Water Oxygen Content Affects Distribution of T and B Lymphocytes in Lymphoid Tissues of Farmed Sea Bass (Dicentrarchus Labrax)
Abstract
1. Introduction
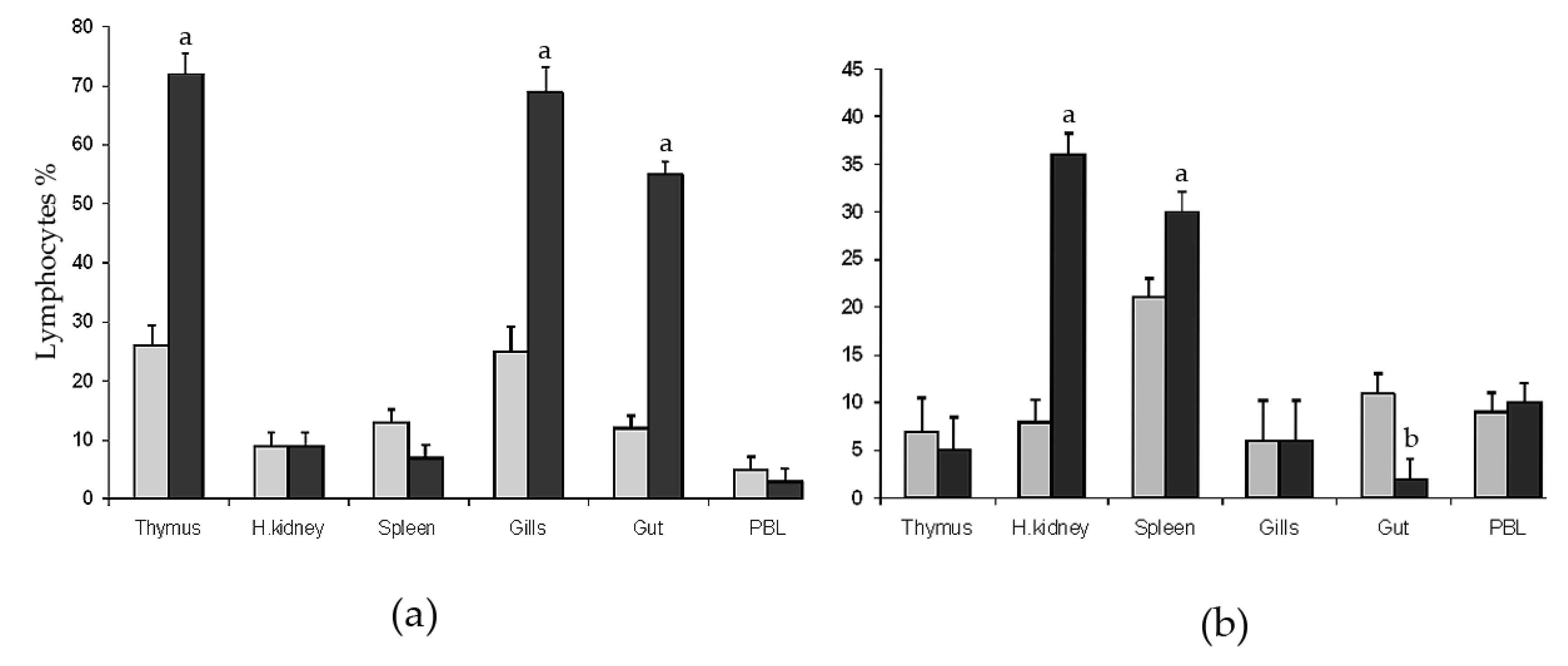
2. Results
2.1. Flow Cytometry
2.2. Immuno-Histochemistry
3. Discussion
4. Materials and Methods
4.1. Fish Experimental Groups
4.2. Cell Suspensions
4.3. Indirect Immunofluorescence and Flow Cytometry
4.4. Immuno-Histochemistry
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nehr, O.; Blancheton, I.P.; Alliot, E. Development of an intensive culture system for sea bass (Dicentrarchus labrax) larvae in sea enclosures. Aquaculture 1996, 143, 43–58. [Google Scholar] [CrossRef]
- Scapigliati, G.; Romano, N.; Buonocore, F.; Picchietti, S.; Baldassini, M.R.; Prugnoli, D.; Galice, A.; Meloni, S.; Secombes, C.J.; Mazzini, M.; et al. The immune system of sea bass, Dicentrarchus labrax, reared in aquaculture. Dev. Comp. Immunol. 2002, 26, 151–160. [Google Scholar] [CrossRef]
- Faggio, C.; Piccione, G.; Marafioti, S.; Arfuso, F.; Trischitta, F.; Fortino, G.; Fazio, F. Monthly variations of haematological parameters of Sparus aurata and Dicentrarchus labrax reared in Mediterranean land off-shore tanks. Cah. Biol. Mar. 2014, 55, 437–443. [Google Scholar]
- Hughes, G.M.; Kikuchi, Y.; Barrington, J. Physiological salines and the mechanical proprieties of trout red blood cells. J. Fish Biol. 1986, 29, 393–402. [Google Scholar] [CrossRef]
- Wells, R.M.G.; Weber, R.E. The spleen in hypoxic and exercised rainbow trout. J. Exp. Biol. 1990, 150, 461–466. [Google Scholar]
- Sørensen, B.; Weber, R.E. Effect of oxygenation and the stress hormones adrenaline and cortisol on the viscosity of blood from the trout Oncorhyncus mykiss. J. Exp. Biol. 1995, 198, 953–959. [Google Scholar]
- Shang, E.H.; Wu, R.S. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ. Sci. Technol. 2004, 38, 4763–4767. [Google Scholar] [CrossRef] [PubMed]
- Van Raaij, M.T.M.; Bakker, E.; Nieveen, M.C.; Zirkzee, H.; van den Thillart, G.E. Energy status and free fatty acid patterns in tissues of common carp (Cyprinus carpio, L.) and rainbow trout (Oncorhynchus mykiss, L) during severe oxygen restriction. Comp. Biochem. Physiol. 1994, 109, 755–767. [Google Scholar] [CrossRef]
- Claireaux, G.; Lagardeáre, J.P. Influence of temperature, oxygen and salinity on the metabolism of the European sea bass. J. Sea Res. 1999, 42, 157–168. [Google Scholar] [CrossRef]
- Caldwell, C.A.; Hinshaw, J. Physiological and haematological response in rainbow trout subjected to supplemental dissolved oxygen in fish culture. Aquaculture 1994, 126, 183–193. [Google Scholar] [CrossRef]
- Smith, G.L.; Hattingh, J. The effect of respiratory stress on carp haemoglobin. Comp. Biochem. Physiol. 1978, 59, 369–374. [Google Scholar] [CrossRef]
- Wood, C.M. Branchial ion and acid base transfer in freshwater teleost fish: Environmental hyperoxia as a probe. Physiol. Zool. 1991, 64, 68–102. [Google Scholar] [CrossRef]
- Soncini, R.; Glass, M.L. The effects of temperature and hydroxia on arterial PO2 and acid-base status in Piaractus mesopotamicus. J. Fish Biol. 1997, 51, 225–233. [Google Scholar]
- Scapigliati, G.; Scalia, D.; Marras, A.; Meloni, S.; Mazzini, M. Immunoglobulin levels in sea bass Dicentrarchus labrax (L.) in relation to age, season and water oxygenation. Aquaculture 1999, 174, 207–212. [Google Scholar] [CrossRef]
- Scapigliati, G.; Romano, N.; Abelli, L. Monoclonal antibodies in teleost fish immunology: Identification, ontogeny and activity of T- and B-lymphocytes. Aquaculture 1999, 172, 3–28. [Google Scholar] [CrossRef]
- Romano, N.; Caccia, E.; Piergentili, R.; Rossi, F.; Ficca, A.G.; Ceccariglia, S.; Mastrolia, L. Antigen-dependent T lymphocytes (TcR β) are primarily differentiated in the thymus rather than in other lymphoid tissues in sea bass (Dicentrarchus Labrax, L.). Fish Shellfish Immunol. 2011, 30, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Scapigliati, G.; Meloni, S.; Buonocore, F.; Bossù, P.; Prugnoli, D.; Secombes, C.J. Immunopurification of B lymphocytes from sea bass Dicentrarchus labrax (L.). Mar. Biotechnol. 2003, 5, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Abelli, L.; Mastrolia, L.; Scapigliati, G. Immunocytochemical detection and cytomorphology of lymphocyte subpopulations in a teleost fish Dicentrarchus labrax (L.). Cell Tissue Res. 1997, 289, 163–171. [Google Scholar] [CrossRef] [PubMed]
- EL-Khaldi, A.T.F. Effect of different stress factors on some physiological parameters of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2010, 17, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Dalla Via, G.J.; Villani, P.; Gasteiger, E.; Niederstätter, H. Oxygen consumption in sea bass fingerling Dicentrarchus labrax exposed to acute salinity and temperature changes: Metabolic basis for maximum stocking density estimations. Aquaculture 1998, 169, 303–313. [Google Scholar] [CrossRef]
- Bergheim, A.; Gausen, M.; Næss, A.; Hølland, P.M.; Krogedal, P.; Crampton, V. A newly developed oxygen injection system for cage farms. Aquac. Eng. 2006, 34, 40–46. [Google Scholar] [CrossRef]
- Bunch, E.C.; Bejerano, I. The effects of environmental factors on the susceptibility of hybrid tilapia Oreochromis niloticus × Oreochromis aureus to Stertococcosis. Isr. J. Aquac. 1997, 49, 67–76. [Google Scholar]
- Fazio, F.; Marafioti, S.; Filiciotto, F.; Buscaino, G.; Panzera, M.; Faggio, C. Blood Hemogram Profiles of Farmed Onshore and Offshore Gilthead Sea Bream (Sparus aurata) from Sicily, Italy. Turk. J. Fish. Aquat. Sci. 2013, 13, 415–422. [Google Scholar] [CrossRef]
- Esteban, M.A.; Meseguer, J.; Ayala, A.G.; Agulleiro, B. Erythropoiesis and thrombopoiesis in the head-kidney of the sea bass (Dicentrarchus labrax L.): An ultrastructural study. Arch. Histol. Cytol. 1989, 52, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Berillis, P.; Mente, E.; Nikouli, E.; Makridis, P.; Grundvig, H.; Bergheim, A.; Gausen, M. Improving aeration for efficient oxygenation in sea bass sea cages. Blood, brain and gill histology. Open Life Sci. 2016, 11, 270–279. [Google Scholar] [CrossRef]
