Hawthorn Polysaccharide Enhances Growth, Immunity, and Intestinal Health in Crucian Carp (Carassius auratus) Challenged with Aeromonas hydrophila
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
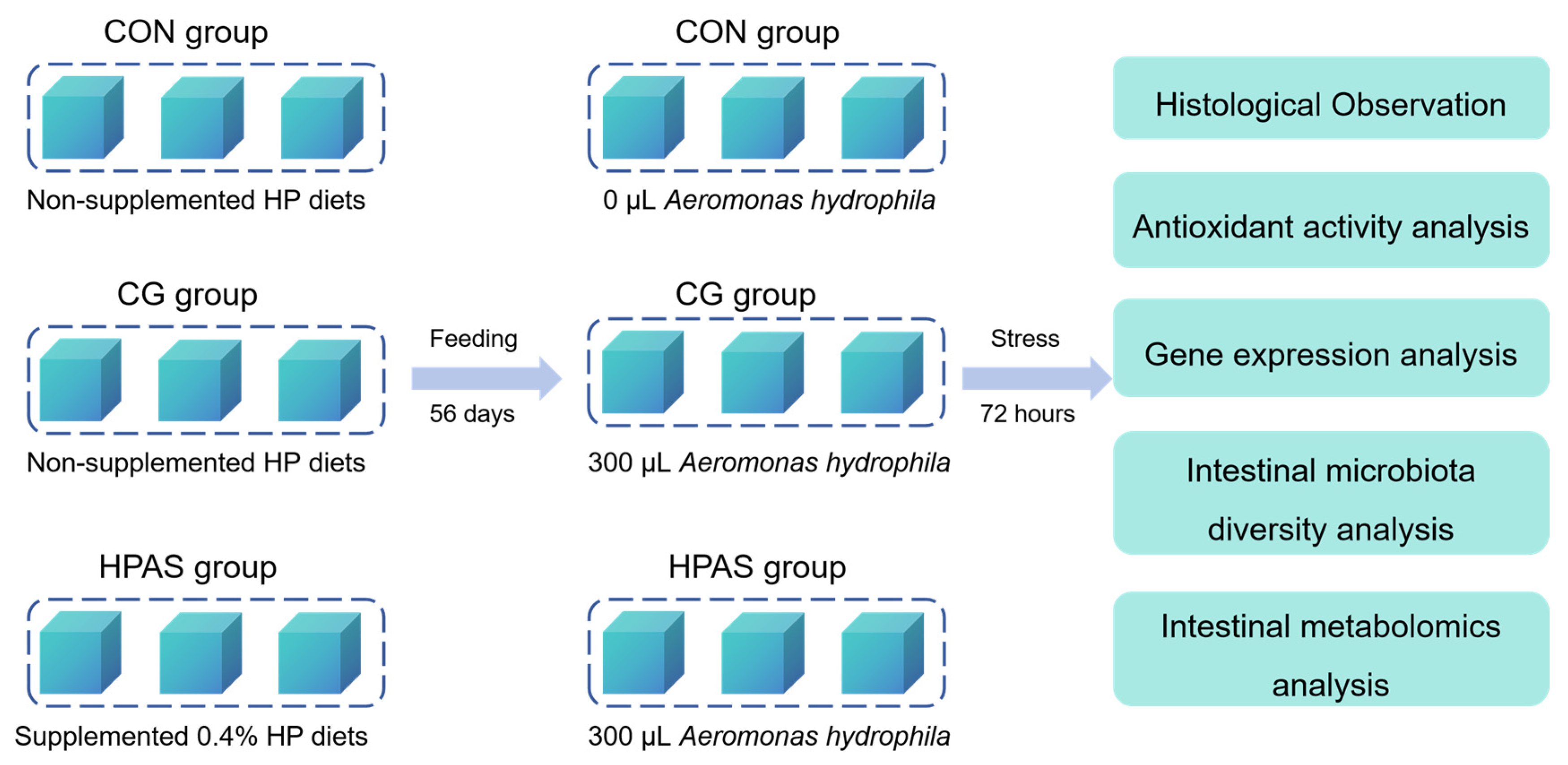
2.2. Experimental Design
2.3. Growth Performance
2.4. Challenge Test
2.5. Sample Collection
2.6. Histological Observation
2.7. Analysis of Liver Antioxidant Activity
2.8. Analysis of Gene Expression
2.9. Analysis of Intestinal Microbiota Diversity
2.10. Analysis of Intestinal Metabolomics
2.11. Statistical Analysis
3. Results
3.1. Growth Performance
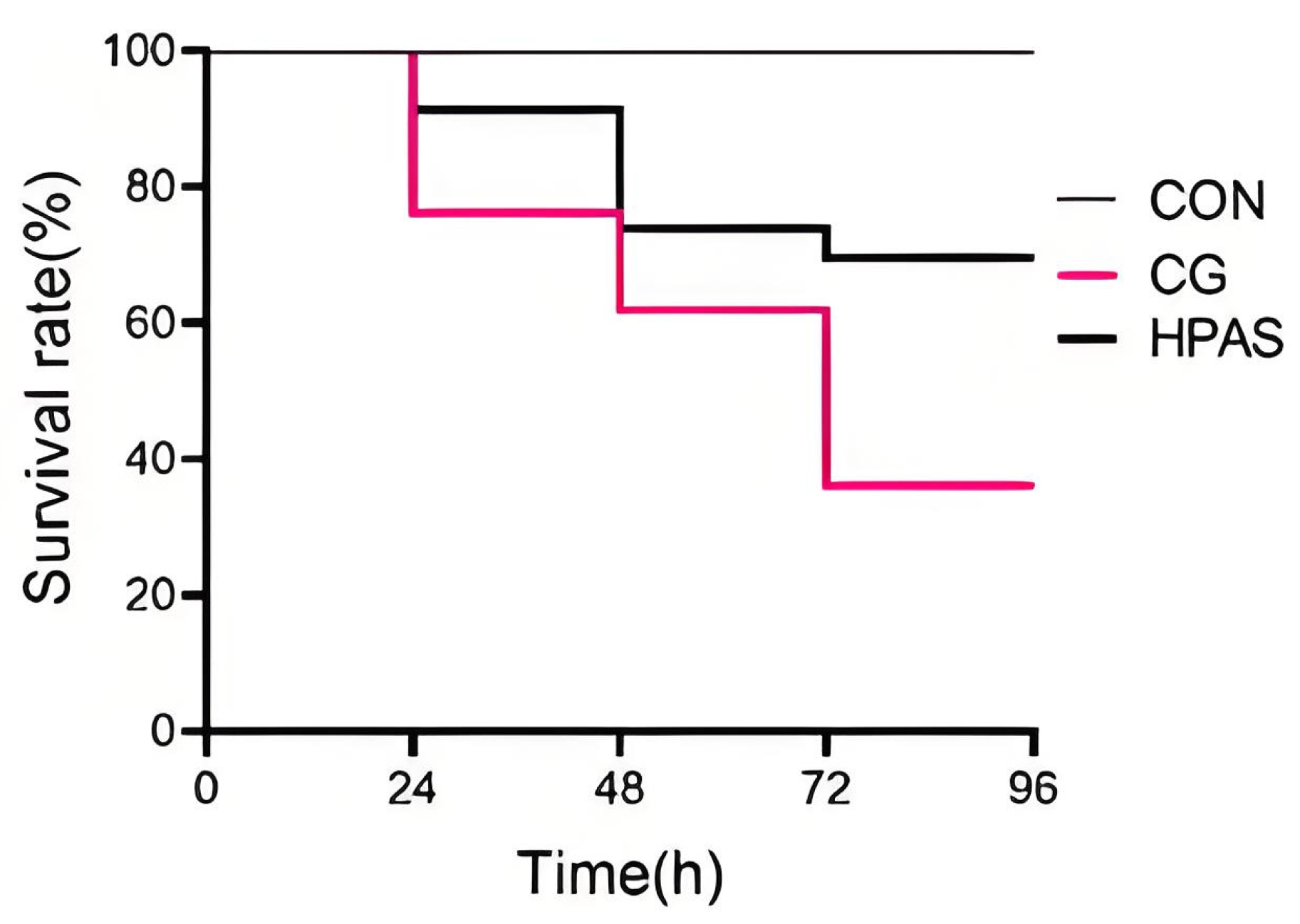
3.2. Challenge Test
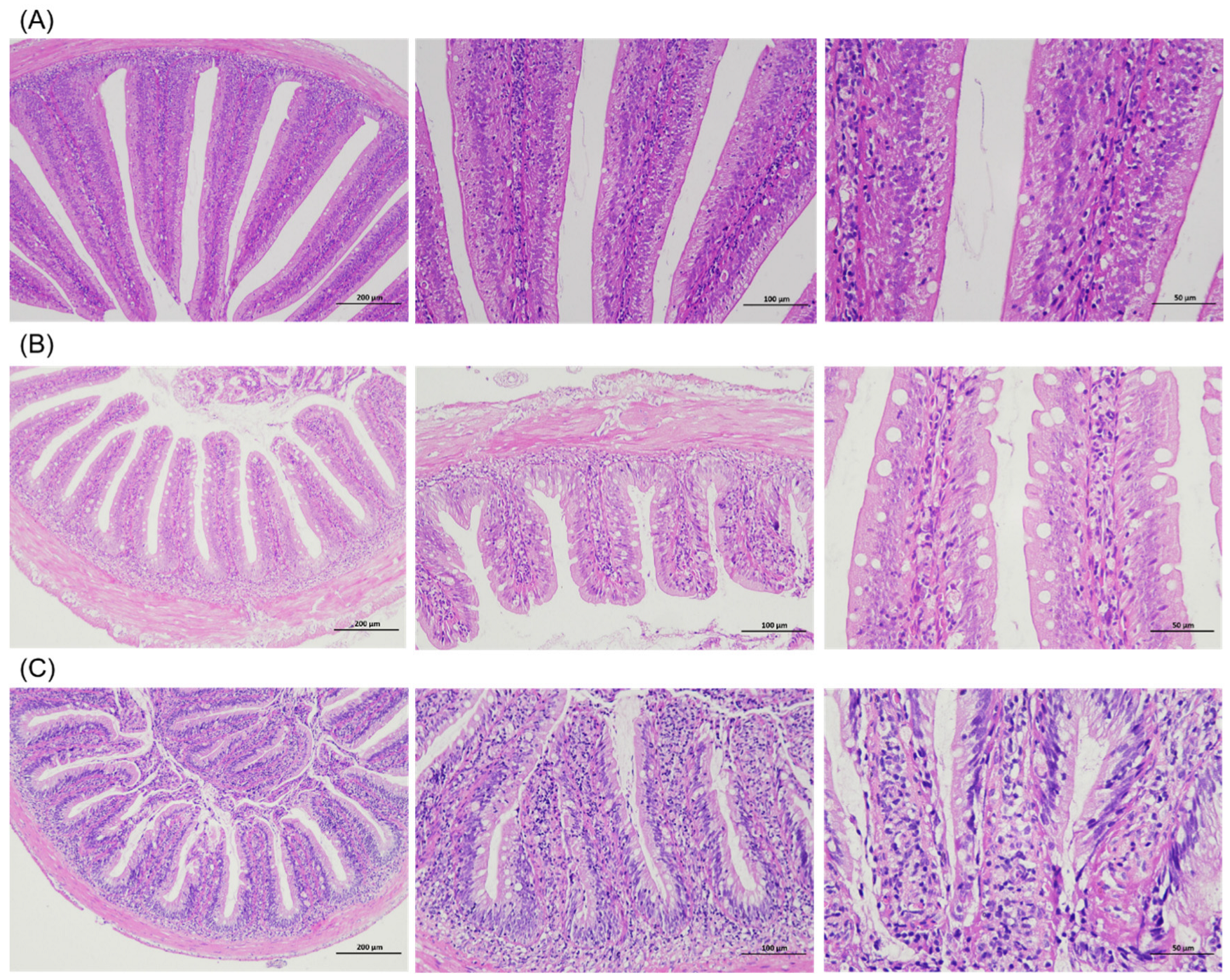
3.3. Intestinal Morphology
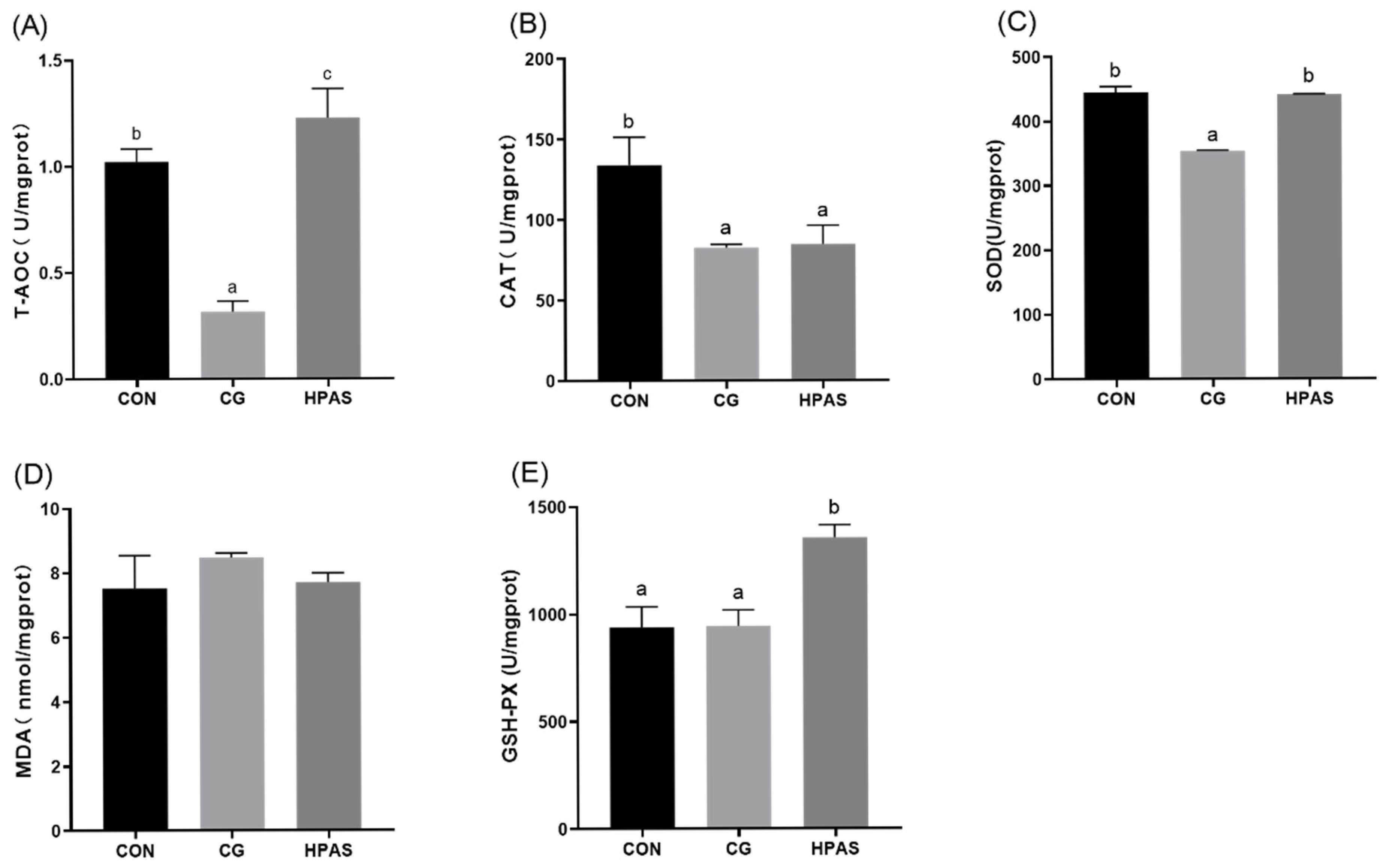
3.4. Antioxidant Capacity
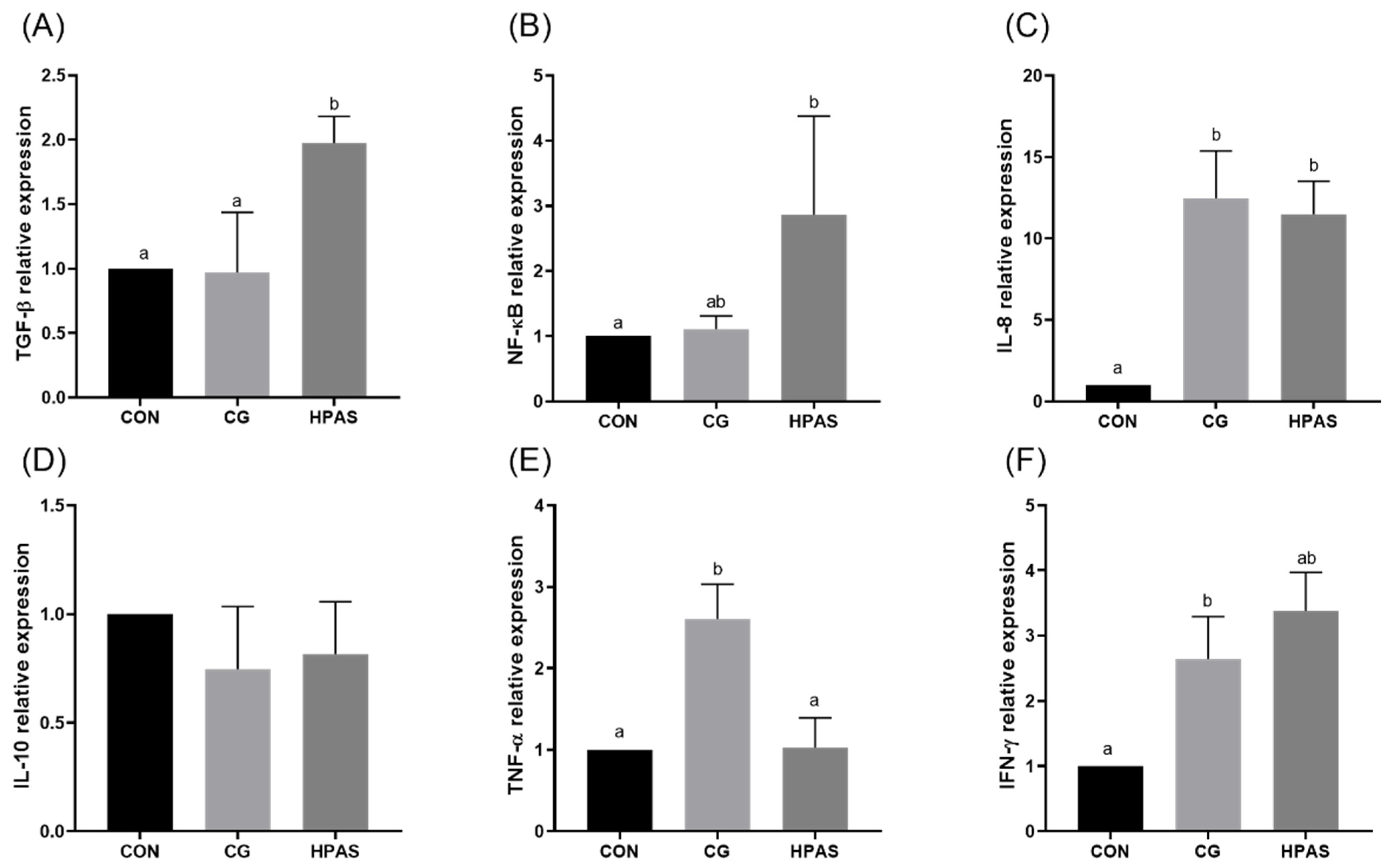
3.5. Gene Expression
3.6. Intestinal Microbiota Diversity and Richness
