Advanced Spatial Modeling to Inform Management of Data-Poor Juvenile and Adult Female Rays
Abstract
1. Introduction
2. Results
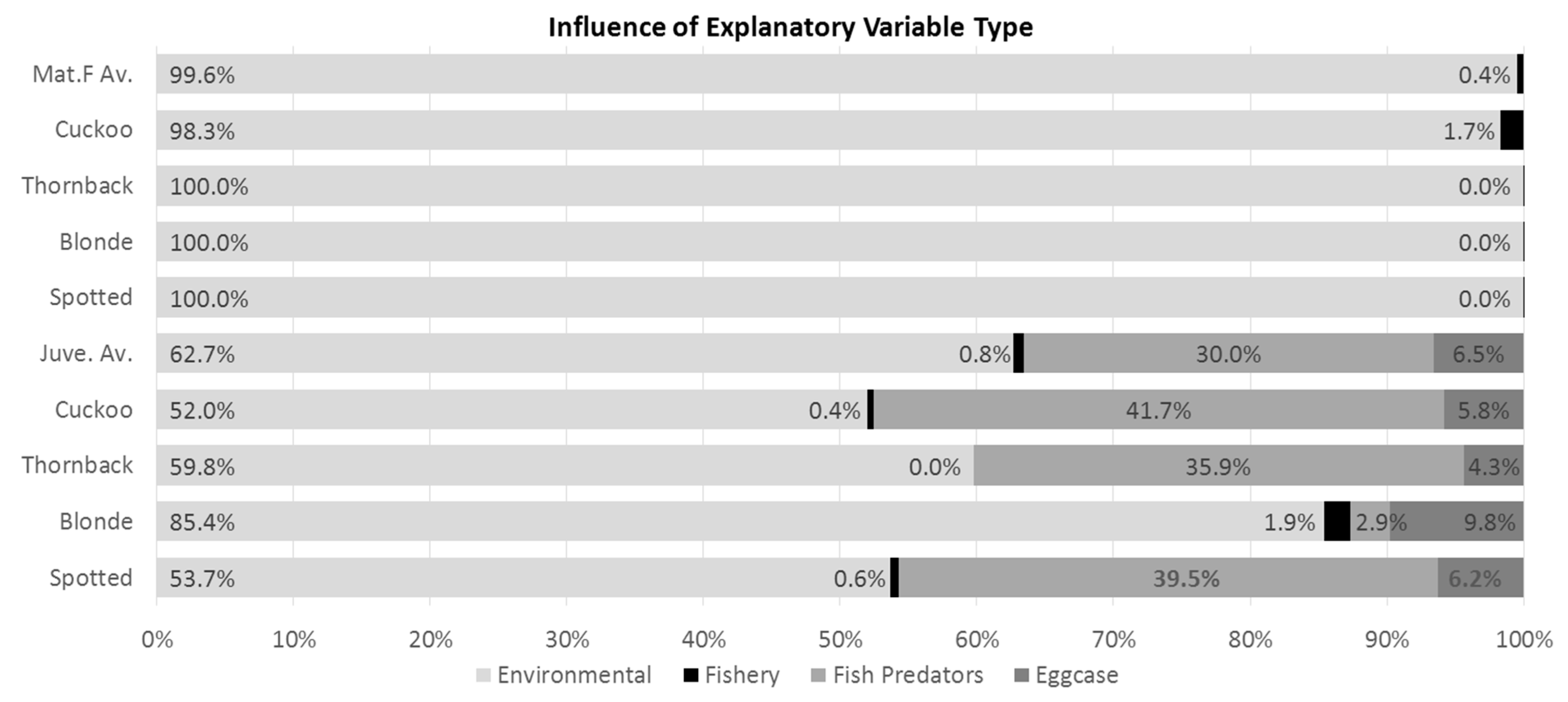
2.1. Relative Importance of Explanatory Variable Types
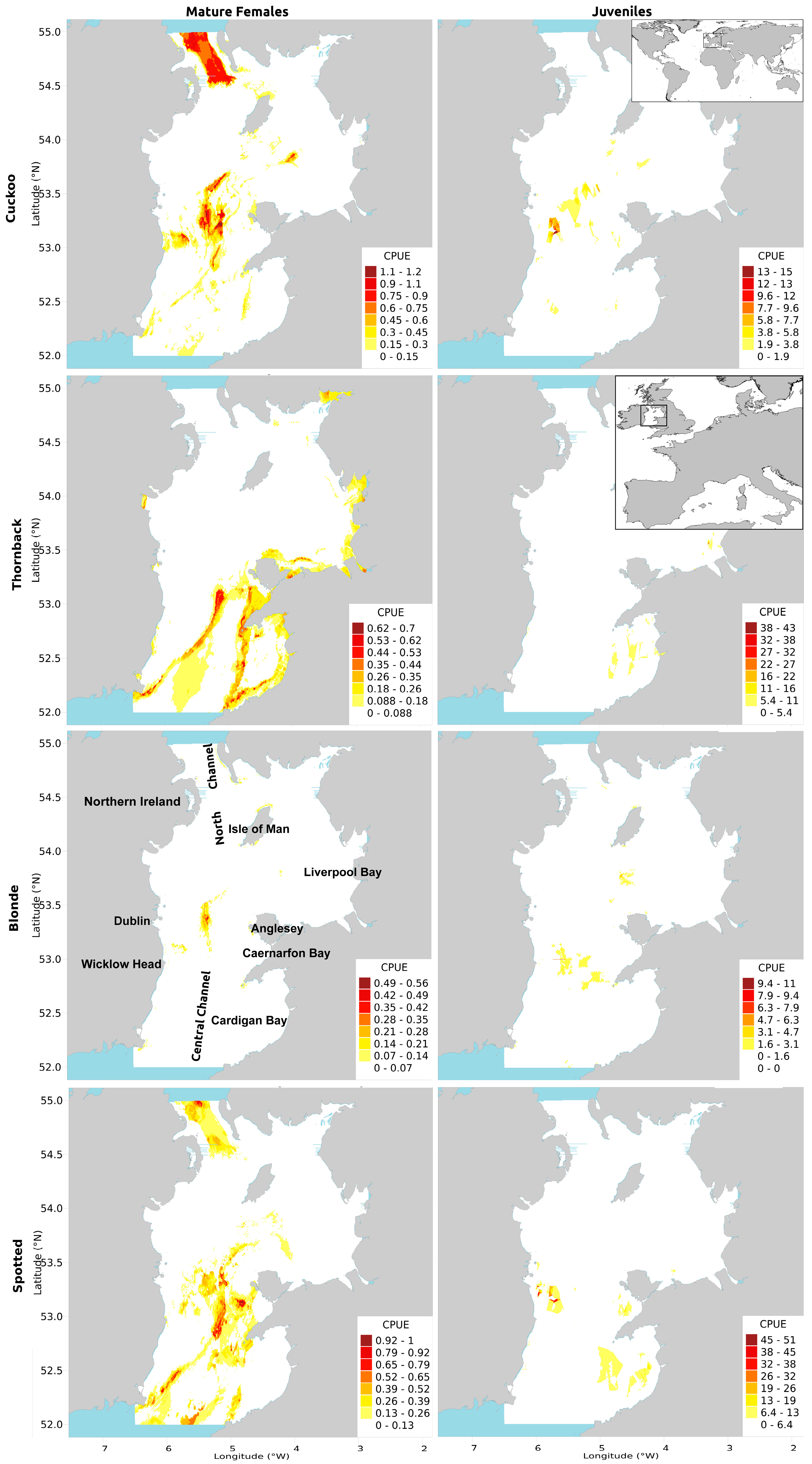
2.2. Influential Variable Relationships and Predicted CPUE Maps
2.2.1. Cuckoo Ray
2.2.2. Thornback Ray
2.2.3. Blonde Ray
2.2.4. Spotted Ray
2.2.5. Fish Predators and Eggcase Removers
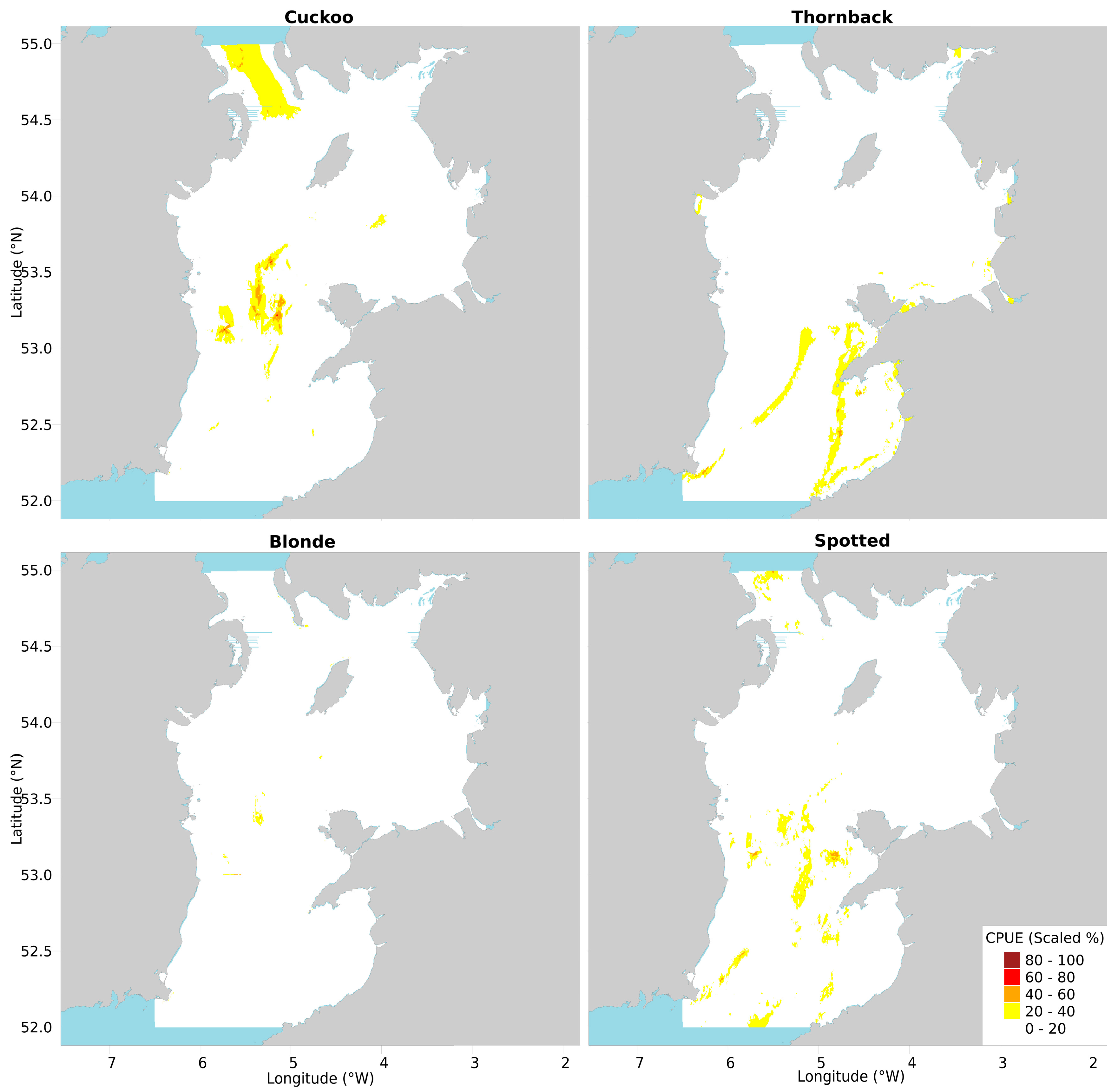
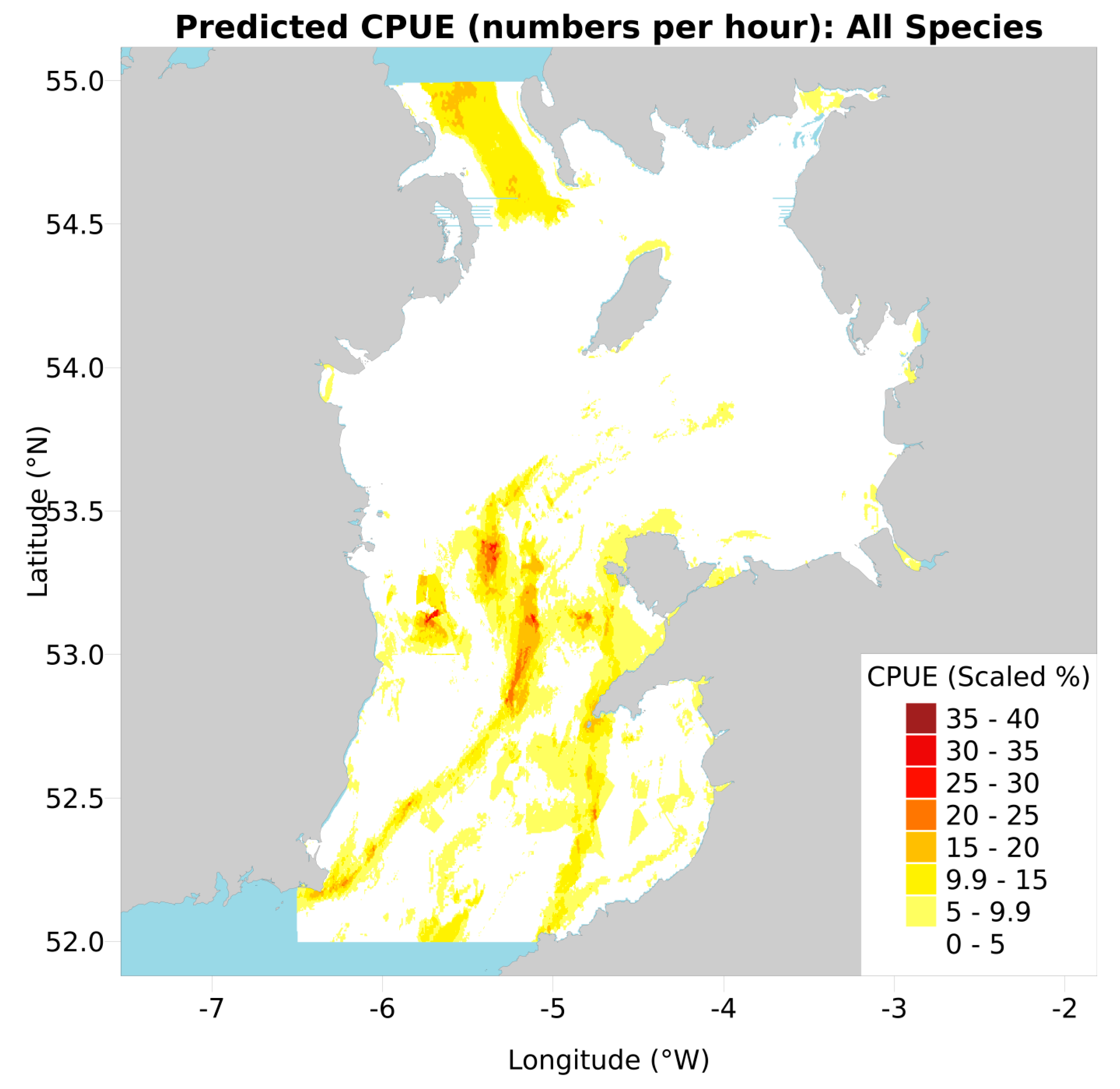
2.3. Conservation Value Maps
2.3.1. Cuckoo Ray
2.3.2. Thornback Ray
2.3.3. Blonde Ray
2.3.4. Spotted Ray
2.4. Model Performance Metrics
3. Discussion
3.1. Overview
3.2. Juvenile Rays and Teleost “Predators”
3.3. Influence of Fishing Pressure
3.4. Modelling Subsets
3.5. Amalgamated and Scaled Maps
3.6. Representativeness and Uncertainty
3.7. Spawning Grounds
3.8. Modelling, Biological and Socioeconomic Context
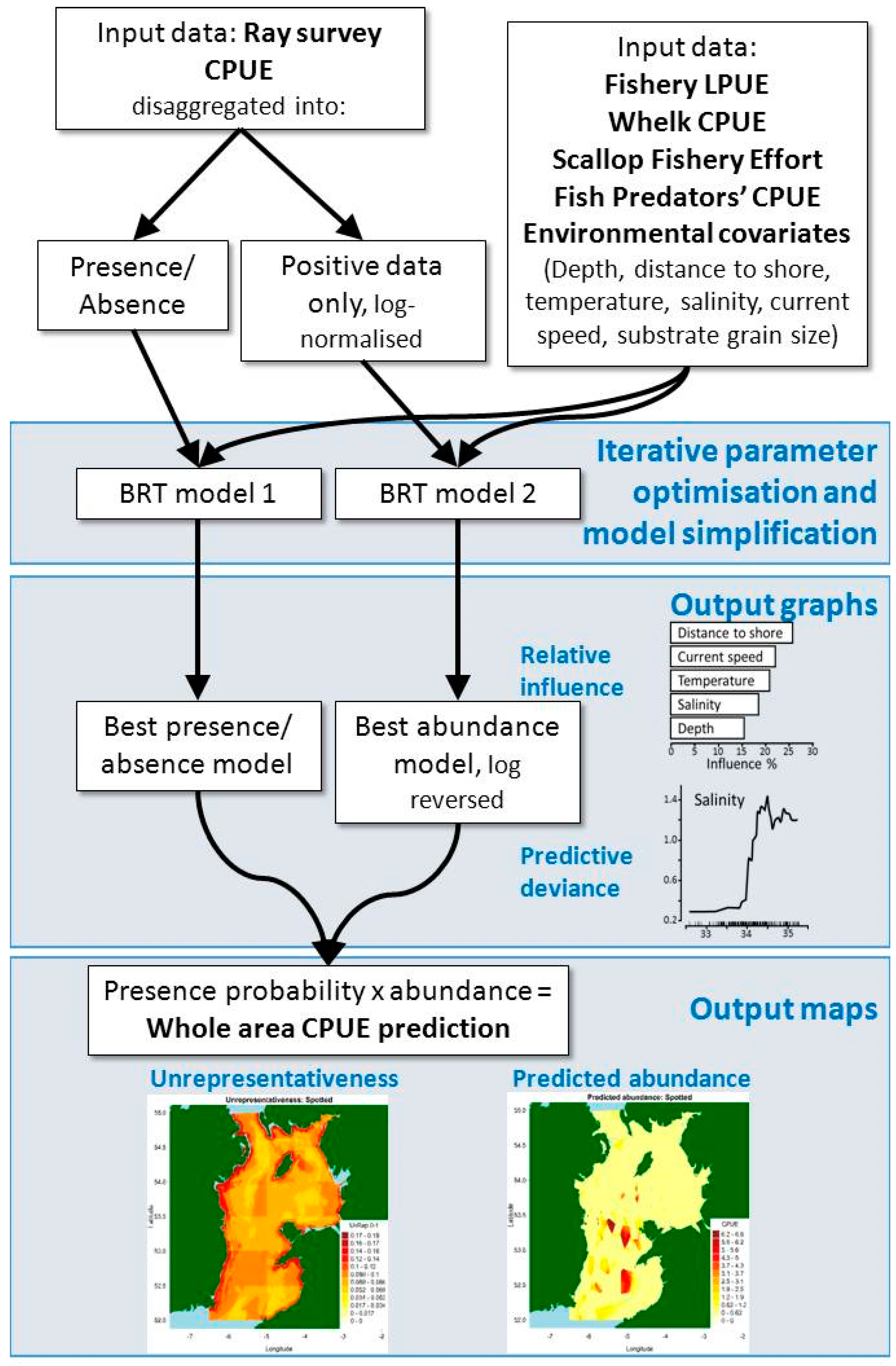
4. Materials and Methods
- Tree complexity, controlling variable interactions, of 2 or 15 for all juveniles, 2 or 6 for all mature females (whelk, scallop, and fish predators are not included in the female model). This allows us to evaluate whether all variables interacting provides a better model result than only two.
- Learning rate, controlling the contribution each tree has to the model, of 0.01 and 0.005 for all rays bar mature female blonde rays where we used 0.01 and 0.001 as this subset has fewer data. Smaller rates processing slower but usually more accurately.
4.1. Database Selection and Processing
4.1.1. Environmental Covariates
4.1.2. Fishery LPUE
4.1.3. Whelk CPUE and Scallop Fishery Effort
4.1.4. Fish Predators’ CPUE
4.1.5. Ray Survey CPUE
4.2. Preliminary Analysis
4.3. Modelling Approach
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
- dismo: Hijmans, R.L., Phillips, S., Leathwick, J. and Elith, J. 2103. dismo: Functions for species distribution modelling, that is, predicting entire geographic distributions from occurrences at a number of sites. R package version: 0.9-3. http://cran.r-project.org/package=dismo
- gam: Hastie, T. 2013. gam: Generalized Additive Models. R package version 1.09. http://CRAN.R-project.org/package=gam
- gbm: Ridgeway, G. 2013. gbm: Generalised Boosted Regression Models. R package version: 2.1. http://cran.r-project.org/package=gbm
- mapplots: Gerritsen, H. 2014. mapplots: Data Visualisation on Maps. R package version 1.5. http://CRAN.R-project.org/package=mapplots
- mgcv: Wood, S.N. 2011. Mgcv: Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society (B) 73(1):3-36. http://CRAN.R-project.org/package=mgcv
- vegan: Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., Minchin, P.R., O’Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H.H. and Wagner, H. 2013. vegan: Community Ecology Package. R package version 2.0-10. http://CRAN.R-project.org/package=vegan
- R package functions gbm.auto, including gbm.map, gbm.rsb, gbm.cons, gbm.valuemap and gbm.bfcheck, written by SD 2012-2016 and available at: https://github.com/SimonDedman/gbm.auto
References
- Rogers, S.; Ellis, J.R. Changes in the demersal fish assemblages of British coastal waters during the 20th century. ICES J. Mar. Sci. 2000, 57, 866–881. [Google Scholar] [CrossRef]
- Ellis, J.R.; Clarke, M.W.; Cortés, E.; Heessen, H.J.; Apostolaki, P.; Carlson, J.K.; Kulla, D.W. Management of elasmobranch fisheries in the North Atlantic. Adv. Fish. Sci. 2008, 50, 184–228. [Google Scholar]
- Walker, P.; Heessen, H. Long-term changes in ray populations in the North Sea. ICES J. Mar. Sci. 1996, 53, 1085–1093. [Google Scholar] [CrossRef]
- Baum, J.K.; Myers, R.A.; Kehler, D.G.; Worm, B.; Harley, S.J.; Doherty, P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science 2003, 299, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.R.; Dulvy, N.K.; Jennings, S.; Parker-Humphreys, M.; Rogers, S.I. Assessing the status of demersal elasmobranchs in UK waters: A review. J. Mar. Biol. Assoc. UK 2005, 85, 1025–1048. [Google Scholar] [CrossRef]
- Worm, B.; Davis, B.; Kettemer, L.; Ward-Paige, C.A.; Chapman, D.; Heithaus, M.R.; Kessel, S.T.; Gruber, S.H. Global catches, exploitation rates, and rebuilding options for sharks. Mar. Policy 2013, 40, 194–204. [Google Scholar] [CrossRef]
- Brander, K. Disappearance of common skate Raia batis from Irish Sea. Nature 1981, 290, 48–49. [Google Scholar] [CrossRef]
- Walker, P.; Hislop, J. Sensitive skates or resilient rays? Spatial and temporal shifts in ray species composition in the central and north-western North Sea between 1930 and the present day. ICES J. Mar. Sci. 1998, 55, 392–402. [Google Scholar] [CrossRef]
- Marine Institute. The Stock Book 2014; Marine Institute: Galway, Ireland, 2014. [Google Scholar]
- International Council for the Exploration of the Sea. Manual for the international bottom trawl surveys in the western and southern areas. In Proceedings of the International Bottom Trawl Survey Working Group, Lisbon, Portugal, 22–26 March 2010. [Google Scholar]
- Dedman, S.; Officer, R.; Brophy, D.; Clarke, M.W.; Reid, D.G. Modelling abundance hotspots for data-poor Irish sea rays. Ecol. Model. 2015, 312, 77–90. [Google Scholar] [CrossRef]
- European Union. Council Regulation (EU) No 2016/72 of 22 January 2016 Fixing for 2016 the Fishing Opportunities for Certain Fish Stocks and Groups of Fish Stocks, Applicable in Union Waters and, to Union Vessels, in Certain Non-Union waters, and Amending Regulation (EU) 2015/104. Off. J. Eur. Union 2016, 22, 1–165. [Google Scholar]
- North Western Waters Advisory Council. Shark Ray Skate Focus Group Report; North Western Waters Advisory Council: Dublin, Ireland, 2012. [Google Scholar]
- European Commission. No. 1380/2013 of the European Parliament and of the Council of 11 December 2013 on the Common Fisheries Policy, amending Council Regulations (EC) No 1954/2003 and (EC) No 1224/2009 and repealing Council Regulations (EC) No 2371/2002 and (EC) No 639/2004 and Council Decision 2004/585/EC. Off. J. Eur. Union 2013, 354, 22–61. [Google Scholar]
- International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes (ICES WGEF). Report of the Working Group on Elasmobranch Fishes (WGEF); International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes: Lisbon, Portugal, 2012. [Google Scholar]
- International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes (ICES WGEF). Report of the Working Group on Elasmobranch Fishes (WGEF); International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes: Lisbon, Portugal, 2015. [Google Scholar]
- International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes (ICES WGEF). ICES Advice: Rays and skates in Subarea VI and Divisions VIIa–c, e–j (Celtic Sea and west of Scotland); International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes: Lisbon, Portugal, 2012. [Google Scholar]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.R.; Silva, J.F.; McCully, S.R.; Evans, M.; Catchpole, T. UK Fisheries for Skates (Rajidae): History and Development of the Fishery, Recent Management Actions and Survivorship of Discards; International Council for the Exploration of the Sea: Lisbon, Portugal, 2010. [Google Scholar]
- Abeare, S.M. Comparisons of Boosted Regression Tree, GLM and GAM Performance in the Standardization of Yellowfin Tuna Catch-Rate Data from the Gulf of Mexico Longline Fishery. Ph.D. Thesis, University of Pretoria, Gauteng, South Africa, 2009. [Google Scholar]
- Lo, N.C.; Jacobson, L.D.; Squire, J.L. Indices of relative abundance from fish spotter data based on delta-lognormal models. Can. J. Fish. Aquat. Sci. 1992, 49, 2515–2526. [Google Scholar] [CrossRef]
- De Raedemaecker, F.; Brophy, D.; O’Connor, I.; Comerford, S. Habitat characteristics promoting high density and condition of juvenile flatfish at nursery grounds on the west coast of Ireland. J. Sea Res. 2012, 73, 7–17. [Google Scholar] [CrossRef]
- Ball, I.R.; Possingham, H.P. MARXAN—A Reserve System Selection Tool. 2013. Available online: http://www.ecology.uq.edu.au/marxan.htm (accessed on 13 June 2013).
- Vincent, M.A.; Atkins, S.M.; Lumb, C.M.; Golding, N.; Lieberknecht, L.M.; Webster, M. Marine Nature Conservation and Sustainable Development—The Irish Sea Pilot; Joint Nature Conservation Committee: Peterborough, UK, 2004. [Google Scholar]
- Loos, S.A. Exploration of MARXAN for Utility in Marine Protected Area Zoning. Master’s Thesis, University of Victoria, Victoria, BC, Canada, 2006. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, Canada, July 2004; pp. 655–662. [Google Scholar]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Johnston, G.; Tetard, A.; Santos, A.R.; Kelly, E.; Clarke, M.W. Spawning and Nursery Areas of Selected Rays and Skate Species in the Celtic Seas; Marine Institute: Galway, Ireland, 2014. [Google Scholar]
- Hijmans, R.J.; Elith, J. Species Distribution Modeling with R, R package version 08-11; 2013. [Google Scholar]
- Froeschke, J.; Drymon, M. Atlantic Sharpnose Shark: Standardized Index of Relative Abundance Using Boosted Regression Trees and Generalized Linear Models; SEDAR34-WP-12; SEDAR: North Charleston, SC, USA, 2013; 31p. [Google Scholar]
- Lane, J.Q.; Raimondi, P.T.; Kudela, R.M. Development of a logistic regression model for the prediction of toxigenic Pseudo-nitzschia blooms in Monterey Bay, California. Mar. Ecol. Prog. Ser. 2009, 383, 37–51. [Google Scholar] [CrossRef]
- Bergmann, M.; Hinz, H.; Blyth, R.; Kaiser, M.; Rogers, S.; Amrstrong, M. Using knowledge from fishers and fisheries scientists to identify possible groundfish “Essential Fish Habitats”. Fish. Res. 2004, 66, 373–379. [Google Scholar] [CrossRef]
- Frank, K.T.; Petrie, B.; Shackell, N.L. The ups and downs of trophic control in continental shelf ecosystems. Trends Ecol. Evol. 2007, 22, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Pinnegar, J.K. DAPSTOM—An Integrated Database & Portal for Fish Stomach Records; Centre for Environment, Fisheries & Aquaculture Science: Lowestoft, UK, 2014. [Google Scholar]
- Shephard, S.; Gerritsen, H.; Kaiser, M.J.; Reid, D.G. Spatial heterogeneity in fishing creates de facto refugia for endangered celtic sea elasmobranchs. PLoS ONE 2012, 7, e49307. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.I.; Maxwell, D.; Rijnsdorp, A.D.; Damm, U.; Vanhee, W. Fishing effects in northeast Atlantic shelf seas: Patterns in fishing effort, diversity and community structure. IV. Can comparisons of species diversity be used to assess human impacts on demersal fish faunas? Fish. Res. 1999, 40, 135–152. [Google Scholar] [CrossRef]
- Kaiser, M.; Bergmann, M.; Hinz, H.; Galanidi, M.; Shucksmith, R.; Rees, E.I.S.; Darbyshire, T.; Ramsay, K. Demersal fish and epifauna associated with sandbank habitats. Estuar. Coast. Shelf Sci. 2004, 60, 445–456. [Google Scholar] [CrossRef]
- Shelmerdine, R.L.; Stone, D.; Leslie, B.; Robinson, M. Implications of defining fisheries closed areas based on predicted habitats in Shetland: A proactive and precautionary approach. Mar. Policy 2013, 43, 184–199. [Google Scholar] [CrossRef]
- Murphy, H.M.; Jenkins, G.P. Observational methods used in marine spatial monitoring of fishes and associated habitats: A review. Mar. Freshwater Res. 2010, 61, 236–252. [Google Scholar] [CrossRef]
- Brown, K.; Adger, W.N.; Tompkins, E.; Bacon, P.; Shim, D.; Young, K. Trade-off analysis for marine protected area management. Ecol. Econ. 2001, 37, 417–434. [Google Scholar] [CrossRef]
- International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes (ICES WGEF). Report of the Working Group on Elasmobranch Fishes (WGEF); International Council for the Exploration of the Sea Working Group on Elasmobranch Fishes: Lisbon, Portugal, 2014. [Google Scholar]
- Heupel, M.R.; Carlson, J.K.; Simpfendorfer, C.A. Shark nursery areas: Concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 2007, 337, 287–297. [Google Scholar] [CrossRef]
- Queirós, A.M.; Huebert, K.B.; Keyl, F.; Fernandes, J.A.; Stolte, W.; Maar, M.; Kay, S.; Jones, M.C.; Hamon, K.G.; Hendriksen, G.; et al. Solutions for ecosystem-level protection of ocean systems under climate change. Glob. Chang. Biol. 2016, 22, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Erzini, K.; Serra-Pereira, B.; Figueiredo, I. Reproductive biology of cuckoo ray Leucoraja naevus. J. Fish Biol. 2012, 81, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.S. Rays and Skates (Raiæ) No. 1.—Egg-Capsules and Young. J. Mar. Biol. Assoc. 1922, 12, 578–643. [Google Scholar] [CrossRef]
- Holden, M. The fecundity of Raja clavata in British waters. ICES J. Mar. Sci. 1975, 36, 110–118. [Google Scholar] [CrossRef]
- Steven, G. Migrations and growth of the thornback ray. J. Mar. Biol. Assoc. 1936, 20, 605–614. [Google Scholar] [CrossRef]
- Ryland, J.; Ajayi, T. Growth and population dynamics of three Raja species (Batoidei) in Carmarthen Bay, British Isles. ICES J. Mar. Sci. 1984, 41, 111–120. [Google Scholar] [CrossRef]
- Walker, P.; Ellis, J.R. Ecology of rays of the north-eastern Atlantic. In Biology of Skates, Proceedings of the Biology of Skates Symposium (New Orleans, 1996); Princeton Press: New Orleans, LA, USA, 1998; pp. 7–29. [Google Scholar]
- Knudby, A.; LeDrew, E.; Brenning, A. Predictive mapping of reef fish species richness, diversity and biomass in Zanzibar using IKONOS imagery and machine-learning techniques. Remote Sens. Environ. 2010, 114, 1230–1241. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Agardy, T.; Di Sciara, G.N.; Christie, P. Mind the gap: Addressing the shortcomings of marine protected areas through large scale marine spatial planning. Mar. Policy 2011, 35, 226–232. [Google Scholar] [CrossRef]
- Buxton, C.; Barrett, N.; Haddon, M.; Gardner, C.; Edgar, G. Evaluating the Effectiveness of Marine Protected Areas as a Fisheries Management Tool; Tasmanian Aquaculture and Fisheries Institute: Hobart, Tasmania, 2006. [Google Scholar]
- Rhodes, J.R.; Callaghan, J.G.; McAlpine, C.A.; de Jong, C.; Bowen, M.E.; Mitchell, D.; Lunney, D.; Possingham, H.P. Regional variation in habitat-occupancy thresholds: A warning for conservation planning. J. Appl. Ecol. 2008, 45, 549–557. [Google Scholar] [CrossRef]
- Dedman, S.; Officer, R.; Brophy, D.; Clarke, M.W.; Reid, D.G. Towards a flexible Decision Support Tool for MSY-based Marine Protected Area design for skates and rays. ICES J. Mar. Sci. 2017, 74, 576–587. [Google Scholar] [CrossRef]
- Klein, C.J.; Tulloch, V.J.; Halpern, B.S.; Selkoe, K.A.; Watts, M.E.; Steinback, C.; Scholz, A.; Possingham, H.P. Tradeoffs in marine reserve design: Habitat condition, representation, and socioeconomic costs. Conserv. Lett. 2013, 6, 324–332. [Google Scholar] [CrossRef]
- Penn, J.W.; Fletcher, W.J. The Efficacy of Sanctuary Areas for the Management of Fish Stocks and Biodiversity in WA Waters; Government of Western Australia Department of Fisheries: North Beach, Western Australia, Australia, 2010.
- Little, A.S.; Needle, C.L.; Hilborn, R.; Holland, D.S.; Marshall, C.T. Real-time spatial management approaches to reduce bycatch and discards: Experiences from Europe and the United States. Fish Fish. 2014. [Google Scholar] [CrossRef]
- Dedman, S.; Officer, R.; Brophy, D.; Clarke, M.W.; Reid, D.G. Gbm.auto: Automated delta log-normal boosted regression tree spatial modelling and MPA generation in R. PLoS ONE 2017. (In Press)
- Parisien, M.-A.; Moritz, M. Environmental controls on the distribution of wildfire at multiple spatial scales. Ecol. Monogr. 2009, 79, 127–154. [Google Scholar] [CrossRef]
- Duan, N. Smearing estimate: A nonparametric retransformation method. J. Am. Stat. Assoc. 1983, 78, 605–610. [Google Scholar] [CrossRef]
- Connor, D.W.; Gilliland, P.M.; Golding, N.; Robinson, P.; Tod, D.; Verling, E. UKSeaMap: The Mapping of Seabed and Water Column Features of UK Seas; JNCC, Ed.; Joint Nature Conservation Committee: Peterborough, UK, 2006. [Google Scholar]
- Boelens, R.; Maloney, D.; Parsons, A.; Walsh, A. Ireland’s Marine and Coastal Areas and Adjacent Seas: An Environmental Assessment; Boelens, R.G.V., Maloney, D.M., Parsons, A.P., Walsh, A.R., Eds.; Marine Institute: Dublin, Ireland, 1999. [Google Scholar]
- EMODnet. EMODnet Biological Data Products. 2014. Available online: http://bio.emodnet.eu (accessed on 14 November 2012).
- British Geological Survey. Digital Geological Map of Great Britain’s Sea Bed Sediments 1:250,000 Scale (DiGSBS250K) Data [CD-ROM], 3rd ed.; British Geological Survey: Nottingham, UK, 2011. [Google Scholar]
- ICES. ICES Database of Trawl Surveys 1990–2014. 2015. Available online: http://datras.ices.dk (accessed on 13 February 2015).
- Martin, C.; Vaz, S.; Ellis, J.R.; Lauria, V.; Coppin, F.; Carpentier, A. Modelled distributions of ten demersal elasmobranchs of the eastern English Channel in relation to the environment. J. Exp. Mar. Biol. Ecol. 2012, 418, 91–103. [Google Scholar] [CrossRef]
- Ellis, J.R.; Cruz-Martinez, A.; Rackham, B.; Rogers, S. The distribution of chondrichthyan fishes around the British Isles and implications for conservation. J. Northwest Atl. Fish. Sci. 2005, 35, 195–213. [Google Scholar] [CrossRef]
- Potts, J.M.; Elith, J. Comparing species abundance models. Ecol. Model. 2006, 199, 153–163. [Google Scholar] [CrossRef]
- Minchin, D. Biological observations on young scallops, Pecten maximus. J. Mar. Biol. Assoc. UK 1992, 72, 807–819. [Google Scholar] [CrossRef]
- Paul, J.D. Natural settlement and early growth of spat of the queen scallop Chlamys opercularis (L.), with reference to the formation of the first growth ring. J. Molluscan Stud. 1981, 47, 53–58. [Google Scholar] [CrossRef]
- Eggleston, D. Spat of the scallop (Pecten maximus (L.)) from off Port Erin, Isle of Man. Rep. Mar. Biol. Stn. Port Erin 1962, 74, 29–32. [Google Scholar]
- North Pacific Fishery Management Council (NPFMC) and NMFS. Final Environmental Assessment for Amendment 104 to the Fishery Management Plan for Groundfish of the Bering Sea and Aleutian Islands Management Area: Habitat Areas of Particular Concern (HAPC) Areas of Skate Egg Concentration; North Pacific Fishery Management Council and NMFS: Anchorage, AK, USA, 2014. [Google Scholar]
- Hitz, C.R. Observations on egg cases of the big skate (Raja binoculata Girard) found in Oregon coastal waters. J. Fish. Board Can. 1964, 21, 851–854. [Google Scholar] [CrossRef]
- Cox, D.L.; Koob, T.J. Predation on elasmobranch eggs. Environ. Biol. Fish. 1993, 38, 117–125. [Google Scholar] [CrossRef]
- Cox, D.L.; Walker, P.; Koob, T.J. Predation on eggs of the thorny skate. Trans. Am. Fish. Soc. 1999, 128, 380–384. [Google Scholar] [CrossRef]
- Kabat, A.R. Predatory ecology of naticid gastropods with a review of shell boring predation. Malacol. Int. J. Malacol. 1990, 32, 155–193. [Google Scholar]
- Nakazawa, T.; Ushio, M.; Kondoh, M. Scale dependence of predator-prey mass ratio: Determinants and applications. In Advances in Ecological Research; Belgrano, A., Reiss, J., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; Volume 45, pp. 269–302. [Google Scholar]
- Gallagher, M.J. The Fisheries Biology of Commercial Ray Species from Two Geographically Distinct Regions. Ph.D. Thesis, University of Dublin, Dublin, Ireland, 2000. [Google Scholar]
- Coull, K.; Jermyn, A.; Newton, A.; Henderson, G.; Hall, W. Length/Weight Relationships for 88 Species of Fish Encountered in the North East Atlantic; Department of Agriculture and Fisheries for Scotland Marine Laboratory: Aberdeen, UK, 1989. [Google Scholar]
- Compagno, L.J.V. Alternative Life-History Styles of Cartilaginous Fishes in Time and Space. Alternative Life-History Styles of Fishes; Springer: Dordrecht, The Netherlands, 1990; Volume 28, pp. 33–75. [Google Scholar]
- McCully, S.R.; Scott, F.; Ellis, J.R. Lengths at maturity and conversion factors for skates (Rajidae) around the British Isles, with an analysis of data in the literature. ICES J. Mar. Sci. 2012, 69, 1812–1822. [Google Scholar] [CrossRef]
- Quantum GIS Development Team. Quantum GIS Geographic Information System, v2.14.5. 2014. Available online: http://qgis.osgeo.org (accessed on 1 August 2017).
- Folk, R.L. The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J. Geol. 1954, 62, 344–359. [Google Scholar] [CrossRef]
- SearchMESH. Median Grain Size Chart. 2014. Available online: http://www.searchmesh.net/images/gmhm2-38_table-sediment_classifications.jpg (accessed on 13 June 2014).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, v3.4.1. 2013. Available online: www.R-project.org (accessed on 1 August 2017).
- Miller, J.A. Species distribution models: Spatial autocorrelation and non-stationarity. Prog. Phys. Geogr. 2012, 36, 681–692. [Google Scholar] [CrossRef]
- Redfern, J.V.; Ferguson, M.C.; Becker, E.A.; Hyrenbach, K.D.; Good, C.; Barlow, J.; Kaschner, K.; Baumgartner, M.F.; Forney, K.A.; Balance, L.T.; et al. Techniques for cetacean-habitat modeling. Mar. Ecol. Prog. Ser. 2006, 310, 271–295. [Google Scholar] [CrossRef]
- Dormann, F.C.; McPherson, M.J.; Araújo, B.M.; Bivand, R.; Bolliger, J.; Gudrun, C.; Richard, G.D.; Alexandre, H.; Walter, J.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
| Juvenile Ray Species | |||||
|---|---|---|---|---|---|
| Cuckoo | Thornback | Blonde | Spotted | ||
| Predator/Eggcase remover | Cod |  | Negligible influence | Negligible influence | Negligible influence |
| Whiting | 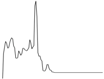 | 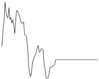 | |||
| Plaice | Negligible influence | 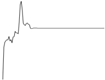 |  | ||
| Scallop |  | Negligible influence |  | Negligible influence | |
| Haddock |  | Negligible influence | |||
| Whelk | Negligible influence |  | |||
| Blonde ray |  | ||||
| Common Skate | Negligible influence | ||||
| Subset | Species | Training AUC | CV AUC | CV AUC se | TAUC − CVAUC = O |
|---|---|---|---|---|---|
| All | Cuckoo | 0.936 | 0.882 | 0.007 | 0.054 |
| Thornback | 0.880 | 0.832 | 0.010 | 0.048 | |
| Blonde | 0.944 | 0.877 | 0.010 | 0.067 | |
| Spotted | 0.923 | 0.861 | 0.008 | 0.062 | |
| Mature Female | Cuckoo | 0.943 | 0.828 | 0.024 | 0.115 |
| Thornback | 0.918 | 0.765 | 0.027 | 0.153 | |
| Blonde | 0.998 | 0.915 | 0.041 | 0.083 | |
| Spotted | 0.960 | 0.876 | 0.015 | 0.084 | |
| Juvenile | Cuckoo | 0.991 | 0.949 | 0.008 | 0.042 |
| Thornback | 0.981 | 0.936 | 0.006 | 0.045 | |
| Blonde | 0.965 | 0.882 | 0.011 | 0.083 | |
| Spotted | 0.989 | 0.952 | 0.006 | 0.037 | |
| All | Average | 0.921 | 0.863 | 0.009 | 0.058 |
| Mature Female | 0.955 | 0.846 | 0.027 | 0.109 | |
| Juvenile | 0.981 | 0.930 | 0.008 | 0.052 |
| Environmental Dataset | Spatial Resolution | Source |
|---|---|---|
| Depth | 275 × 455 m grids | EMODnet (European Marine Observation and Data Network) [65] |
| Average Monthly sea bottom temperatures 2010–2012 (°C), | 1185 × 1680 m grids | Marine Institute, 2014 (http://www.marine.ie/Home/site-area/data-services/data-services) |
| Average Monthly sea bottom salinities 2010–2012 (ppm), | ||
| Maximum monthly 2 dimensional velocity (m s−1) | ||
| Substrate (grain size in mm) | ≥250 m2 grids | British Geological Survey, 2011 [66] |
| Distance to shore (m) | 275 × 455 m grids | via European coastline layer (freely available) |
| Fishing & Predation Dataset | Spatial Resolution | Source |
| Surveyed ray CPUE (numbers per hour), 1990–2014 | Point data (n = 1447) | ICES DATRAS [67] |
| Surveyed fish predator CPUE (numbers per hour), 1990–2014 | Point data | ICES DATRAS [67] |
| Standardized average annual ray LPUE from demersal trawls (Kg−Hr), 2006–2012 (all rays combined) | 0.02° lat x 0.03° lon grids | Marine Institute, 2014 |
| Average annual whelk LPUE (Kg−KwH), 2009–2013 | 0.5° lat x 1° lon ICES rectangles | Marine Management Organisation, 2015 |
| Average annual scallop dredging effort (KwH), 2006–2013/2014 | 0.5° lat x 1° lon ICES rectangles | Marine Management Organisation, and Marine Institute, 2015 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedman, S.; Officer, R.; Brophy, D.; Clarke, M.; Reid, D.G. Advanced Spatial Modeling to Inform Management of Data-Poor Juvenile and Adult Female Rays. Fishes 2017, 2, 12. https://doi.org/10.3390/fishes2030012
Dedman S, Officer R, Brophy D, Clarke M, Reid DG. Advanced Spatial Modeling to Inform Management of Data-Poor Juvenile and Adult Female Rays. Fishes. 2017; 2(3):12. https://doi.org/10.3390/fishes2030012
Chicago/Turabian StyleDedman, Simon, Rick Officer, Deirdre Brophy, Maurice Clarke, and David G. Reid. 2017. "Advanced Spatial Modeling to Inform Management of Data-Poor Juvenile and Adult Female Rays" Fishes 2, no. 3: 12. https://doi.org/10.3390/fishes2030012
APA StyleDedman, S., Officer, R., Brophy, D., Clarke, M., & Reid, D. G. (2017). Advanced Spatial Modeling to Inform Management of Data-Poor Juvenile and Adult Female Rays. Fishes, 2(3), 12. https://doi.org/10.3390/fishes2030012






