Narrowing the Range of Environmental Salinities Where Juvenile Meagre (Argyrosomus regius) Can Be Cultured Based on an Osmoregulatory Pilot Study
Abstract
:1. Introduction
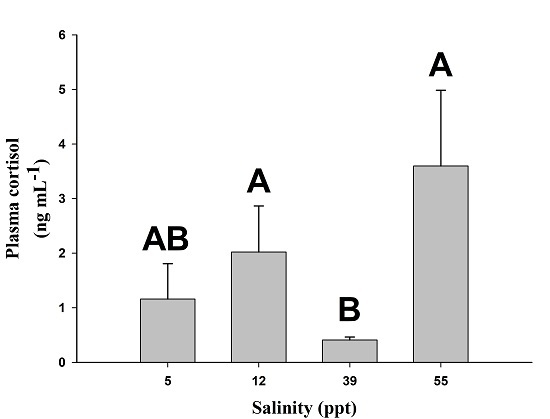
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Blood and Tissue Sampling
4.3. Plasma Measurements
4.4. Gill Na+/K+-ATPase Activity
4.5. Liver Metabolite Levels
4.6. Growth Parameters
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflict of interest
References
- McCormick, S.D.; Farrell, A.P.; Brauner, C.J. Euryhaline Fishes; Academic Press: Oxford, UK, 2013; Volume 32. [Google Scholar]
- Evans, D.H. Teleost fish osmoregulation: What have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol. 2008, 295, R704–R713. [Google Scholar] [CrossRef] [PubMed]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Gonçalves, O.; Pâscoa, I.; Martín del Río, M.P.; Mancera, J.M. Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup, 1858) to osmotic challenge: Implications for osmoregulation, energy metabolism and growth. Aquaculture 2009, 287, 419–426. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Gregorio, S.F.; Gaetano, P.; Trischitta, F.; Fuentes, J. High rates of intestinal bicarbonate secretion in seawater tilapia (Oreochromis mossambicus). Comp. Biochem. Physiol. A 2017, 207, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jarabo, I.; González-Wevar, C.A.; Oyarzún, R.; Fuentes, J.; Poulin, E.; Bertrán, C.; Vargas-Chacoff, L. Isolation driven divergence in osmoregulation in Galaxias maculatus (Jenyns, 1848) (Actinopterygii: Osmeriformes). PLoS ONE 2016, 11, e0154766. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, J.; Kaneko, T.; Seikai, T.; Tanaka, M. Developmental sequence of chloride cells in the body skin and gills of Japanese flounder (Paralichthys olivaceus) larvae. Zool. Sci. 1998, 15, 455–460. [Google Scholar] [PubMed]
- Carvalho, E.S.; Gregorio, S.F.; Power, D.M.; Canario, A.V.; Fuentes, J. Water absorption and bicarbonate secretion in the intestine of the sea bream are regulated by transmembrane and soluble adenylyl cyclase stimulation. J. Comp. Physiol. B 2012, 182, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Bystriansky, J.S.; Schulte, P.M. Changes in gill H+-ATPase and Na+/K+-ATPase expression and activity during freshwater acclimation of Atlantic salmon (Salmo salar). J. Exp. Biol. 2011, 214, 2435–2442. [Google Scholar] [CrossRef]