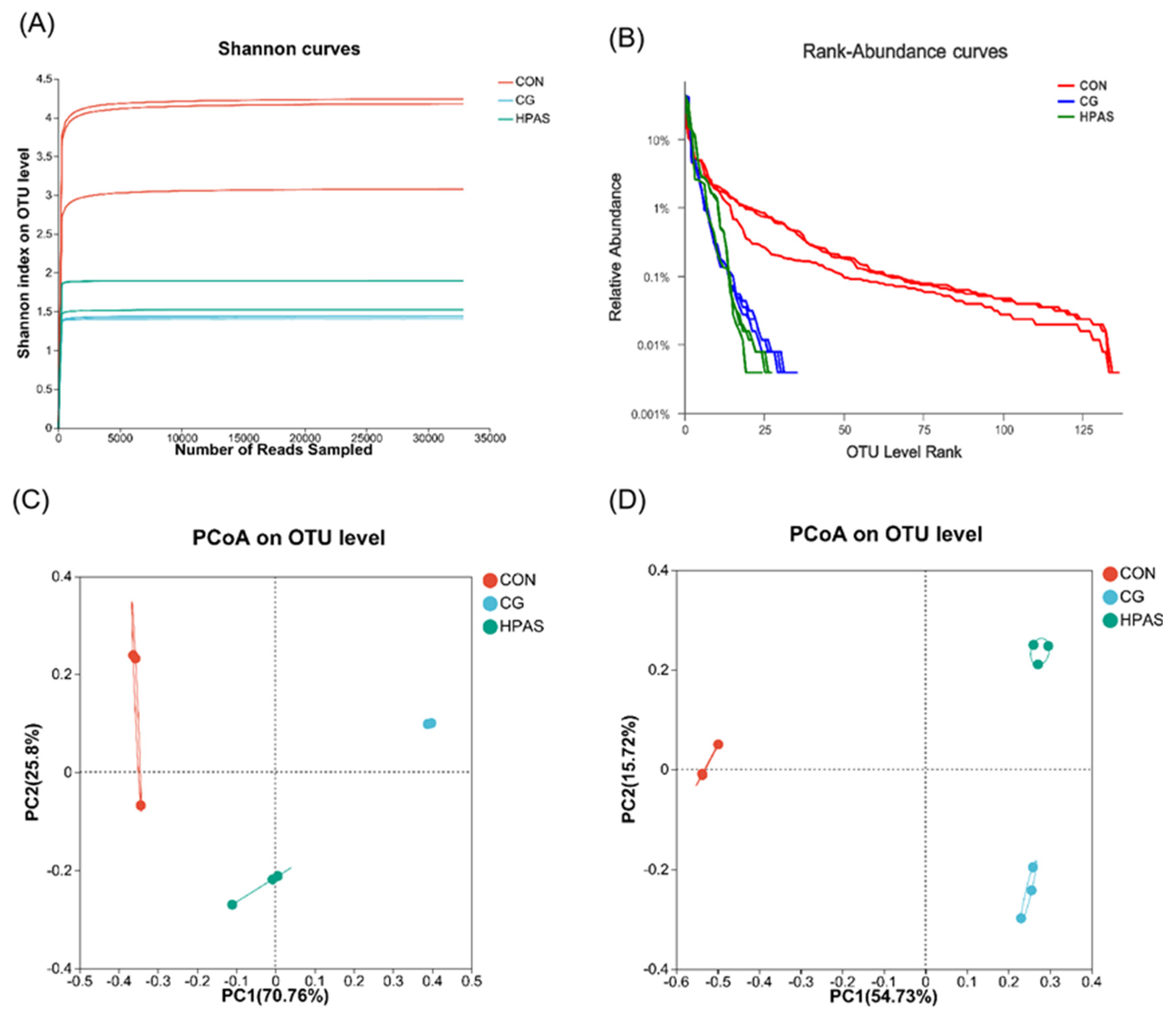
3.6.1. Diversity Analysis of the Intestinal Microbiota
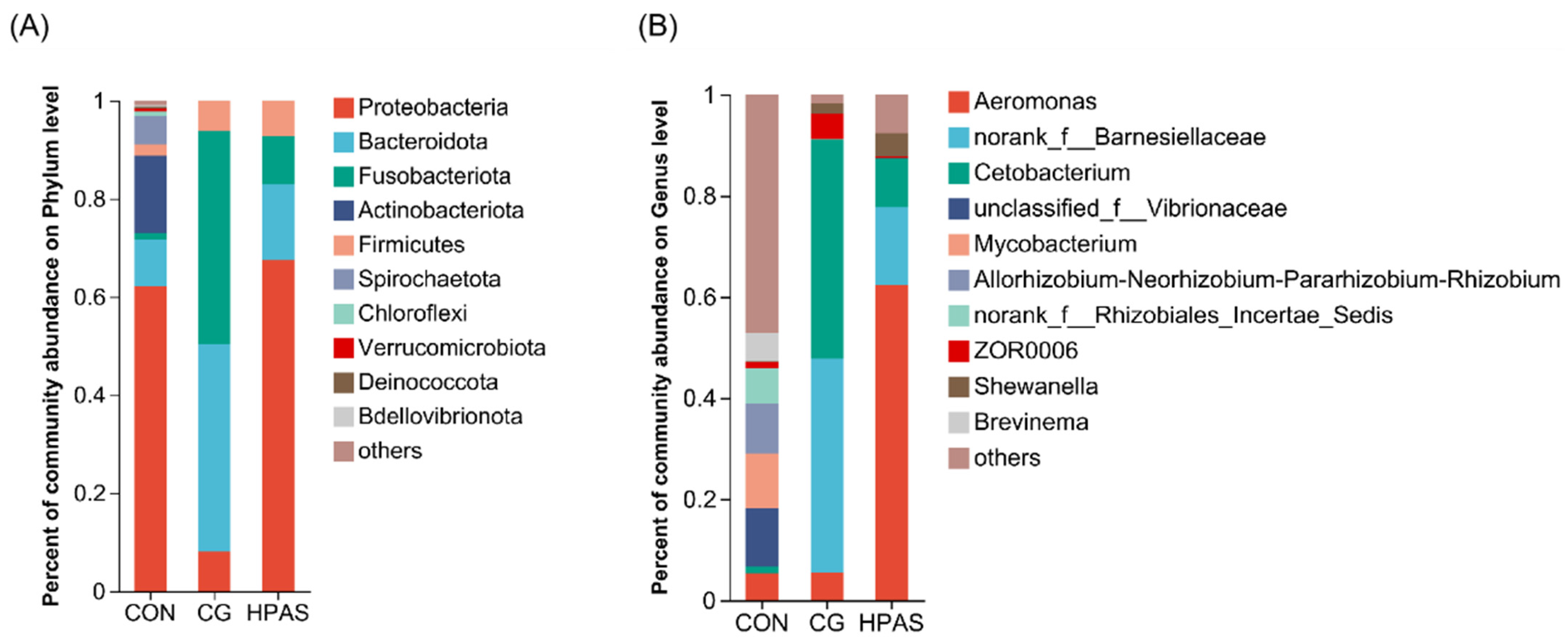
3.6.2. Intestinal Microbiota Structure and Composition
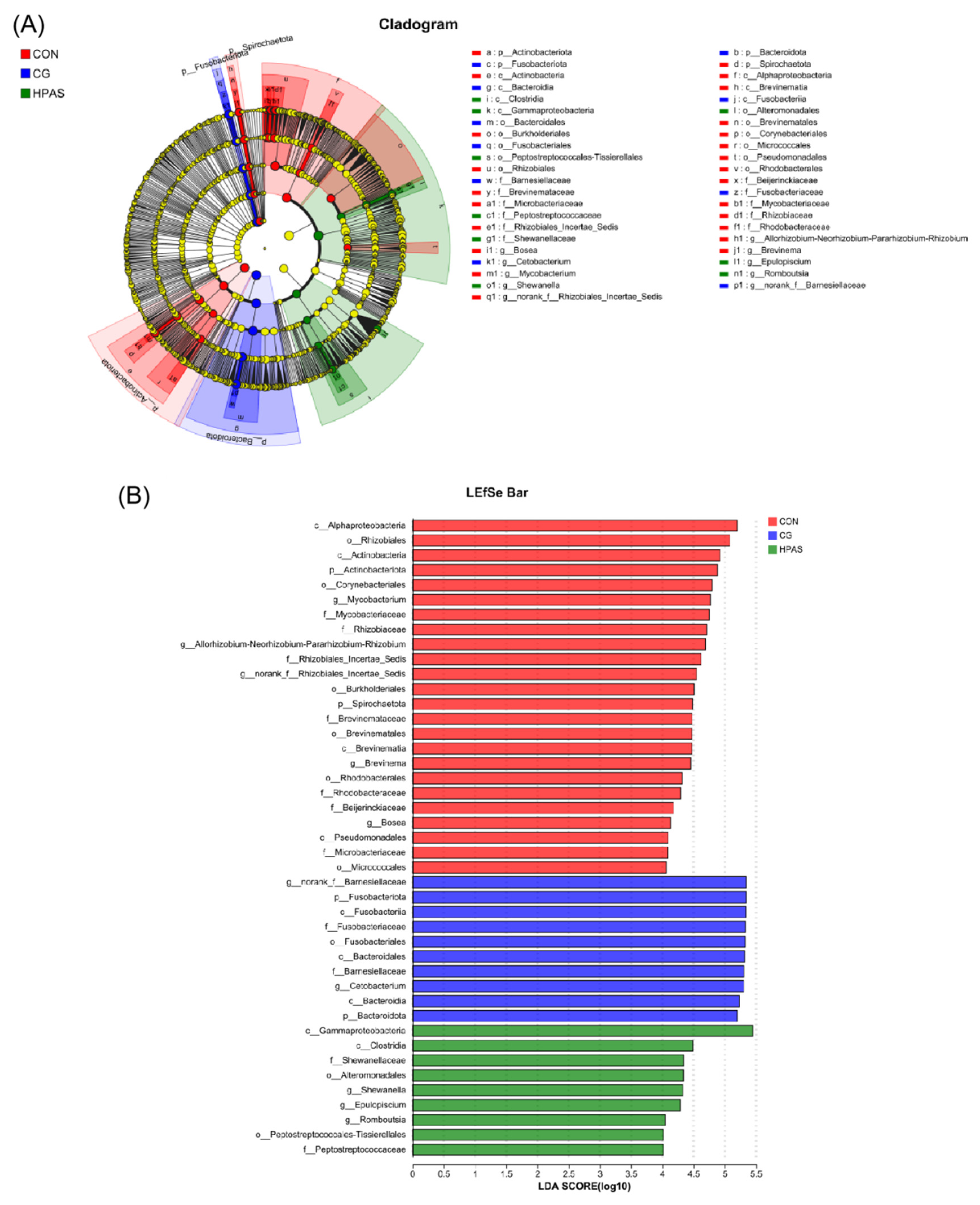
3.7. Differential Analysis of Intestinal Microbiota
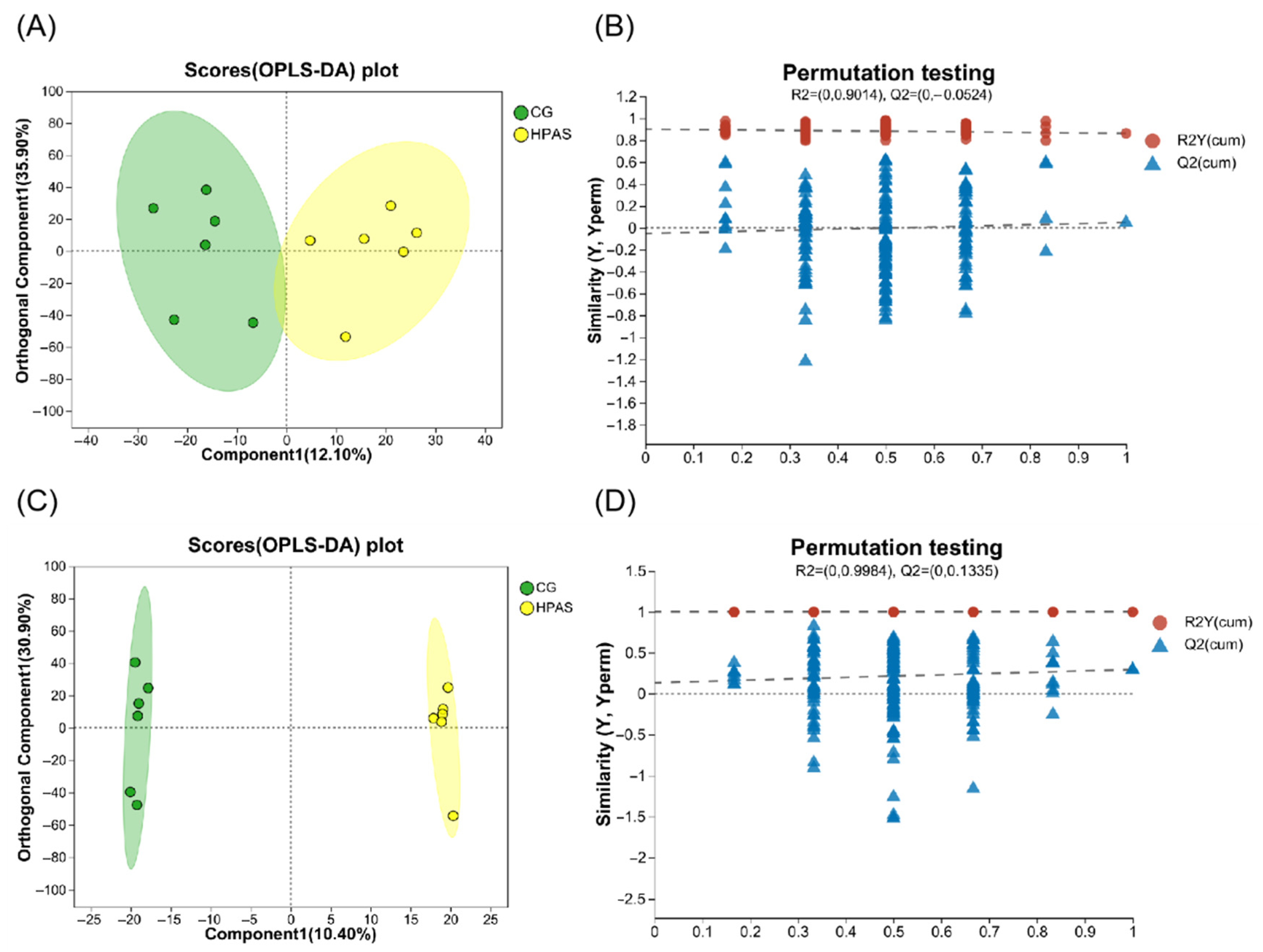
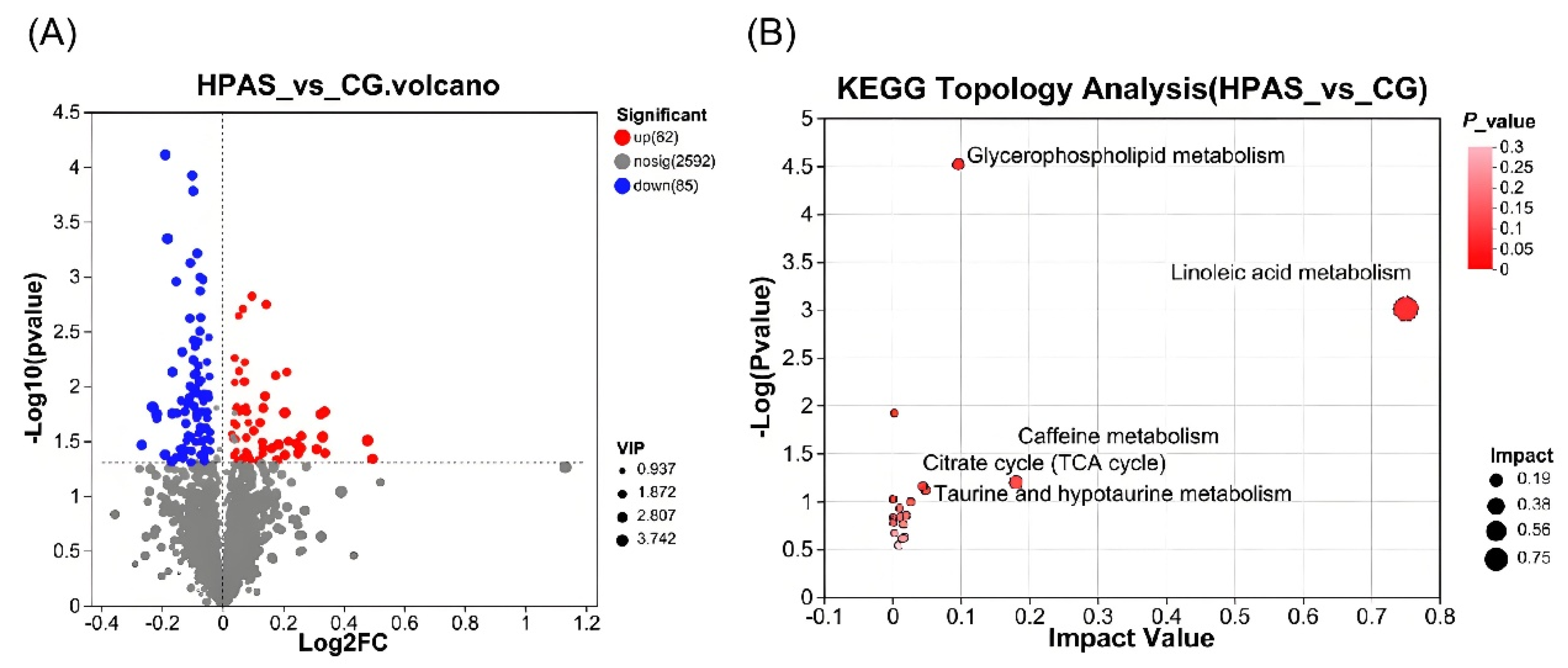
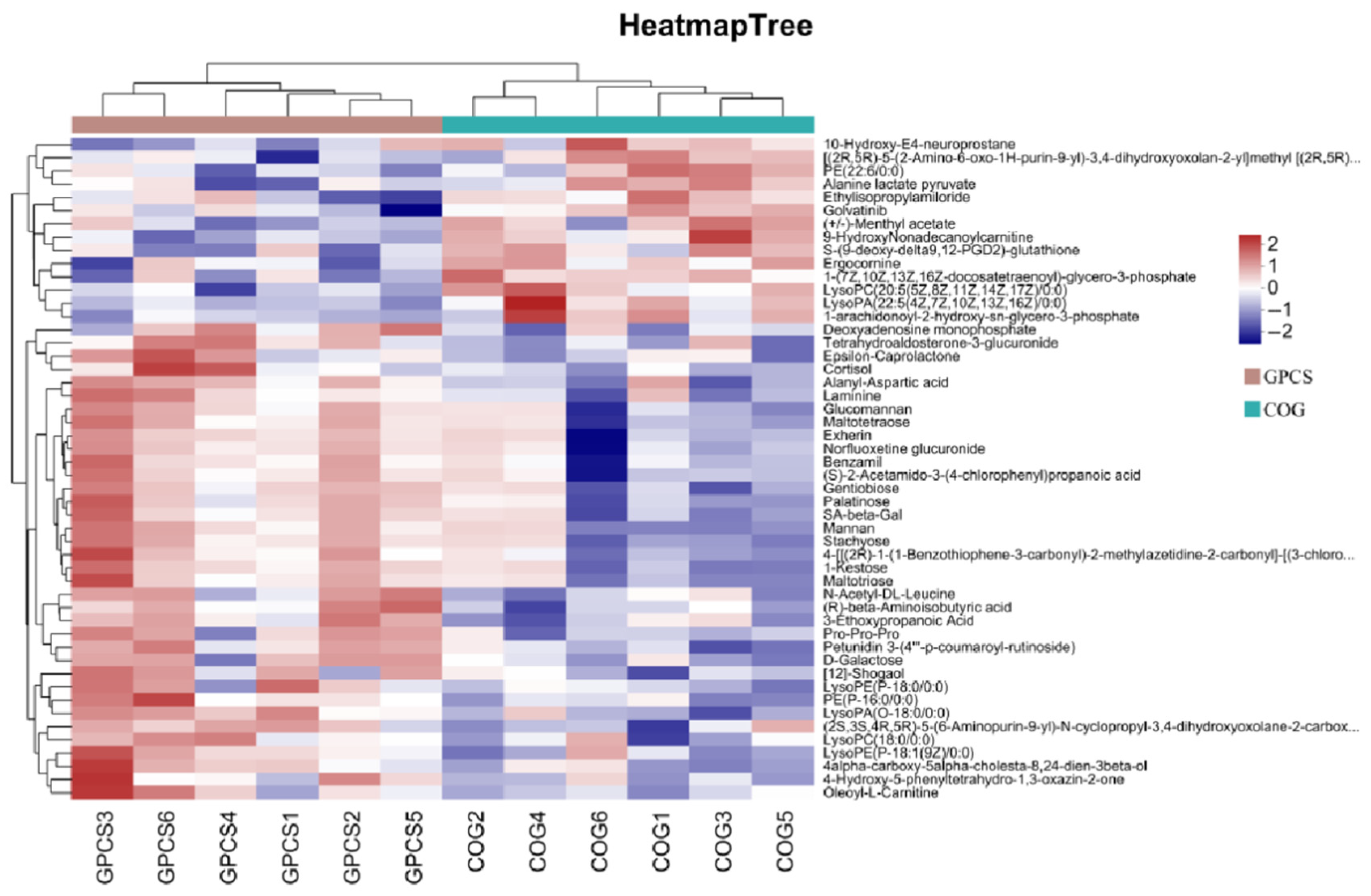
3.8. Analysis of Intestinal Metabolism of Crucian Carp
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Z.; Yang, L.; Feng, L.; Jiang, W.; Wu, P.; Liu, Y.; Zhang, L.; Yang, J.; Zhou, X. An emerging role of guanidine acetic acid in rescuing immune function injured by Aeromonas hydrophila in grass carp (Ctenopharyngodon idella). Aquac. Rep. 2024, 35, 101950. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Jiang, W.; Wu, P.; Liu, Y.; Ren, H.; Zhang, L.; Mi, H.; Tang, L.; Zhong, C.; et al. Emerging role of vitamin D3 in alleviating intestinal structure injury caused by Aeromonas hydrophila in grass carp (Ctenopharyngodon idella). Anim. Nutr. 2024, 16, 202–217. [Google Scholar] [CrossRef]
- Daskalov, H. The importance of Aeromonas hydrophila in food safety. Food Control 2006, 17, 474–483. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Chen, J.; Wang, Y.; Gu, Y.; Jiu, M. Antibacterial mechanisms and antivirulence activities of oridonin against pathogenic Aeromonas hydrophila AS 1.1801. Microorganisms 2024, 12, 415. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Lv, A.; Guan, Z. Skin immune response to Aeromonas hydrophila infection in crucian carp Carassius auratus revealed by multi-omics analysis. Fish Shellfish Immunol. 2022, 127, 866–875. [Google Scholar] [CrossRef]
- Cao, Y.; Kou, T.; Peng, L.; Munang’andu, H.M.; Peng, B. Fructose promotes crucian carp survival against Aeromonas hydrophila infection. Front. Immunol. 2022, 13, 865560. [Google Scholar] [CrossRef]
- Meng, X.; Luo, L.; Zhao, Z.; Wang, S.; Zhang, R.; Guo, K. Ginger polysaccharide alleviates the effects of acute exposure to carbonate in crucian carp (Carassius auratus) by regulating immunity, intestinal microbiota, and intestinal metabolism. Ecotoxicol. Environ. Saf. 2024, 273, 116127. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Wang, G.; Ling, F. The oral protective efficacy of magnolol against Aeromonas hydrophila and A. veronii infection via enhancing anti-inflammatory ability in goldfish (Carassius auratus). J. Fish Dis. 2023, 46, 1413–1423. [Google Scholar] [CrossRef]
- Wang, E.; Chen, X.; Liu, T.; Wang, K. Effect of dietary Ficus carica polysaccharides on the growth performance, innate immune response and survival of crucian carp against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2021, 120, 434–440. [Google Scholar] [CrossRef]
