Ethmalosa fimbriata (Bowdich 1825), a Clupeid Fish That Exhibits Elevated Batch Fecundity in Hypersaline Waters
Abstract
:1. Introduction
2. Results
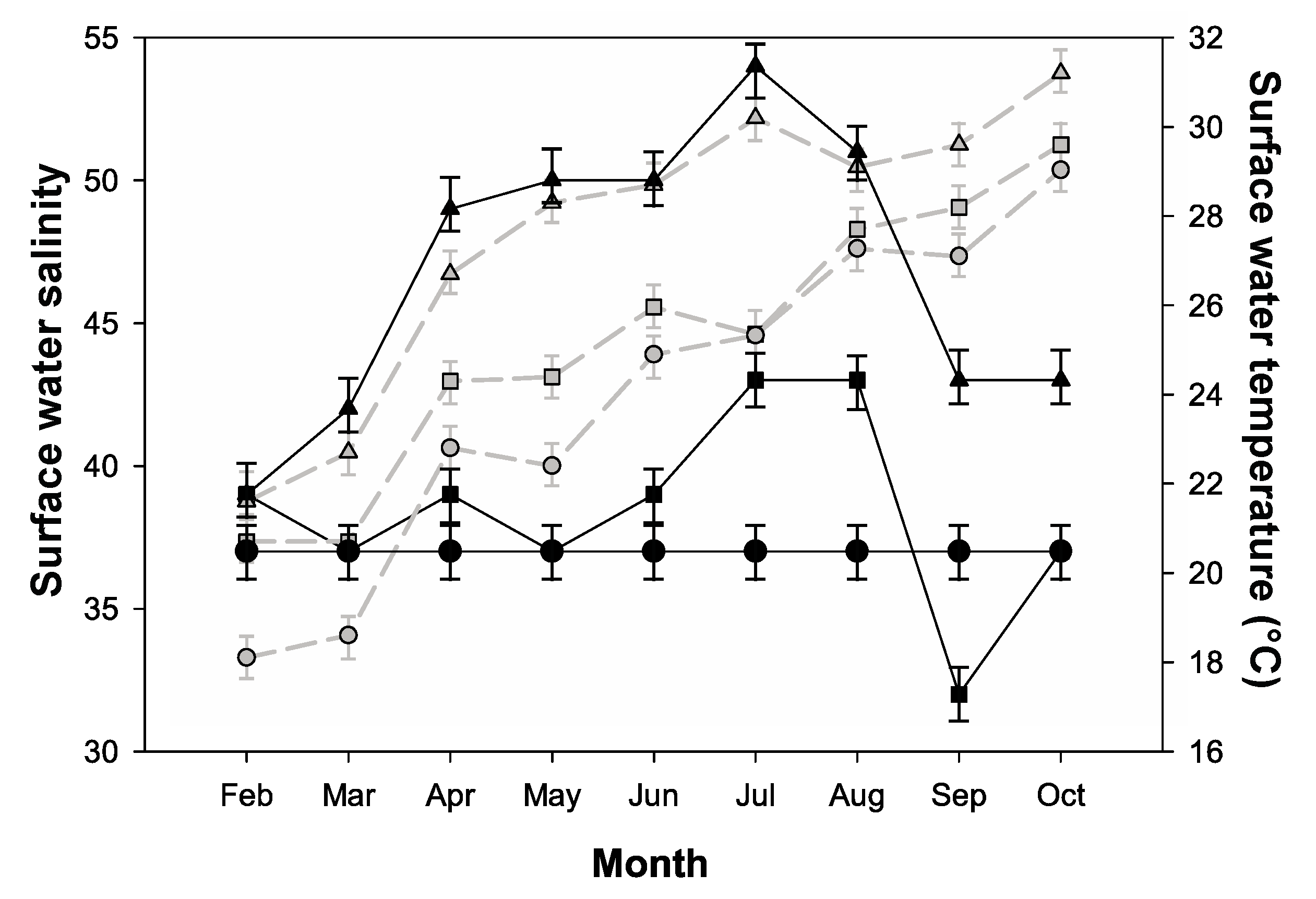
2.1. Environmental Parameters
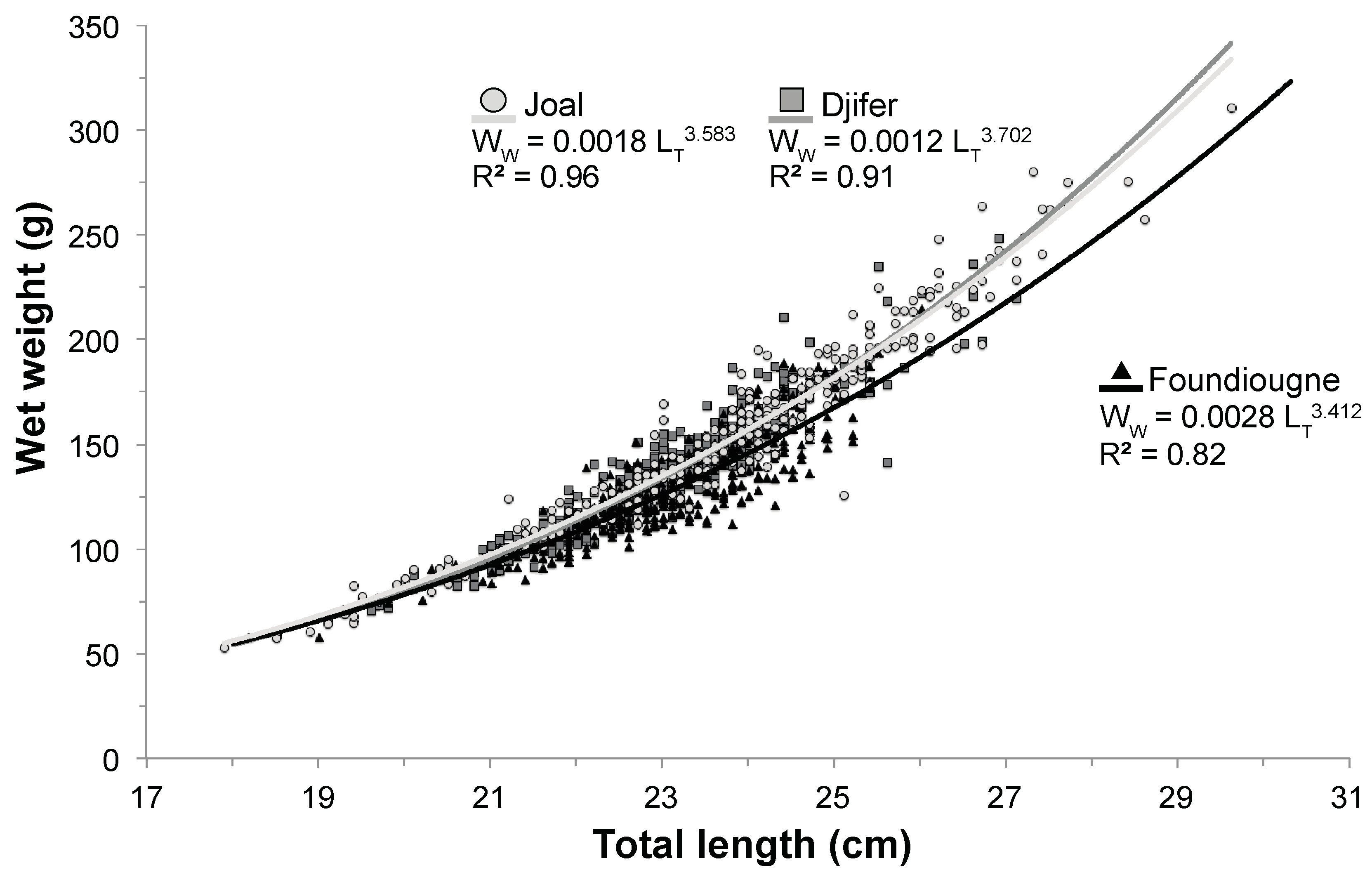
2.2. Length-Weight Relationship
2.3. Reproductive Parameters
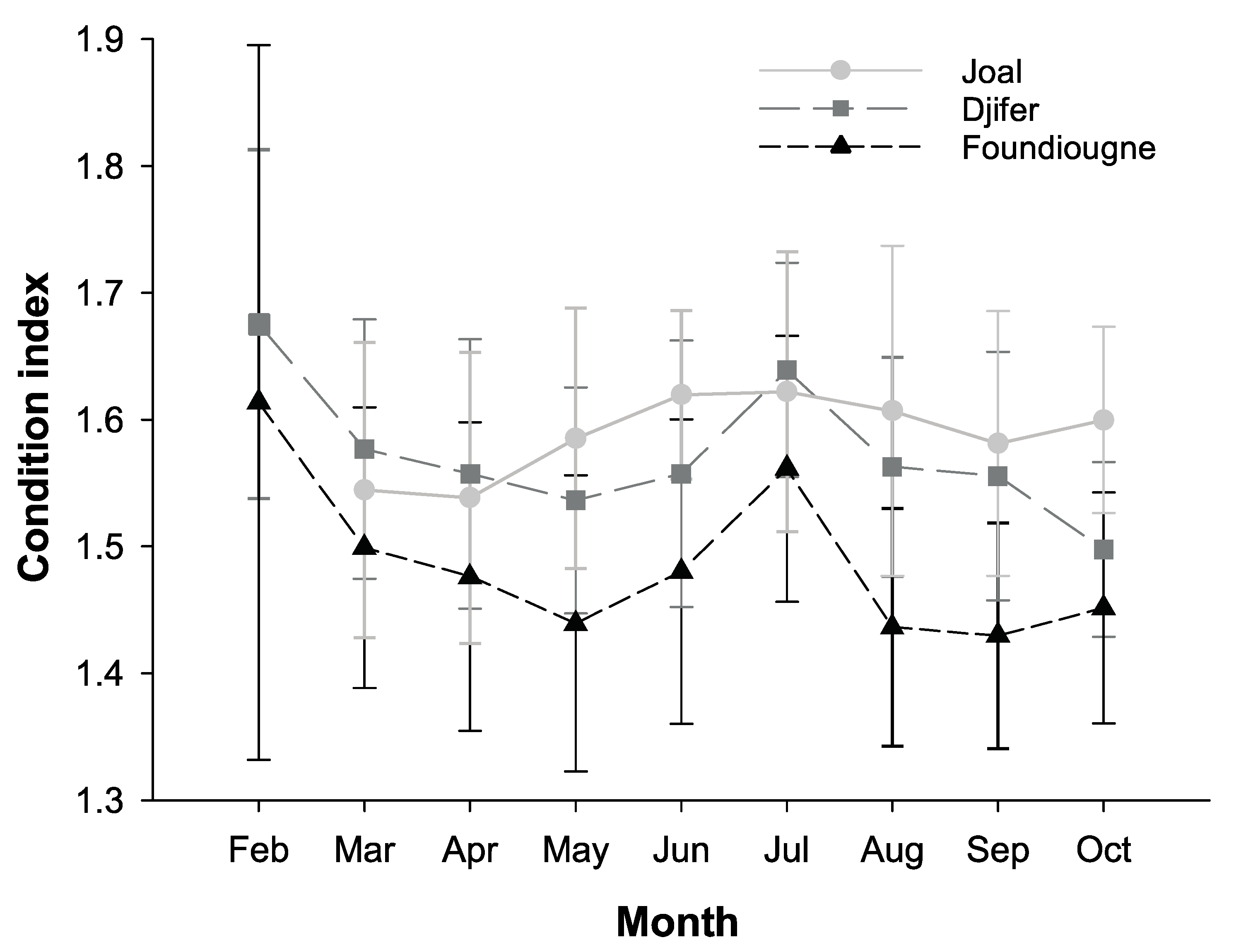
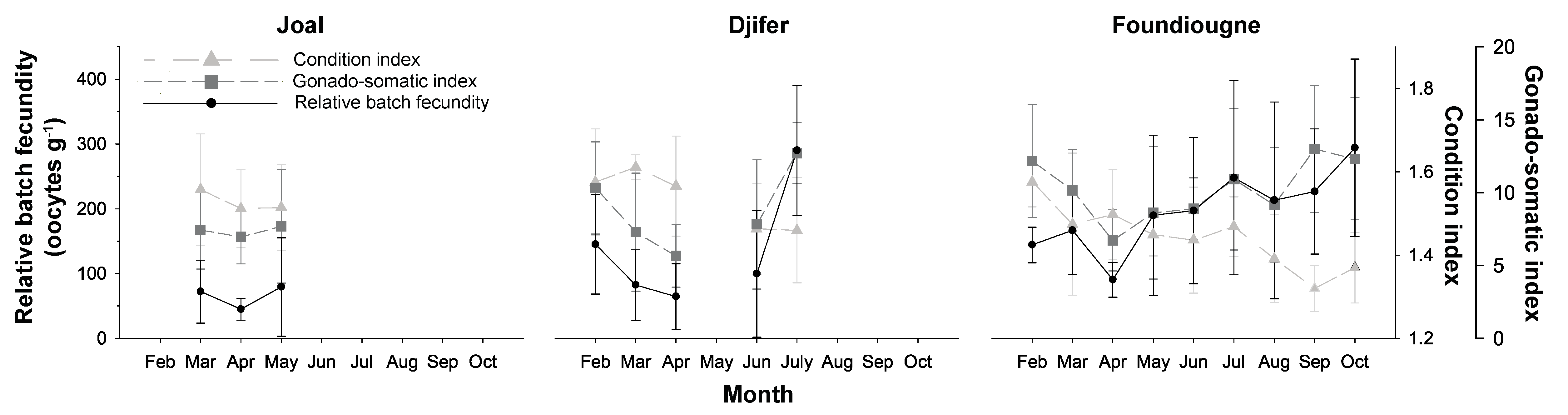
2.3.1. The Condition Index and the Gonado-Somatic Index
2.3.2. Batch Fecundities
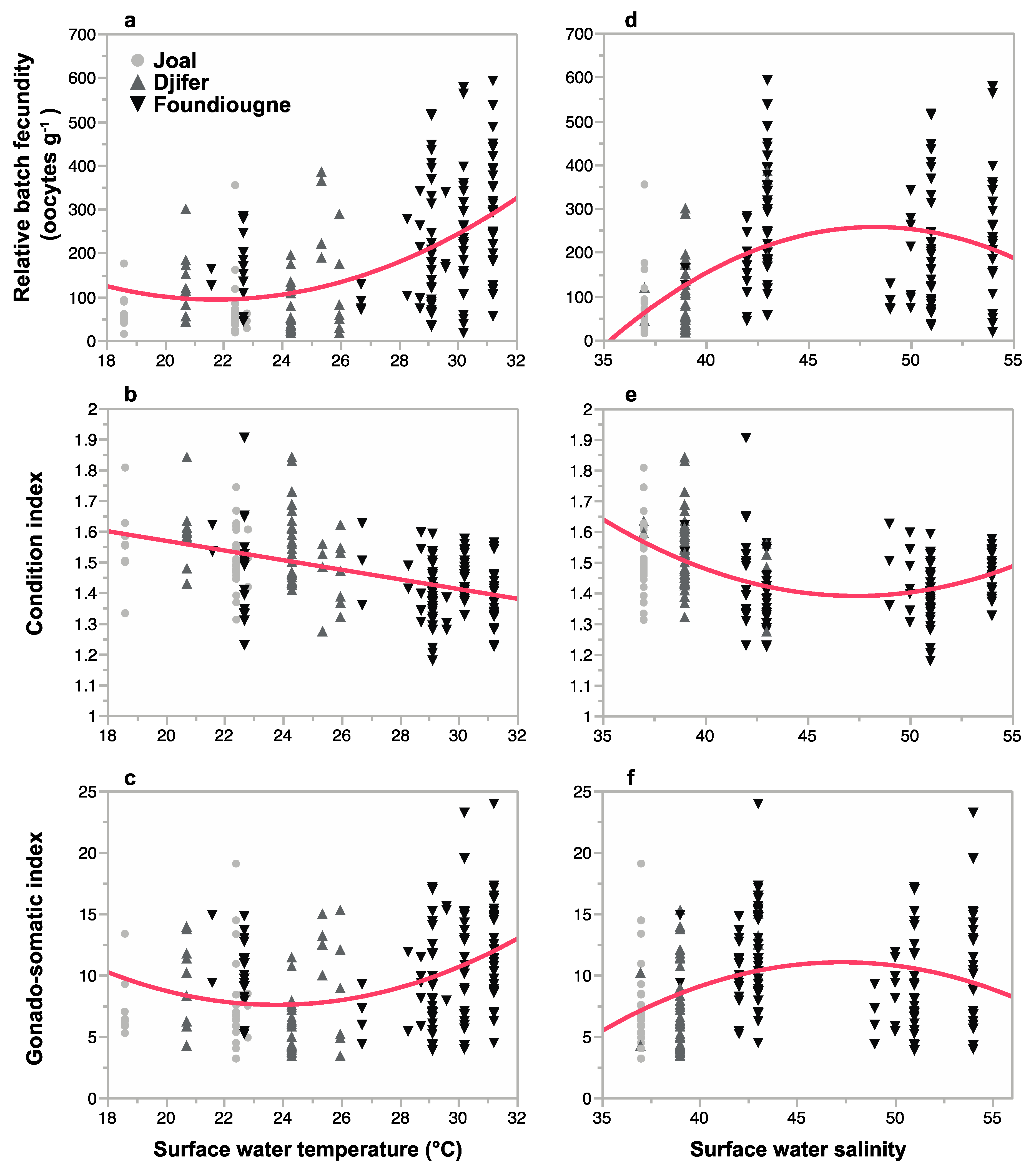
2.3.3. Correlation of Reproductive Parameters with Temperature and Salinity
3. Discussion
4. Materials and Methods
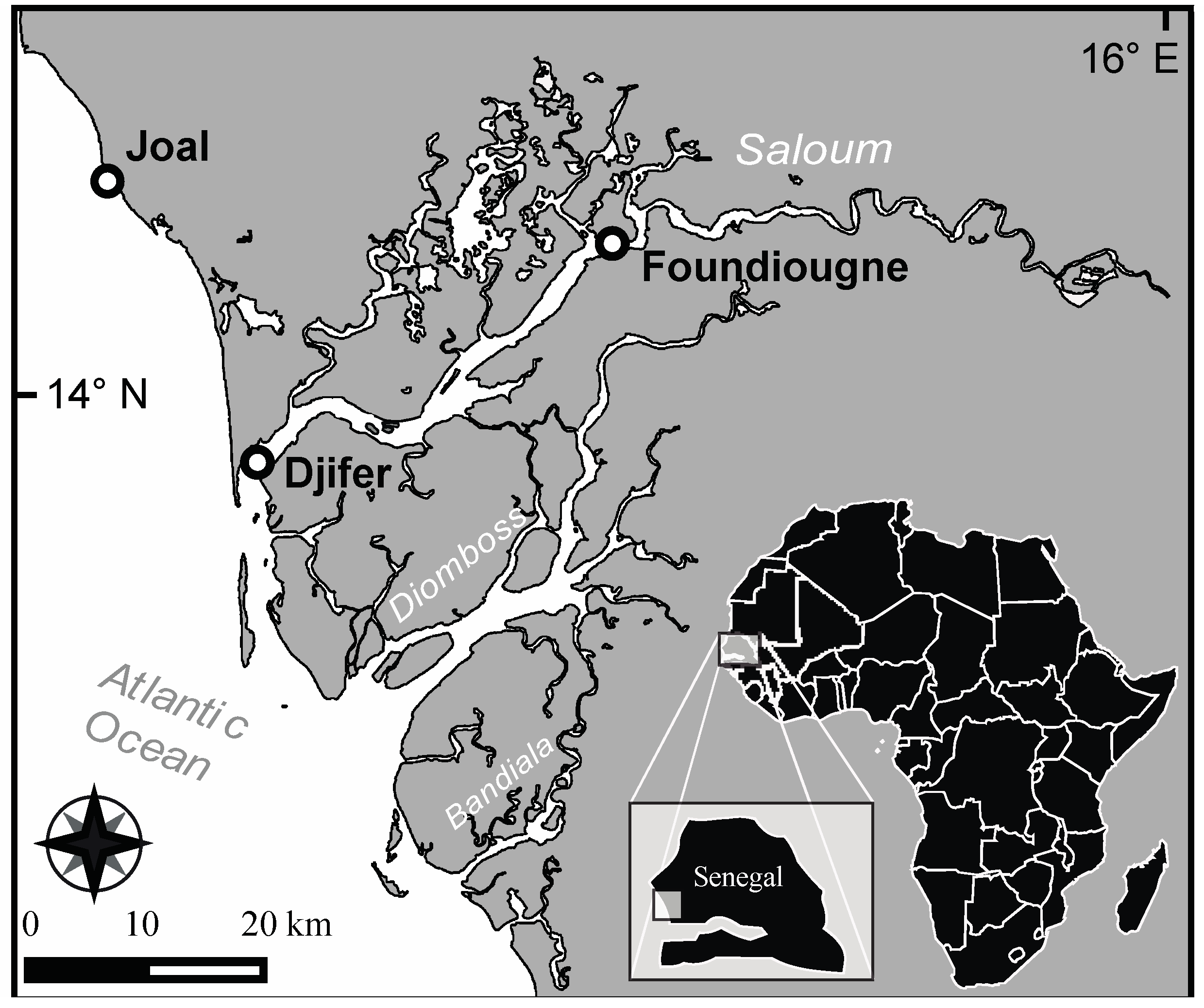
4.1. Sampling Sites
4.2. Environmental Parameters
4.3. Fish Sampling
4.4. The Condition Index and the Gonado-Somatic Index
4.5. Absolute Batch Fecundity and Relative Batch Fecundity
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Site | LT (cm) | February | March | April | May | June | July | August | September | October | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Joal | 23.1–27.5 | Khyd | 1.56 (±0.12) | 1.51 (±0.09) | 1.51 (±0.10) | ||||||
| IG | 7.43 (±4.32) | 6.97 (±2.05) | 7.68 (±3.90) | ||||||||
| RBF | 72.21 (±144.48) | 44.74 (±16.73) | 79.33 (±76.024) | ||||||||
| ABF | 3337–31,052 | 4824–10,877 | 3316–66,375 | ||||||||
| Djifer | 20.8–26.9 | Khyd | 1.58 (±0.13) | 1.61 (±0.03) | 1.57 (±0.12) | 1.57 (±0.12) | 1.46 (±0.11) | ||||
| IG | 10.32 (±3.08) a | 7.20 (±4.19) ab | 5.66 (±2.16) b | 7.82 (±4.44) ab | 12.70 (±2.08) a | ||||||
| RBF | 145.05 (±76.78) ab | 82.29 (±54.61) bc | 64.53 (±50.97) c | 99.68 (±97.95) bc | 290.15 (±100.05) a | ||||||
| ABF | 6941–39,490 | 6126–17,630 | 2200–36,745 | 2724–27,222 | 17,151–35,693 | ||||||
| Foundiougne | 19.9–28.3 | Khyd | 1.58 (±0.06) a | 1.47 (±0.17) a | 1.49 (±0.11) ab | 1.45 (±0.05) abc | 1.44 (±0.13) abc | 1.47 (±0.07) a | 1.39 (±0.11) bc | 1.32 (±0.06) c | 1.37 (±0.08) c |
| IG | 12.17 (±3.88) abc | 10.16 (±2.90) abc | 6.73 (±2.11) c | 8.61 (±4.55) abc | 8.91 (±2.11) abc | 10.92 (±4.84) abc | 9.15 (±3.95) bc | 13.00 (±4.36) ab | 12.33 (±4.19) a | ||
| RBF | 144.36 (±27.62) abc | 167.08 (±68.93) bc | 90.36 (±26.89) c | 189.96 (±123.84) abc | 197.15 (±112.92) abc | 247.87 (±150.02) ab | 213.04 (±152.06) bc | 226.78 (±96.53) abc | 294.05 (±136.71) a | ||
| ABF | 16,388–23,160 | 6470–54,205 | 6344–13,298 | 13,173–29,889 | 11,229–36,199 | 3160–85,409 | 4321–85,045 | 19,501–37,985 | 6162–61,270 |
Appendix B
References
- Kucera, C.J.; Faulk, C.K.; Holt, G.J. The effect of parental acclimation to spawning salinity on the survival of larval Cynoscion nebulosus. J. Fish Biol. 2002, 61, 726–738. [Google Scholar] [CrossRef]
- Nissling, A.; Johansson, U.; Jacobsson, M. Effects of salinity and temperature conditions on the reproductive success of turbot (Scophthalmus maximus) in the Baltic Sea. Fish. Res. 2006, 80, 230–238. [Google Scholar] [CrossRef]