- Romano, N.; Rossi, F.; Caccia, E.; Abelli, L.; Piergentili, R.; Mastrolia, L.; Randelli, E.; Buonocore, F. Majority of TcRβ+ T-lymphocytes located in thymus and midgut of the bony fish Dicentrarchus labrax (L.). Cell Tissue Res. 2007, 329, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Abelli, L.; Pichietti, S.; Romano, N.; Mastrolia, L.; Scapigliati, G. Immunocytochemical detection of thymocyte antigenic determinant in developing lymphoid organs of sea bass, Dicentrarchus labrax L. Fish Shellfish Immunol. 1996, 6, 493–509. [Google Scholar] [CrossRef]
- Khan, D.; Ansar Ahmed, S. The immune system is a natural target for estrogen action: Opposing effects of estrogen in two prototypical autoimmune diseases. Front. Immunol. 2015, 6, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Cabas, I.; Liarte, S.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; Garciía-Ayala, A. 17α-ethynylestradiol alters the immune response of the teleost gilthead seabream (Sparus aurata L.) both in vivo and in vitro. Dev. Comp. Immunol. 2012, 36, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, S.J.; McAllister, P.E.; Hetrick, F.M.; Anderson, D.P. Effect of exogenous corticosteroids on circulating virus and neutralizing antibodies in striped bass (Morone saxalis) infected with infectious pancreatic necrosis virus. Vet. Immunol. Immunopathol. 1986, 12, 305–311. [Google Scholar] [CrossRef]
- Maule, A.G.; Schreck, C.B. Glucocorticoids receptors in leukocytes and gill of juvenile Coho salmon (Oncorhynchus kisutch). Gen. Comp. Endocrinol. 1990, 77, 448–455. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [PubMed]
- Weyts, F.A.A.; Verburg-van Kemenade, B.M.L.; Flik, G. Characterisation of Glucocorticoid Receptors in Peripheral Blood Leukocytes of Carp, Cyprinus carpio L. Gen. Comp. Endocrinol. 1998, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Groups | NO | HO | ||
|---|---|---|---|---|
| DLT15 | DLIg3 | DLT15 | DLIg3 | |
| H. kidney | 675 ± 274 | 480 ± 187 | 316 ± 118 ** | 305 ± 118 * |
| Spleen | 206 ± 159 | 232 ± 112 | 363 ± 260 | 275 ± 55 |
| Gut | 802 ± 431 | 281 ± 158 a | 1401 ± 280 ** | 434 ± 151 b |
| Thymus | 51233 ± 18485 | 6 ± 2.3 | 69477 ± 14745 ** | 15 ± 5 * |
| Gills | 520 ± 70 | 12 ± 5 b | 1178 ± 97 ** | 45 ± 36 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, N.; Scapigliati, G.; Abelli, L. Water Oxygen Content Affects Distribution of T and B Lymphocytes in Lymphoid Tissues of Farmed Sea Bass (Dicentrarchus Labrax). Fishes 2017, 2, 16. https://doi.org/10.3390/fishes2030016
Romano N, Scapigliati G, Abelli L. Water Oxygen Content Affects Distribution of T and B Lymphocytes in Lymphoid Tissues of Farmed Sea Bass (Dicentrarchus Labrax). Fishes. 2017; 2(3):16. https://doi.org/10.3390/fishes2030016
Chicago/Turabian StyleRomano, Nicla, Giuseppe Scapigliati, and Luigi Abelli. 2017. "Water Oxygen Content Affects Distribution of T and B Lymphocytes in Lymphoid Tissues of Farmed Sea Bass (Dicentrarchus Labrax)" Fishes 2, no. 3: 16. https://doi.org/10.3390/fishes2030016
APA StyleRomano, N., Scapigliati, G., & Abelli, L. (2017). Water Oxygen Content Affects Distribution of T and B Lymphocytes in Lymphoid Tissues of Farmed Sea Bass (Dicentrarchus Labrax). Fishes, 2(3), 16. https://doi.org/10.3390/fishes2030016