- Wu, Z.; Pang, S.; Liu, J.; Zhang, Q.; Fu, S.; Du, J.; Chen, X. Coriolus versicolor polysaccharides enhance the immune response of crucian carp (Corassius auratus gibelio) and protect against Aeromonas hydrophila. J. Appl. Ichthyol. 2013, 29, 562–568. [Google Scholar] [CrossRef]
- Li, T.; Fu, S.; Huang, X.; Zhang, X.; Cui, Y.; Zhang, Z.; Ma, Y.; Zhang, X.; Yu, Q.; Yang, S.; et al. Biological properties and potential application of hawthorn and its major functional components: A review. J. Funct. Foods 2022, 90, 104988. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, Q.; Li, C.; Zheng, J.; Wang, Y.; Duan, B. Preparation, structural characterization, bioactivities, and applications of Crataegus spp. polysaccharides: A review. Int. J. Biol. Macromol. 2023, 253, 126671. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Meng, X.; Chen, M.; Li, D.; Liu, R.; Sun, T. Isolation, structural properties and bioactivities of polysaccharides from Crataegus pinnatifida. J. Ethnopharmacol. 2023, 323, 117688. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, Q.; Han, X.; Gao, Z.; Peng, R.; Lin, X.; Cheng, X.; Zhao, W. In vitro digestion and fecal fermentation of polysaccharides from hawthorn and its impacts on human gut microbiota. Processes 2022, 10, 1922. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Yan, M.; Zhang, R.; Hu, B.; Sun, S.; Zhang, D.; Zheng, Y.; Wu, L. Structural characterization of a heteropolysaccharide from the fruit of Crataegus pinnatifida and its bioactivity on the gut microbiota of immunocompromised mice. Food Chem. 2023, 413, 135658. [Google Scholar] [CrossRef]