- Kirschner, L.B. The energetics of osmotic regulation in ureotelic and hypoosmotic fishes. J. Exp. Zool. 1993, 267, 19–26. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Laiz-Carrión, R.; Guzmán, J.M.; Martin del Río, M.P.; Miguez, J.M.; Mancera, J.M.; Soengas, J.L. Acclimation of S. aurata to various salinities alters energy metabolism of osmoregulatory and nonosmoregulatory organs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Holliday, F.G.T.; Blaxter, J.H.S. The effects of salinity on the developing eggs and larvae of the herring. J. Mar. Biol. Assoc. U. K. 1960, 39, 591–603. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Q.; Goudie, C.A.; Simco, B.A. Survival, growth, and feed utilization of pre- and postmetamorphic american shad exposed to increasing salinity. N. Am. J. Aquac. 2009, 71, 197–205. [Google Scholar] [CrossRef]
- Leggett, W.C.; Carscadden, J.E. Latitudinal variation in reproductive characteristics of American shad (Alosa sapidissima): Evidence for population specific life history strategies in fish. J. Fish. Res. Board Can. 1978, 35, 1469–1478. [Google Scholar] [CrossRef]
- McBride, R.S.; Somarakis, S.; Fitzhugh, G.R.; Albert, A.; Yaragina, N.A.; Wuenschel, M.J.; Alonso-Fernández, A.; Basilone, G. Energy acquisition and allocation to egg production in relation to fish reproductive strategies. Fish Fish. 2015, 16, 23–57. [Google Scholar] [CrossRef]
- Panfili, J.; Thior, D.; Ecoutin, J.-M.; Ndiaye, P.; Albaret, J.J. Influence of salinity on the size at maturity for fish species reproducing in contrasting West African estuaries. J. Fish Biol. 2006, 69, 95–113. [Google Scholar] [CrossRef]
- Aykanat, T.; Thrower, F.P.; Heath, D.D. Rapid evolution of osmoregulatory function by modification of gene transcription in steelhead trout. Genetica 2011, 139, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Rogers, P.J.; Ward, T.M. Life history strategy of sandy sprat Hyperlophus vittatus (Clupeidae): A comparison with clupeoids of the Indo-Pacific and southern Australia. J. Appl. Ichthyol. 2007, 23, 583–591. [Google Scholar] [CrossRef]
- Alheit, J. Reproductive biology of sprat (Sprattus sprattus): Factors determining annual egg production. ICES J. Mar. Sci. 1988, 44, 162–168. [Google Scholar] [CrossRef]
- Müller, A.; Muhsin, K.; Köster, F.W. Ovarian Maturation and Batch Fecundity in Baltic Sprat from the Bornholm Basin, 1988; ICES C. M.: Copenhagen, Denmark, 1990. [Google Scholar]
- Alekseev, F.E.; Alekseeva, E.I. Batch fecundity and daily egg production of the Baltic sprat Sprattus sprattus balticus (Clupeidae) from the southeastern part of the Baltic Sea. J. Ichthyol. 2005, 45, 93–102. [Google Scholar]
- Haslob, H.; Tomkiewicz, J.; Hinrichsen, H.-H.; Kraus, G. Spatial and interannual variability in Baltic sprat batch fecundity. Fish. Res. 2011, 110, 289–297. [Google Scholar] [CrossRef]
- Döring, J.; Hauss, H.; Haslob, H. Spatial and seasonal variability in reproductive investment of Baltic sprat. Fish. Res. 2017. in revision. [Google Scholar]
- Tanasichuk, R.W.; Ware, D.M. Influence of interannual variations in winter sea temperature on fecundity and egg size in Pacific herring (Clupea harengus pallasi). Can. J. Fish. Aquat. Sci. 1987, 44, 1485–1495. [Google Scholar] [CrossRef]
- Milton, D.A.; Blaber, S.J.M.; Rawlinson, N.J.F. Fecundity and egg production of four species of short-lived clupeoid from Solomon Islands, Tropical South Pacific. ICES J. Mar. Sci. 1995, 52, 111–125. [Google Scholar] [CrossRef]
- Leonard, J.B.K.; Norieka, J.F.; Kynard, B.; McCormick, S.D. Metabolic rates in an anadromous clupeid, the American shad (Alosa sapidissima). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1999, 169, 287–295. [Google Scholar] [CrossRef]
- Hempel, G. Early Life History of Marine Fish: The Egg Stage; University of Washington Press: Seattle, WA, USA; London, UK, 1979. [Google Scholar]
- Martin, T.J. Interaction of salinity and temperature as a mechanism for spatial separation of three co-existing species of Ambassidae (Cuvier) (Teleostei) in estuaries on the south-east coast of Africa. J. Fish Biol. 1988, 33, 9–15. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Schulte, P.M.; Wood, C.M.; Schiemer, F. Niche dimensions in fishes: An integrative view. Physiol. Biochem. Zool. 2010, 83, 808–826. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.A.; Baumann, H.; Bernreuther, M.; Clemmesen, C.; Herrmann, J.-P.; Haslob, H.; Huwer, B.; Kanstinger, P.; Köster, F.W.; Petereit, C.; et al. The ecophysiology of Sprattus sprattus in the Baltic and North Seas. Prog. Oceanogr. 2012, 103, 42–57. [Google Scholar] [CrossRef]
- Panfili, J.; Durand, J.-D.; Mbow, A.; Guinand, B.; Diop, K.; Kantoussan, J.; Thior, D.; Thiaw, O.T.; Albaret, J.J.; Laë, R. Influence of salinity on life history traits of the bonga shad Ethmalosa fimbriata (Pisces, Clupeida): Comparison between the Gambia and Saloum estuaries. Mar. Ecol. Prog. Ser. 2004, 270, 241–257. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Hwang, P.P. Some insights into energy metabolism for osmoregulation in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, A.D. Nutrition and reproductive capacity in fish. Proc. Nutr. Soc. 1960, 19, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hempel, G. Egg production and egg mortality in herring. Rapports et Proces-verbaux des Réunions, ICES 1971, 160, 8–11. [Google Scholar]
- Marshall, C.T.; Frank, K.T. The effect of interannual variation in growth and condition on haddock recruitment. Can. J. Fish. Aquat. Sci. 1999, 56, 347–355. [Google Scholar] [CrossRef]
- Somarakis, S.; Schismenou, E.; Siapatis, A.; Giannoulaki, M.; Kallianiotis, A.; Machias, A. High variability in the Daily Egg Production Method parameters of an eastern Mediterranean anchovy stock: Influence of environmental factors, fish condition and population density. Fish. Res. 2012, 117–118, 12–21. [Google Scholar] [CrossRef]
- De Vlaming, V.L. The effects of food deprivation and salinity changes on reproductive function in the estuarine gobiid Ffsh, Gillichthys mirabilis. Biol. Bull. 1971, 141, 458–471. [Google Scholar] [CrossRef]
- Morgan, M.J. Integrating reproductive biology into scientific advice for fisheries management. J. Northwest Atl. Fish. Sci. 2008, 41, 37–51. [Google Scholar] [CrossRef]
- Bainbridge, V. Food of Ethmalosa dorsalis (Cuvier and Valenciennes). Nature 1957, 179, 874–875. [Google Scholar] [CrossRef]
- Bainbridge, V. The early life history of the bonga, Ethmalosa dorsalis (Cuvier and Valenciennes). ICES J. Mar. Sci. 1961, 3, 347–353. [Google Scholar] [CrossRef]
- Bainbridge, V. The food, feeding habits and distribution of the bonga Ethmalosa dorsalis (Cuvier & Valenciennes). ICES J. Mar. Sci. 1963, 28, 270–283. [Google Scholar]
- Fagade, S.O.; Olaniyan, C.I.O. The biology of the West African shad Ethmalosa fimbriata (Bowdich) in the Lagos Lagoon, Nigeria. J. Fish Biol. 1972, 4, 519–533. [Google Scholar] [CrossRef]
- Charles-Dominique, E.; Albaret, J.J. African Shads, with emphasis on the West African Shad Ethmalosa fimbriata. In Biodiversity, Status, and Conservation of the World’s Shads; Limburg, K.E., Waldman, J.R., Eds.; American Fisheries Society Symposium 35: Bethesda, MD, USA, 2003; pp. 27–48. [Google Scholar]
- Lozano-Ray, L. Etude systématique des Clupéidés et des Engraulidés de l`Espagne, du Maroc et du Sahara Espagnols. Rapports et Proces-verbaux des Réunions ICES 1950, 126, 7–20. [Google Scholar]
- Paugy, D.; Lévêque, C.; Teugels, G.G. The Fresh and Brackish Water Fishes of West Africa; Publications Scientifiques du Muséum, MRAC: Paris, France, 2003. [Google Scholar]
- Albaret, J.J.; Charles-Dominique, E. Observation d’une taille a la premiere maturation sexuelle exceptionnellement faible chez Ethmalosa fimbriata Bowdich dans une baie polluee de la lagune ebrie (Cote D’Ivorie). Doc. Sc. Cent. Rech. Océanogr. Abidjan 1982, 13, 23–31. [Google Scholar]
- Deme, M.; Diadhiou, H.D.; Thiam, D.; Ndiaye, V. Effort de Peche, Captures Specifiques et Valeurs Economiques des Debarquements de la Peche Continentale Dans le Fleuve Senegal, au Sine Saloum et en Casamance; Union Mondiale pour la Nature, Institut Sénégalais de Recherches Agricoles, Centre de Recherches Océanographique de Dakar-Thiaroye: Dakar, Senegal, 2001. [Google Scholar]
- Moses, B.S.; Udoidiong, O.M.; Okon, A.O. A statistical survey of the artisanal fisheries of south-eastern Nigeria and the influence of hydroclimatic factors on catch and resource productivity. Fish. Res. 2002, 57, 267–278. [Google Scholar] [CrossRef]
- Scheffers, W.J.; Conand, F.; Reizer, C. Étude de Ethmalosa fimbriata (Bowdich) dans la région Sénégamibienne. 1ère note: Reproduction et lieux de ponte dans le fleuve Sénégal et la région de Saint-Louis. Doc. Sc. Cent. Rech. Océanogr. Dakar-Thiaroye, 1972, 44, 22. [Google Scholar]
- Albaret, J.J.; Gerlotto, F. Biologie de l’Ethmalose (Ethmalosa fimbriata Bowdich) en Côte D’Ivoire. I.-Description de la reproduction et des premiers stades larvaires. Doc. Sci. Cent. Rech. Océanogr. 1976, 7, 113–133. [Google Scholar]
- Pagès, J.; Citeau, J. Rainfall and salinity of a sahelian estuary between 1927 and 1987. J. Hydrol. 1990, 113, 325–341. [Google Scholar] [CrossRef]
- Wolanski, E. An evaporation-driven salinity maximum zone in Australian tropical estuaries. Estuar. Coast. Shelf Sci. 1986, 22, 415–424. [Google Scholar] [CrossRef]
- Ridd, P.V.; Stieglitz, T. Dry season salinity changes in arid estuaries fringed by mangroves and saltflats. Estuar. Coast. Shelf Sci. 2002, 54, 1039–1049. [Google Scholar] [CrossRef]
- Boely, T.; Elwertowski, J. Observations préliminaires sur la pêche de Ethmalosa fimbriata (Bowdich) des eaux sénegalaises et son aspect biologique. Rapports et Proces-verbaux des Réunions ICES 1970, 1959, 182–188. [Google Scholar]
- Nche-Fambo, F.A.; Scharler, U.M.; Tirok, K. Resilience of estuarine phytoplankton and their temporal variability along salinity gradients during drought and hypersalinity. Estuar. Coast. Shelf Sci. 2015, 158, 40–52. [Google Scholar] [CrossRef]
- Döring, J.; Ekau, W. Using oocyte essential fatty acid composition to assess spawner reproductive potential under hypersaline conditions Mar. Ecol. Prog. Ser. 2017, in revision. 2017. in revision. [Google Scholar]
- Blay, J., Jr.; Eyeson, K.N. Feeding activity and food habits of the shad, Ethmalosa fimbriata (Bowdich), in the coastal waters of Cape Coast, Ghana. J. Fish Biol. 1982, 21, 403–410. [Google Scholar] [CrossRef]
- Charles-Dominique, E. Exposé synoptique des données biologiques sur l’ethmalose (Ethmalosa fimbriata S. Bowdich, 1825). Rev. Hydrobiol. Trop. 1982, 14, 373–397. [Google Scholar]
- Glebe, B.; Leggett, W. Latitudinal differences in energy allocation and use during the freshwater migrations of American shad (Alosa sapidissima) and their life history consequences. Can. J. Aquat. Sci. 1981, 38, 806–820. [Google Scholar] [CrossRef]
- Leggett, W.C.; Trump, C.L. Energetics of migration in American shad. In Animal Migration, Navigation, and Homing; Schmidt-Koenig, K., Keeton, W.T., Eds.; Springer: Berlin, Germany, 1978; pp. 370–377. [Google Scholar]
- Navodaru, I.; Waldman, J.R. Shads of Eastern Europe from the Black Sea: Review of species and fisheries. Am. Fish. Soc. Symp. 2003, 35, 69–76. [Google Scholar]
- Casini, M.; Kornilovs, G.; Cardinale, M.; Möllmann, C.; Grygiel, W.; Jonsson, P.; Raid, T.; Flinkman, J.; Feldman, V. Spatial and temporal density dependence regulates the condition of central Baltic Sea clupeids: Compelling evidence using an extensive international acoustic survey. Popul. Ecol. 2011, 53, 511–523. [Google Scholar] [CrossRef]
- Isaac-Nahum, V.J.; Cardoso, R.D.D.; Servo, G.; Rossi-Wongtschowski, C.L.D.B. Aspects of the spawning biology of the Brazilian sardine Sardinella brasiliensis (Steindachner, 1879), (Clupeidae). J. Fish Biol. 1988, 32, 383–396. [Google Scholar] [CrossRef]
- Coombs, S.H.; Smyth, T.J.; Conway, D.V.P.; Halliday, N.C.; Bernal, M.; Stratoudakis, Y.; Alvarez, P. Spawning season and temperature relationships for sardine (Sardina pilchardus) in the eastern North Atlantic. J. Mar. Biol. Assoc. U. K. 2006, 86, 1245–1252. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Antonopoulou, E. Reproductive biology of round sardinella (Sardinella aurita) in the North-Eastern Mediterranean. Sci. Mar. 2006, 70, 281–290. [Google Scholar] [CrossRef]
- Blay, J., Jr.; Eyeson, K.N. Observations on the reproductive biology of the shad, Ethmalosa fimbriata (Bowdich), in the coastal waters of Cape Coast, Ghana. J. Fish Biol. 1982, 21, 485–496. [Google Scholar] [CrossRef]
- Kusemiju, K.; Onadeko, C.A. The seasonal occurrence and bionomics of the bonga, Ethmalosa fimbriata (Bowdich) off Aiyetoro Coast, Nigeria. Fish. Res. 1990, 8, 247–251. [Google Scholar] [CrossRef]
- Jennings, S.; Beverton, R.J.H. Intraspecific variation in the life history tactics of Atlantic herring (Clupea harengus L.) stocks. ICES J. Mar. Sci. 1991, 48, 117–125. [Google Scholar] [CrossRef]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Irwin, E.R.; Bettoli, P.W. Introduced clupeids in a southern reservoir: More evidence for system-specific reproductive styles. Environ. Biol. Fishes 1995, 42, 151–159. [Google Scholar] [CrossRef]
- Blaber, S.J.M.; Milton, D.A.; Pang, J.; Wong, P.; Boon-Teck, O.; Nyigo, L.; Lubim, D. The life history of the tropical shad Tenualosa toli from Sarawak: First evidence of protandry in the Clupeiformes? Environ. Biol. Fishes 1996, 46, 225–242. [Google Scholar] [CrossRef]
- Leal, E.M.; Castro, L.R.; Gabriel, C. Variability in oocyte size and batch fecundity in anchoveta (Engraulis ringens, Jenyns 1842) from two spawning areas off the Chilean coast. Sci. Mar. 2009, 73, 59–66. [Google Scholar]
- Kamler, E. Parent–egg–progeny relationships in teleost fishes: An energetics perspective. Rev. Fish Biol. Fish. 2005, 15, 399–421. [Google Scholar] [CrossRef]
- Hay, D.R.; Brett, J.R. Maturation and fecundity of Pacific herring (Clupea harengus pallasi): An experimental study with comparisons to natural populations. Can. J. Fish. Aquat. Sci. 1988, 45, 399–406. [Google Scholar] [CrossRef]
- Pagès, J.; Gadel, F. Dissolved organic matter and UV absorption in a tropical hyperhaline estuary. Sci. Total Environ. 1990, 99, 173–204. [Google Scholar] [CrossRef]
- Jennerjahn, T.C.; Ittekkot, V. Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwissenschaften 2002, 89, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Castillo, K.D.; Lima, F.P. Comparison of in situ and satellite-derived (MODIS-Aqua/Terra) methods for assessing temperatures on coral reefs. Limnol. Oceanogr. Methods 2010, 8, 107–117. [Google Scholar] [CrossRef]
- Acha, E.M.; Macchi, G.J. Spawning of Brazilian menhaden, Brevoortia aurea, in the Río de la Plata estuary off Argentina and Uruguay. Fish. Bull. 1999, 98, 227–235. [Google Scholar]
- Lambert, Y.; Yaragina, N.A.; Kraus, G.; Marteinsdottir, G.; Wright, P.J. Using environmental and biological indices as proxies for egg and larval production of marine fish. J. Northwest Atl. Fish. Sci. 2004, 33, 115–159. [Google Scholar] [CrossRef]
- Pepin, P. Effect of temperature and size on development, mortality and survival rates of the pelagic early life history stages of marine fish. Can. J. Aquat. Sci. 1991, 48, 503–518. [Google Scholar] [CrossRef]
- Holliday, F.G.T. The effects of salinity on the eggs and larvae of teleosts. Fish Physiol. 1969, 1, 293–311. [Google Scholar]
- Motos, L. Reproductive biology and fecundity of the Bay of Biscay anchovy population (Engraulis encrasicolus L.). Sci. Mar. 1996, 60, 195–207. [Google Scholar]
- Cury, P.; Roy, C. Optimal environmental window and pelagic fish recruitment success in upwelling Areas. Can. J. Fish. Aquat. Sci. 1989, 46, 670–680. [Google Scholar] [CrossRef]
- May, R.C. Larval mortality in marine fishes and the critical period concept. In The Early Life History of Fish; Blaxter, J.H.S., Ed.; Springer: Berlin, Germany, 1974; pp. 3–19. [Google Scholar]
- Rickman, S.J.; Dulvy, N.K.; Jennings, S.; Reynolds, J.D. Recruitment variation related to fecundity in marine fishes. Can. J. Fish. Aquat. Sci. 2000, 57, 116–124. [Google Scholar] [CrossRef]
- Pecquerie, L.; Petitgas, P.; Kooijman, S.A.L.M. Modeling fish growth and reproduction in the context of the Dynamic Energy Budget theory to predict environmental impact on anchovy spawning duration. J. Sea Res. 2009, 62, 93–105. [Google Scholar] [CrossRef]
- Simier, M.; Blanc, L.; Aliaume, C.; Diouf, P.S.; Albaret, J.J. Spatial and temporal structure of fish assemblages in an “inverse estuary”, the Sine Saloum system (Senegal). Estuar. Coast. Shelf Sci. 2004, 59, 69–86. [Google Scholar] [CrossRef]
- Tiedemann, M.; Brehmer, P. Larval fish assemblages across an upwelling front: Indication for active and passive retention. Estuar. Coast. Shelf Sci. 2016, 187, 118–133. [Google Scholar] [CrossRef]
- Sloterdijk, H.; Sadio, O.; Brehmer, P.; Müller, H.; Döring, J.; Ekau, W. Composition and structure of the larval fish community related to environmental parameters in a tropical estuary impacted by climate change. Estuar. Coast. Shelf Sci. 2017, 197, 10–26. [Google Scholar] [CrossRef]
- Saos, J.L.; Pagés, J. Mesure hydrologiques dans le Sine-Saloum. In L’estuaire et la Mangrove du Sine-Saloum: Atelier Régional Unesco-COMAR Dakar (Senégal) du 28 Février au 5 Mars 1983; Atelier Régional UNESCO-COMAR: Dakar, Senegal, 1985; pp. 7–14. [Google Scholar]
- West, G. Methods of assessing ovarian development in fishes: A review. Mar. Freshw. Res. 1990, 41, 199–222. [Google Scholar] [CrossRef]
- Ter Hofstede, R.; Dickey-Collas, M.; Mantingh, I.T.; Wague, A. The link between migration, the reproductive cycle and condition of Sardinella aurita off Mauritania, north-west Africa. J. Fish Biol. 2007, 71, 1293–1302. [Google Scholar] [CrossRef]
- Lloret, J.; Shulman, G.; Love, R.M. Description of condition indicators. In Condition and Health Indicators of Exploited Marine Fishes; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–16. [Google Scholar]
- Zydlewski, J.; McCormick, S.D.; Kunkel, J.G. Late migration and seawater entry is physiologically disadvantageous for American shad juveniles. J. Fish Biol. 2003, 63, 1521–1537. [Google Scholar] [CrossRef]
- Hunter, J.R.; Lo, N.C.H.; Leong, R.J.H. Batch fecundity in multiple spawning fishes. NOAA Tech. Rep. NMFS 1985, 36, 67–77. [Google Scholar]
- Alheit, J. Use ot the Daily Egg Production Method for estimating biomass of clupeoid fishes: A review and evaluation. Bull. Mar. Sci. 1993, 53, 750–767. [Google Scholar]
- Olney, J.E.; McBride, R.S. Intraspecific variation in batch fecundity of American shad: Revisiting the paradigm of reciprocal latitudinal trends in reproductive traits. Am. Fish. Soc. Symp. 2003, 35, 185–192. [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics, 2nd ed.; Sparky House Publishing: Baltimore, MA, USA, 2009. [Google Scholar]
- Brown, M.B.; Forsythe, A.B. The small sample behavior of some statistics which test the equality of several means. Technometrics 1974, 16, 129–132. [Google Scholar] [CrossRef]
- Schmider, E.; Ziegler, M.; Danay, E.; Beyer, L.; Bühner, M. Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodol. Eur. J. Res. Methods Behav. Soc. Sci. 2010, 6, 147–151. [Google Scholar] [CrossRef]
- Huey, R.B.; Stevenson, R.D. Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Integr. Comp. Biol. 1979, 19, 357–366. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
| Sampling Site | Sampling Month | Stage I–IV | Stage V |
|---|---|---|---|
| Joal | February | ||
| March | 30 | 8 | |
| April | 24 | 3 | |
| May | 44 | 20 | |
| June | 35 | ||
| July | 42 | ||
| August | 26 | ||
| September | 34 | ||
| October | 29 | ||
| Djifer | February | 16 | 8 |
| March | 57 | 2 | |
| April | 19 | 25 | |
| May | 38 | ||
| June | 32 | 7 | |
| July | 37 | 4 | |
| August | 37 | ||
| September | 24 | ||
| October | 27 | ||
| Foundiougne | February | 52 | 2 |
| March | 20 | 15 | |
| April | 66 | 4 | |
| May | 44 | 2 | |
| June | 40 | 5 | |
| July | 26 | 23 | |
| August | 42 | 29 | |
| September | 51 | 3 | |
| October | 27 | 29 |
| Relative Batch Fecundity | Condition Index | Gonado-Somatic Index | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | |
| Intercept | 2.1680917 | 0.3079701 | <0.0001 | ||||||
| T | −5.725703 | 1.254891 | <0.0001 | −0.0053596 | 0.0014593 | <0.001 | −0.5523669 | 0.1249885 | <0.0001 |
| S | 3.378869 | 0.718607 | <0.0001 | −0.0365113 | 0.0143465 | <0.05 | 0.3900672 | 0.0715741 | <0.0001 |
| T2 | 0.118154 | 0.023322 | <0.0001 | 0.0108684 | 0.0023229 | <0.0001 | |||
| S2 | −0.035223 | 0.007655 | <0.0001 | 0.0004007 | 0.0001541 | <0.05 | −0.0041510 | 0.0007624 | <0.0001 |
| R2 | 0.90 | 0.25 | 0.97 | ||||||
| RSE | 4.1560 | 0.0458 | 0.4140 | ||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Döring, J.; Tiedemann, M.; Stäbler, M.; Sloterdijk, H.; Ekau, W. Ethmalosa fimbriata (Bowdich 1825), a Clupeid Fish That Exhibits Elevated Batch Fecundity in Hypersaline Waters. Fishes 2017, 2, 13. https://doi.org/10.3390/fishes2030013
Döring J, Tiedemann M, Stäbler M, Sloterdijk H, Ekau W. Ethmalosa fimbriata (Bowdich 1825), a Clupeid Fish That Exhibits Elevated Batch Fecundity in Hypersaline Waters. Fishes. 2017; 2(3):13. https://doi.org/10.3390/fishes2030013
Chicago/Turabian StyleDöring, Julian, Maik Tiedemann, Moritz Stäbler, Hans Sloterdijk, and Werner Ekau. 2017. "Ethmalosa fimbriata (Bowdich 1825), a Clupeid Fish That Exhibits Elevated Batch Fecundity in Hypersaline Waters" Fishes 2, no. 3: 13. https://doi.org/10.3390/fishes2030013
APA StyleDöring, J., Tiedemann, M., Stäbler, M., Sloterdijk, H., & Ekau, W. (2017). Ethmalosa fimbriata (Bowdich 1825), a Clupeid Fish That Exhibits Elevated Batch Fecundity in Hypersaline Waters. Fishes, 2(3), 13. https://doi.org/10.3390/fishes2030013