- McCormick, S.D.; Regish, A.M.; Christensen, A.K. Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J. Exp. Biol. 2009, 212, 3994–4001. [Google Scholar] [CrossRef]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C 2001, 130, 411–423. [Google Scholar]
- Chen, Q.L.; Luo, Z.; Pan, Y.X.; Zheng, J.L.; Zhu, Q.L.; Sun, L.D.; Zhuo, M.Q.; Hu, W. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquat. Toxicol. 2013, 136, 72–78. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Toni, C.; Martos-Sitcha, J.A.; Ruiz-Jarabo, I.; Mancera, J.M.; Martinez-Rodriguez, G.; Pinheiro, C.G.; Heinzmann, B.M.; Baldisserotto, B. Stress response in silver catfish (Rhamdia quelen) exposed to the essential oil of Hesperozygis Ringens. Fish Physiol. Biochem. 2015, 41, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Costas, B.; Conceicao, L.; Aragao, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.; Afonso, A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Mancera, J.M.; Calduch-Giner, J.A.; Yufera, M.; Martinez-Rodriguez, G.; Perez-Sanchez, J. Unraveling the tissue-specific gene signatures of gilthead sea bream (Sparus aurata L.) after hyper- and hypo-osmotic challenges. PLoS ONE 2016, 11, e0148113. [Google Scholar] [CrossRef] [PubMed]
- Costas, B.; Aragao, C.; Ruiz-Jarabo, I.; Vargas-Chacoff, L.; Arjona, F.; Mancera, J.M.; Dinis, M.T.; Conceicao, L. Different environmental temperatures affect amino acid metabolism in the eurytherm teleost Senegalese sole (Solea senegalensis Kaup, 1858) as indicated by changes in plasma metabolites. Amino Acids 2012, 43, 327–335. [Google Scholar] [CrossRef]
- Aragao, C.; Costas, B.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Dinis, M.T.; Mancera, J.M.; Conceicao, L.E. Changes in plasma amino acid levels in a euryhaline fish exposed to different environmental salinities. Amino Acids 2010, 38, 311–317. [Google Scholar] [CrossRef]
- Wunderink, Y.S.; Engels, S.; Halm, S.; Yufera, M.; Martinez-Rodriguez, G.; Flik, G.; Klaren, P.H.M.; Mancera, J.M. Chronic and acute stress responses in Senegalese sole (Solea senegalensis): The involvement of cortisol, CRH and CRH-BP. Gen. Comp. Endocrinol. 2011, 171, 203–210. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Martin del Rio, M.P.; Mancera, J.M. Osmoregulatory response of Senegalese sole (Solea senegalensis) to changes in environmental salinity. Comp. Biochem. Physiol. A 2007, 148, 413–421. [Google Scholar] [CrossRef]
- Laiz-Carrion, R.; Guerreiro, P.M.; Fuentes, J.; Canario, A.V.M.; Martin Del Rio, M.P.; Mancera, J.M. Branchial osmoregulatory response to salinity in the gilthead sea bream, Sparus Auratus. J. Exp. Zool. A 2005, 303A, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Vargas-Chacoff, L.; Hachero, I.; Ruiz-Jarabo, I.; Rodiles, A.; Navas, J.I.; Mancera, J.M. Osmoregulatory changes in wedge sole (Dicologoglossa cuneata Moreau, 1881) after acclimation to different environmental salinities. Aquac. Res. 2009, 40, 762–771. [Google Scholar] [CrossRef]
- Cardenas, S. Crianza de la Corvina (Argyrosomus regius); Fundacion Observatorio Español de Acuicultura: Madrid, Spain, 2010; Volume 3, p. 97. [Google Scholar]
- Gonzalez-Silvera, D.; Herrera, M.; Giráldez, I.; Esteban, M.A. Effects of the dietary tryptophan and aspartate on the immune response of meagre (Argyrosomus regius) after stress. Fishes 2018, 3, 6. [Google Scholar] [CrossRef]
- Carvalho, M.; Peres, H.; Saleh, R.; Fontanillas, R.; Rosenlund, G.; Oliva-Teles, A.; Izquierdo, M. Dietary requirement for n-3 long-chain polyunsaturated fatty acids for fast growth of meagre (Argyrosomus regius, asso 1801) fingerlings. Aquaculture 2018, 488, 105–113. [Google Scholar] [CrossRef]
- Magnoni, L.J.; Salas-Leiton, E.; Peixoto, M.-J.; Pereira, L.; Silva-Brito, F.; Fontinha, F.; Gonçalves, J.F.M.; Wilson, J.M.; Schrama, J.W.; Ozório, R.O.A. Dietary electrolyte balance affects growth performance, amylase activity and metabolic response in the meagre (Argyrosomus regius). Comp. Biochem. Physiol. B 2017, 211, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Millán-Cubillo, A.F.; Martos-Sitcha, J.A.; Ruiz-Jarabo, I.; Cárdenas, S.; Mancera, J.M. Low stocking density negatively affects growth, metabolism and stress pathways in juvenile specimens of meagre (Argyrosomus regius, Asso 1801). Aquaculture 2016, 451, 87–92. [Google Scholar] [CrossRef]
- Cárdenas, C.; Toni, C.; Martos-Sitcha, J.A.; Cárdenas, S.; de la Heras, V.; Baldisserotto, B.; Heinzmann, B.M.; Vázquez, R.; Mancera, J.M. Effects of clove oil, essential oil of Lippia alba and 2-phe anaesthesia on juvenile meagre, Argyrosomus regius (Asso, 1801). J. Appl. Ichthyol. 2016, 32, 693–700. [Google Scholar] [CrossRef]
- Poli, B.M.; Parisi, G.; Zampacavallo, G.; Iurzan, F.; Mecatti, M.; Lupi, P.; Bonelli, A. Preliminary results on quality and quality changes in reared meagre (Argyrosomus regius): Body and fillet traits and freshness changes in refrigerated commercial-size fish. Aquac. Int. 2003, 11, 301–311. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Geffen, A.J.; Perez-Mayol, S.; Palmer, M.; Gonzalez-Quiros, R.; Grau, A. Seasonal and ontogenic migrations of meagre (Argyrosomus regius) determined by otolith geochemical signatures. Fish. Res. 2012, 127–128, 154–165. [Google Scholar] [CrossRef]
- Gonzalez-Quiros, R.; del Arbol, J.; Garcia-Pacheco, M.D.; Silva-Garcia, A.J.; Naranjo, J.M.; Morales-Nin, B. Life-history of the meagre Argyrosomus regius in the Gulf of Cadiz (SW Iberian Peninsula). Fish. Res. 2011, 109, 140–149. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Pascoa, I.; Goncalves, O.; Mancera, J.M. Yearly growth and metabolic changes in earthen pond-cultured meagre Argyrosomus Regius. Sci. Mar. 2014, 78, 193–202. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Pavlidis, M.; Papandroulakis, N.; Zaiss, M.M.; Tsafarakis, D.; Papadakis, I.E.; Varsamos, S. Growth performance and osmoregulation in the shi drum (Umbrina cirrosa) adapted to different environmental salinities. Aquaculture 2009, 287, 203–210. [Google Scholar] [CrossRef]
- Jensen, M.K.; Madsen, S.S.; Kristiansen, K. Osmoregulation and salinity effects on the expression and activity of Na+,K+-ATPpase in the gills of European sea bass, Dicentrarchus labrax (L.). J. Exp. Zool. 1998, 282, 290–300. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Saavedra, E.; Oyarzún, R.; Martínez-Montaño, E.; Pontigo, J.P.; Yáñez, A.; Ruiz-Jarabo, I.; Mancera, J.M.; Ortiz, E.; Bertrán, C. Effects on the metabolism, growth, digestive capacity and osmoregulation of juvenile of sub-Antarctic nototheniod fish Eleginops maclovinus acclimated at different environmental salinities. Fish Physiol. Biochem. 2015, 41, 1369–1381. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Klaren, P.H.M.; Louro, B.; Martos-Sitcha, J.A.; Pinto, P.I.S.; Vargas-Chacoff, L.; Flik, G.; Martínez-Rodríguez, G.; Power, D.M.; Mancera, J.M.; et al. Characterization of the peripheral thyroid system of gilthead seabream acclimated to different ambient salinities. Comp. Biochem. Physiol. A 2017, 203, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.; Gunnarsson, S.; Foss, A.; Stefansson, S.O. Gill Na+, K+-ATPase activity, plasma chloride and osmolality in juvenile turbot (Scophthalmus maximus) reared at different temperatures and salinities. Aquaculture 2003, 218, 671–683. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Calvo, A.; Ruiz-Jarabo, I.; Villarroel, F.; Munoz, J.L.; Tinoco, A.B.; Cardenas, S.; Mancera, J.M. Growth performance, osmoregulatory and metabolic modifications in red porgy fry, Pagrus pagrus, under different environmental salinities and stocking densities. Aquac. Res. 2011, 42, 1269–1278. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Herrera, M.; Hachero-Cruzado, I.; Vargas-Chacoff, L.; Mancera, J.M.; Arjona, F.J. Environmental salinity and osmoregulatory processes in cultured flatfish. Aquac. Res. 2015, 46, 10–29. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Barany-Ruiz, A.; Jerez-Cepa, I.; Mancera, J.M.; Fuentes, J. Intestinal response to salinity challenge in the Senegalese sole (Solea senegalensis). Comp. Biochem. Physiol. A 2017, 204, 57–64. [Google Scholar] [CrossRef]
- Lisboa, V.; Barcarolli, I.F.; Sampaio, L.A.; Bianchini, A. Effect of salinity on survival, growth and biochemical parameters in juvenile lebranch mullet Mugil liza (Perciformes: Mugilidae). Neotrop. Ichthyol. 2015, 13, 447–452. [Google Scholar] [CrossRef]
- Armesto, P.; Infante, C.; Cousin, X.; Ponce, M.; Manchado, M. Molecular and functional characterization of seven Na+/K+-ATPase beta subunit paralogs in Snegalese sole (Solea senegalensis Kaup, 1858). Comp. Biochem. Physiol. B 2015, 182, 14–26. [Google Scholar] [CrossRef]
- Armesto, P.; Campinho, M.A.; Rodriguez-Rua, A.; Cousin, X.; Power, D.M.; Manchado, M.; Infante, C. Molecular characterization and transcriptional regulation of the Na+/K+ ATPase alpha subunit isoforms during development and salinity challenge in a teleost fish, the Senegalese sole (Solea senegalensis). Comp. Biochem. Physiol. B 2014, 175, 23–38. [Google Scholar] [CrossRef]
- Hsu, H.H.; Lin, L.Y.; Tseng, Y.C.; Horng, J.L.; Hwang, P.P. A new model for fish ion regulation: Identification of ionocytes in freshwater- and seawater-acclimated medaka (Oryzias latipes). Cell Tissue Res. 2014, 357, 225–243. [Google Scholar] [CrossRef]
- Hiroi, J.; McCormick, S.D. New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir. Physiol. Neurobiol. 2012, 184, 257–268. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine control of osmoregulation in teleost fish. Am. Zool. 2001, 41, 781–794. [Google Scholar] [CrossRef]
- Cadiz, L.; Roman-Padilla, J.; Gozdowska, M.; Kulczykowska, E.; Martinez-Rodriguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Cortisol modulates vasotocinergic and isotocinergic pathways in the gilthead sea bream. J. Exp. Biol. 2015, 218, 316–325. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; Lopez-Olmeda, J.; Spedicato, M.T.; Tort, L.; Overli, O.; Martins, C.I. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef]
- Das, C.; Thraya, M.; Vijayan, M.M. Nongenomic cortisol signaling in fish. Gen. Comp. Endocrinol. 2018, 265, 121–127. [Google Scholar] [CrossRef]
- Menezes, C.; Ruiz-Jarabo, I.; Martos-Sitcha, J.A.; Toni, C.; Salbego, J.; Becker, A.; Loro, V.L.; Martinez-Rodriguez, G.; Mancera, J.M.; Baldiserotto, B. The influence of stocking density and food deprivation in silver catfish (Rhamdia quelen): A metabolic and endocrine approach. Aquaculture 2015, 435, 257–264. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Martinez, D.; Oyarzun, R.; Nualart, D.; Olavarria, V.; Yanez, A.; Bertran, C.; Ruiz-Jarabo, I.; Mancera, J.M. Combined effects of high stocking density and Piscirickettsia salmonis treatment on the immune system, metabolism and osmoregulatory responses of the Sub-Antarctic Notothenioid fish Eeginops Maclovinus. Fish Shellfish Immunol. 2014, 40, 424–434. [Google Scholar] [CrossRef]
- Laiz-Carrion, R.; Viana, I.R.; Cejas, J.; Ruiz-Jarabo, I.; Jerez, S.; Martos, J.A.; Almansa, E.; Mancera, J.M. Influence of food deprivation and high stocking density on energetic metabolism and stress response in red porgy, Pagrus pagrus L. Aquac. Int. 2012, 20, 585–599. [Google Scholar] [CrossRef]
- Herrera, M.; Ruiz-Jarabo, I.; Hachero, I.; Vargas-Chacoff, L.; Amo, A.; Mancera, J. Stocking density affects growth and metabolic parameters in the brill (Scophthalmus rhombus). Aquac. Int. 2012, 20, 1041–1052. [Google Scholar] [CrossRef]
- Herrera, M.; Vargas-Chacoff, L.; Hachero, I.; Ruiz-Jarabo, I.; Rodiles, A.; Navas, J.I.; Mancera, J.M. Physiological responses of juvenile wedge sole Dicologoglossa cuneata (Moreau) to high stocking density. Aquac. Res. 2009, 40, 790–797. [Google Scholar] [CrossRef]
- Soengas, J.L.; Sangiao-Alvarellos, S.; Laiz-Carrion, R.; Mancera, J.M. Energy metabolism and osmotic acclimation in teleost fish. In Fish Osmoregulation; Baldiserotto, B., Mancera, J.M., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 277–307. [Google Scholar]
- Bernatzeder, A.K.; Cowley, P.D.; Hecht, T. Do juveniles of the estuarine-dependent dusky kob, Argyrosomus japonicus, exhibit optimum growth indices at reduced salinities? Estuar. Coast. Shelf Sci. 2010, 90, 111–115. [Google Scholar] [CrossRef]
- Mohammed-Geba, K.; Gonzalez, A.A.; Suarez, R.A.; Galal-Khallaf, A.; Martos-Sitcha, J.A.; Ibrahim, H.M.; Martinez-Rodriguez, G.; Mancera, J.M. Molecular performance of Prl and Gh/igf1 axis in the Mediterranean meager, Argyrosomus regius, acclimated to different rearing salinities. Fish Physiol. Biochem. 2017, 43, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Guzman, J.M.; Laiz-Carrion, R.; Miguel, J.M.; Martin del Rio, M.P.; Mancera, J.M.; Soengas, J.L. Actions of 17 β-estradiol on carbohydrate metabolism in liver, gills, and brain of gilthead sea bream Sparus auratus during acclimation to different salinities. Mar. Biol. 2005, 146, 607–617. [Google Scholar] [CrossRef]
- Lagardere, J.P.; Mariani, A. Spawning sounds in meagre Argyrosomus regius recorded in the Gironde estuary, Fance. J. Fish Biol. 2006, 69, 1697–1708. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Bianchini, A. Salinity effects on osmoregulation and growth of the euryhaline flounder Paralichthys Orbignyanus. J. Exp. Mar. Biol. Ecol. 2002, 269, 187–196. [Google Scholar] [CrossRef]
- Moore, S. Amino acid analysis: Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem. 1968, 243, 6281–6283. [Google Scholar]
- Martos-Sitcha, J.A.; Wunderink, Y.S.; Straatjes, J.; Skrzynska, A.K.; Mancera, J.M.; Martinez-Rodriguez, G. Different stressors induce differential responses of the CRH-stress system in the gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. A 2014, 177, 49–61. [Google Scholar] [CrossRef]
- McCormick, S.D. Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can. J. Fish. Aquat. Sci. 1993, 50, 656–658. [Google Scholar] [CrossRef]
- Mancera, J.M.; Laiz Carrion, R.; Martín del Río, M.P. Osmoregulatory action of PRL, GH, and cortisol in the gilthead seabream (Sparus aurata l.). Gen. Comp. Endocrinol. 2002, 129, 95–103. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Arjona, F.J.; Laiz-Carrion, R.; Flik, G.; Klaren, P.H.M.; Mancera, J.M. Energy metabolism of hyperthyroid gilthead sea bream Sparus aurata L. Comp. Biochem. Physiol. A 2016, 191, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Decker, K. Glycogen determination with amyloglucosidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; Volume 3, pp. 1127–1131. [Google Scholar]
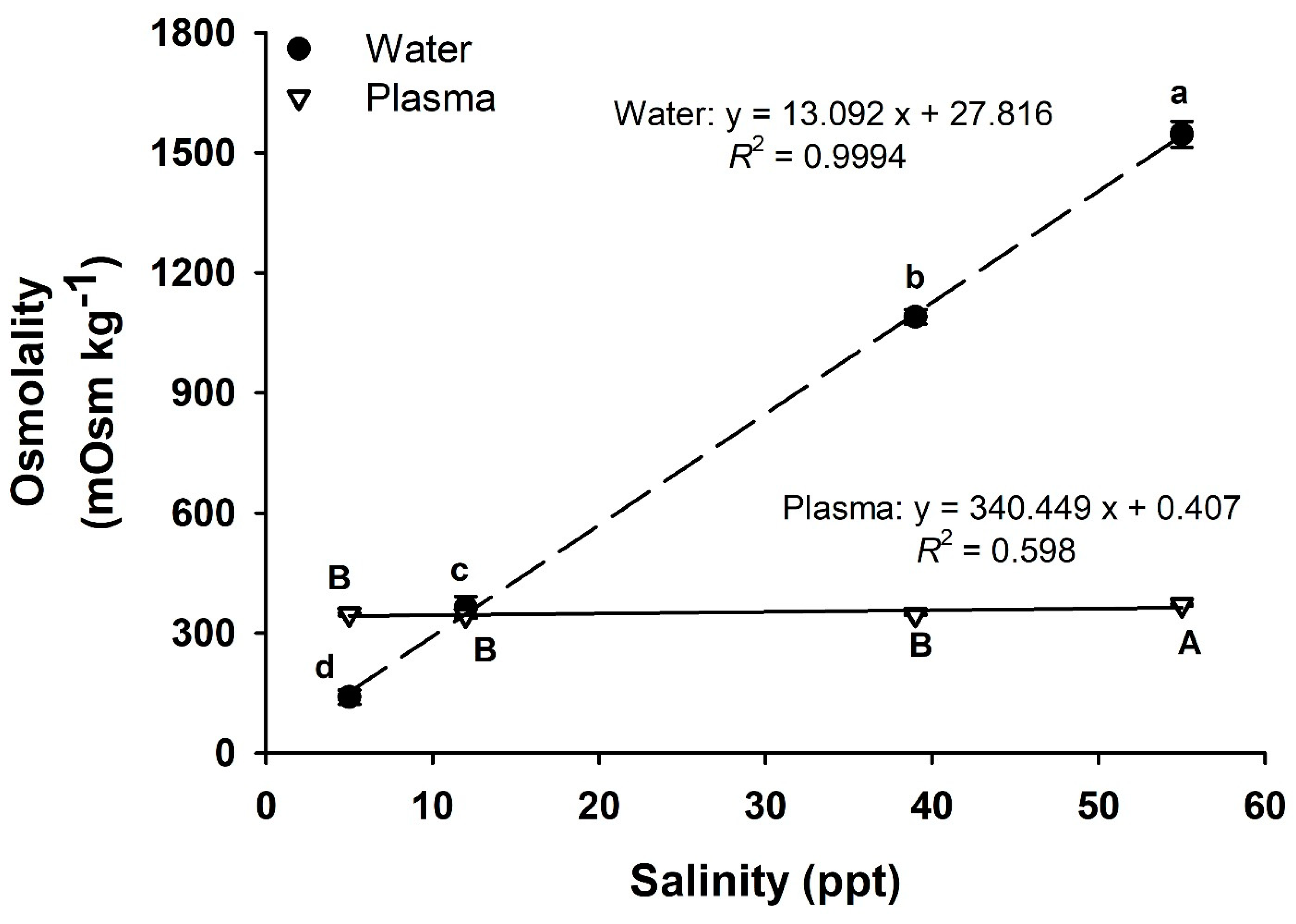
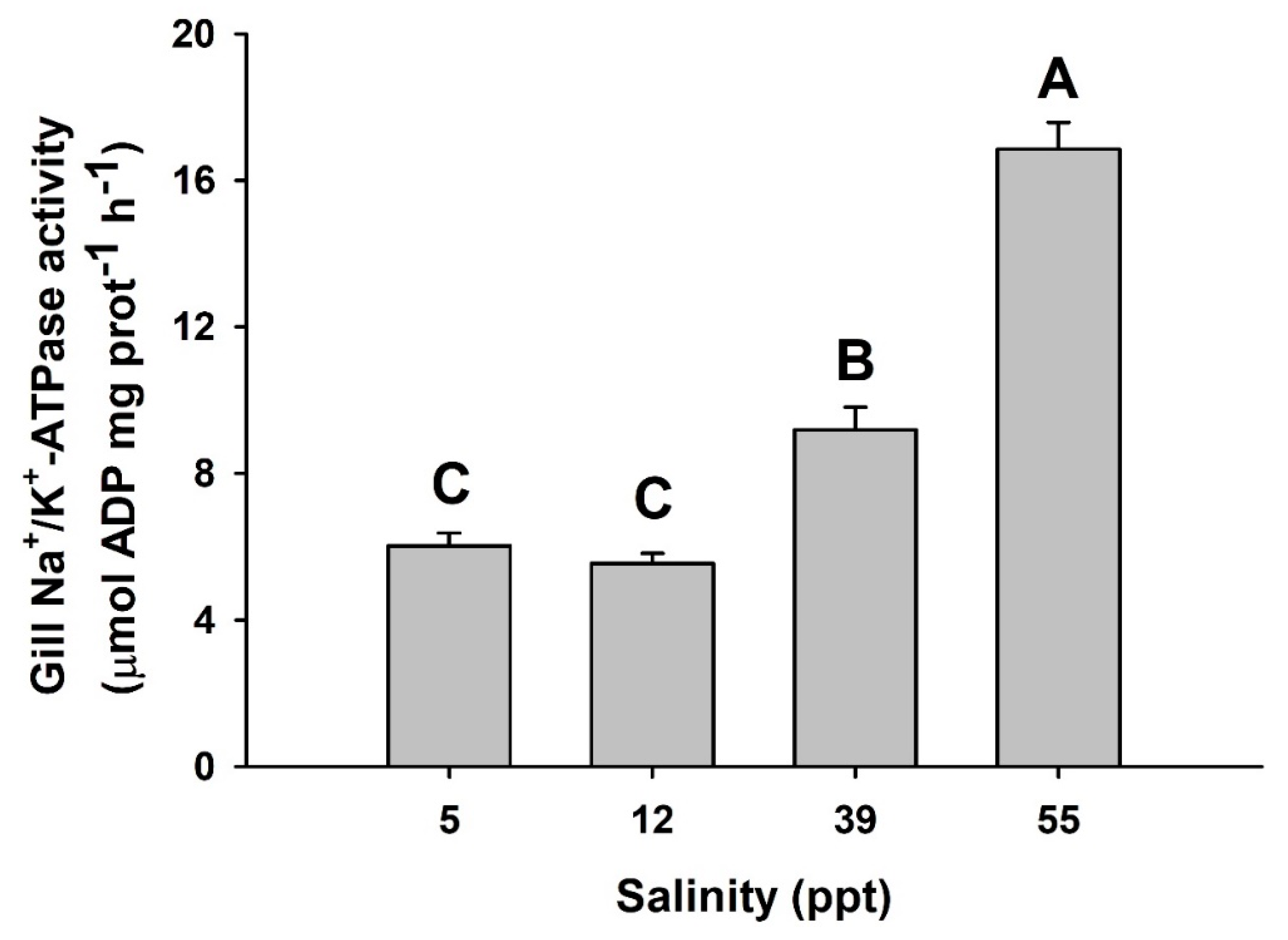
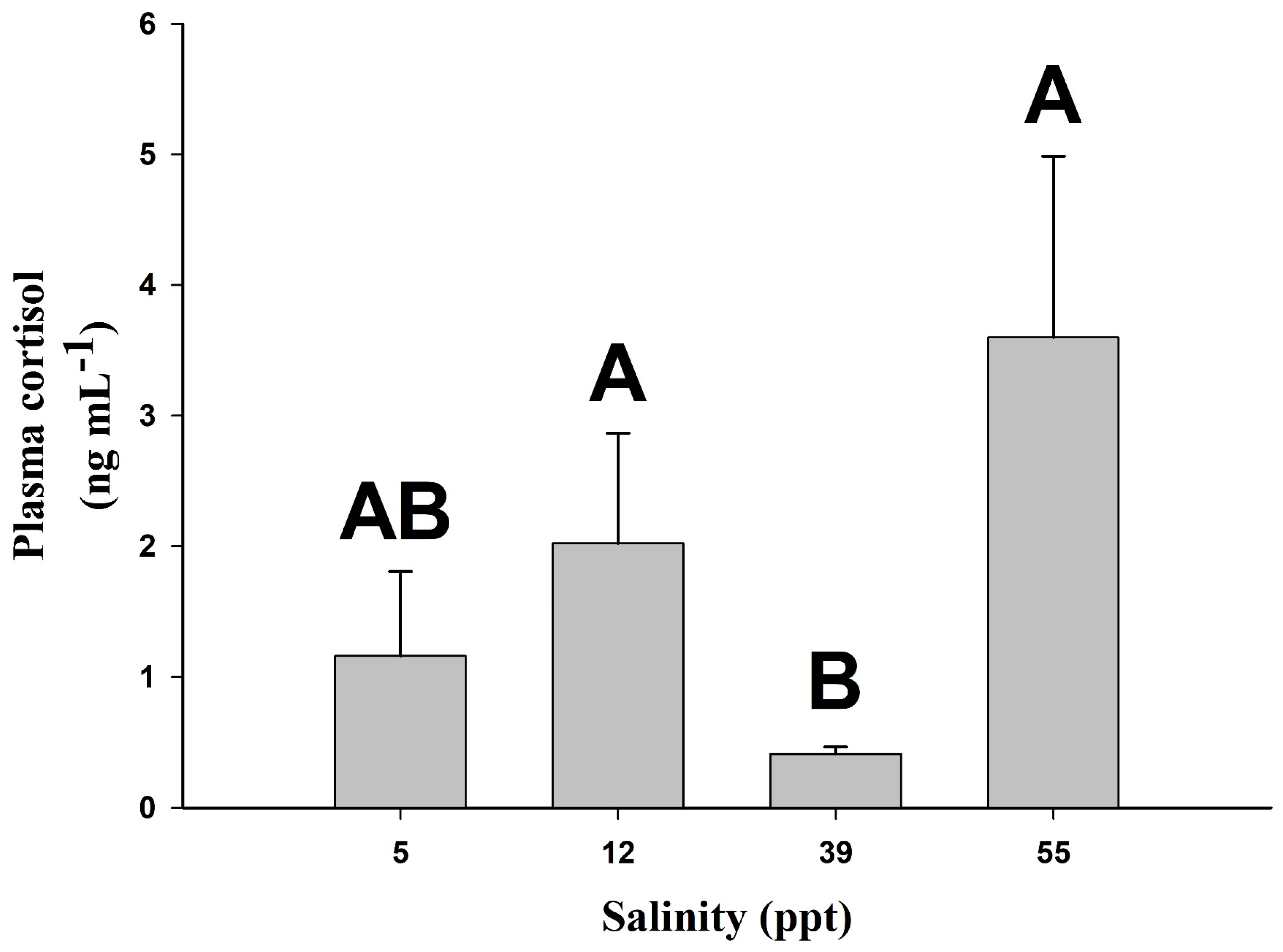
| Variable | 5 ppt | 12 ppt | 39 ppt | 55 ppt |
|---|---|---|---|---|
| Glucose (mM) | 2.8 ± 0.1 c | 3.6 ± 0.2 bc | 5.1 ± 0.4 a | 3.8 ± 0.2 b |
| Lactate (mM) | 1.4 ± 0.2 ab | 1.5 ± 0.1 a | 0.9 ± 0.1 b | 1.5 ± 0.2 a |
| Proteins (g dL−1) | 30.1 ± 1.2 b | 31.5 ± 0.6 ab | 30.4 ± 0.8 b | 33.5 ± 0.7 a |
| Amino acid (mM) | 16.0 ± 0.8 a | 15.7 ± 0.4 a | 13.1 ± 0.5 b | 13.9 ± 0.5 ab |
| TAG (mM) | 0.9 ± 0.1 b | 1.1 ± 0.1 ab | 1.0 ± 0.1 ab | 1.4 ± 0.1 a |
| FFA (mM) | 1.7 ± 0.2 b | 2.6 ± 0.2 b | 2.6 ± 0.2 b | 5.4 ± 0.4 a |
| Variable | 5 ppt | 12 ppt | 39 ppt | 55 ppt |
|---|---|---|---|---|
| HSI (%) | 2.0 ± 0.2 ab | 1.8 ± 0.2 ab | 2.2 ± 0.2 a | 1.5 ± 0.1 b |
| Glycogen (mg liver−1) | 36.8 ± 2.2 ab | 34.1 ± 2.2 b | 36.6 ± 2.8 a | 26.6 ± 4.0 c |
| Glucose (mg liver−1) | 5.1 ± 0.3 ab | 4.6 ± 0.3 b | 4.3 ± 0.4 b | 6.1 ± 0.6 a |
| TAG (mg liver−1) | 4.5 ± 0.3 | 3.7 ± 0.2 | 4.9 ± 0.5 | 4.3 ± 0.2 |
| Amino acid (μmol liver−1) | 258 ± 16 a | 267 ± 9 a | 289 ± 18 a | 172 ± 14 b |
| Variable | 5 ppt | 12 ppt | 39 ppt | 55 ppt |
|---|---|---|---|---|
| WG (%) | 25.4 ± 10.7 a | 16.9 ± 6.4 a | 20.0 ± 9.9 a | −12.6 ± 7.0 b |
| K (no units) | 0.99 ± 0.01 a | 0.96 ± 0.01 a | 0.99 ± 0.01 a | 0.89 ± 0.01 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Jarabo, I.; Márquez, P.; Vargas-Chacoff, L.; Martos-Sitcha, J.A.; Cárdenas, S.; Mancera, J.M. Narrowing the Range of Environmental Salinities Where Juvenile Meagre (Argyrosomus regius) Can Be Cultured Based on an Osmoregulatory Pilot Study. Fishes 2018, 3, 48. https://doi.org/10.3390/fishes3040048
Ruiz-Jarabo I, Márquez P, Vargas-Chacoff L, Martos-Sitcha JA, Cárdenas S, Mancera JM. Narrowing the Range of Environmental Salinities Where Juvenile Meagre (Argyrosomus regius) Can Be Cultured Based on an Osmoregulatory Pilot Study. Fishes. 2018; 3(4):48. https://doi.org/10.3390/fishes3040048
Chicago/Turabian StyleRuiz-Jarabo, Ignacio, Pura Márquez, Luis Vargas-Chacoff, Juan Antonio Martos-Sitcha, Salvador Cárdenas, and Juan Miguel Mancera. 2018. "Narrowing the Range of Environmental Salinities Where Juvenile Meagre (Argyrosomus regius) Can Be Cultured Based on an Osmoregulatory Pilot Study" Fishes 3, no. 4: 48. https://doi.org/10.3390/fishes3040048
APA StyleRuiz-Jarabo, I., Márquez, P., Vargas-Chacoff, L., Martos-Sitcha, J. A., Cárdenas, S., & Mancera, J. M. (2018). Narrowing the Range of Environmental Salinities Where Juvenile Meagre (Argyrosomus regius) Can Be Cultured Based on an Osmoregulatory Pilot Study. Fishes, 3(4), 48. https://doi.org/10.3390/fishes3040048