- Yu, W.; Wen, G.; Lin, H.; Yang, Y.; Huang, X.; Zhou, C.; Li, T. Effects of dietary Spirulina platensis on growth performance, hematological and serum biochemical parameters, hepatic antioxidant status, immune responses and disease resistance of Coral trout Plectropomus leopardus (Lacepede, 1802). Fish Shellfish Immunol. 2018, 74, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F. Application of natural polysaccharides and their derivatives biomaterials. Shandong Chem. Ind. 2020, 49, 93–94, 100. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, S.; Du, Y.; He, Y.; Shi, H.; Wang, K.; Dou, T.; Liu, L.; Shen, Z.; Jia, J.; et al. Application and research progress of plant polysaccharides in fish breeding. J. Shanxi Agric. Sci. 2022, 50, 272–280. [Google Scholar]
- Wang, E.; Chen, X.; Wang, K.; Wang, J.; Chen, D.; Geng, Y.; Lai, W.; Wei, X. Plant polysaccharides used as immunostimulants enhance innate immune response and disease resistance against Aeromonas hydrophila infection in fish. Fish Shellfish Immunol. 2016, 59, 196–202. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.; Guo, Y.; Zhao, H.; Sun, J.; Ma, S.; Xing, K. Dietary polysaccharide from Angelica sinensis enhanced cellular defence responses and disease resistance of grouper Epinephelus malabaricus. Aquacult. Int. 2011, 19, 945–956. [Google Scholar] [CrossRef]
- Rajendran, P.; Subramani, P.A.; Michael, D. Polysaccharides from marine macroalga, Padina gymnospora improve the nonspecific and specific immune responses of Cyprinus carpio and protect it from different pathogens. Fish Shellfish Immunol. 2016, 58, 220–228. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, G.; Shen, Y.; Tan, X.; Tang, T.; Li, R. Clinical research progress of ginger umbilical application. Guangming J. Chin. Med. 2022, 37, 2933–2936. [Google Scholar] [CrossRef]
- Jing, Y.; