat length, df = 3) + s(days at sea, df = 5.5) + s(Chl a, df = 9.4) + s(SST, df = 9); family = negative binomial (θ = 1.8) | NA | 20,403.25 |
| Parameter | Degree of Freedom | Sum of Square | Mean of Square | % MS | F Value | p-Value |
|---|---|---|---|---|---|---|
| ANOVA for Parametric Effects | ||||||
| Month | 11 | 309.93 | 28.1752 | 35.64 | 27.88 | >0.0001 |
| Year | 5 | 65.61 | 13.1227 | 16.60 | 12.9852 | 1.63 × 10−12 |
| Chl a | 1 | 5.32 | 5.3237 | 6.73 | 5.2679 | 0.022 |
| Boat length | 1 | 6.85 | 6.8485 | 8.66 | 6.7768 | 0.009 |
| SST | 1 | 0.08 | 0.0838 | 0.11 | 0.0829 | 0.773458 |
| Days at sea | 1 | 24.49 | 24.492 | 30.98 | 24.2354 | 9.02 × 10−07 |
| Residuals | 2787 | 2816.51 | 1.0106 | 1.28 | ||
| ANOVA for Nonparametric Effects | ||||||
| Month | ||||||
| Year | ||||||
| Chl a | 8.4 | 2.1923 | 0.02299 | |||
| Boat length | 2 | 2.8834 | 0.05611 | |||
| SST | 8.1 | 6.072 | 7.65 × 10−08 | |||
| Days at sea | 4.5 | 22.0429 | <2.2 × 10−16 | |||
| Variable | Type | Description |
|---|---|---|
| Year | Categorical | Year of trip (2012–2017) |
| Month | Categorical | Month of trip (January–December) |
| Days at sea | Numeric | Total day of fishing trip |
| Boat length | Numeric | Length of boat in meters |
| Fish aggregating device (FAD) | Categorical | Fishing on FAD or not |
| Fishing Ground | Categorical | Grid code of fishing areas (Figure 1) |
| Catch | Numeric | Number of fish with weight ≥10 kg caught on each fishing trip |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiryawan, B.; Loneragan, N.; Mardhiah, U.; Kleinertz, S.; Wahyuningrum, P.I.; Pingkan, J.; Wildan; Timur, P.S.; Duggan, D.; Yulianto, I. Catch per Unit Effort Dynamic of Yellowfin Tuna Related to Sea Surface Temperature and Chlorophyll in Southern Indonesia. Fishes 2020, 5, 28. https://doi.org/10.3390/fishes5030028
Wiryawan B, Loneragan N, Mardhiah U, Kleinertz S, Wahyuningrum PI, Pingkan J, Wildan, Timur PS, Duggan D, Yulianto I. Catch per Unit Effort Dynamic of Yellowfin Tuna Related to Sea Surface Temperature and Chlorophyll in Southern Indonesia. Fishes. 2020; 5(3):28. https://doi.org/10.3390/fishes5030028
Chicago/Turabian StyleWiryawan, Budy, Neil Loneragan, Ulfah Mardhiah, Sonja Kleinertz, Prihatin Ika Wahyuningrum, Jessica Pingkan, Wildan, Putra Satria Timur, Deirdre Duggan, and Irfan Yulianto. 2020. "Catch per Unit Effort Dynamic of Yellowfin Tuna Related to Sea Surface Temperature and Chlorophyll in Southern Indonesia" Fishes 5, no. 3: 28. https://doi.org/10.3390/fishes5030028
APA StyleWiryawan, B., Loneragan, N., Mardhiah, U., Kleinertz, S., Wahyuningrum, P. I., Pingkan, J., Wildan, Timur, P. S., Duggan, D., & Yulianto, I. (2020). Catch per Unit Effort Dynamic of Yellowfin Tuna Related to Sea Surface Temperature and Chlorophyll in Southern Indonesia. Fishes, 5(3), 28. https://doi.org/10.3390/fishes5030028







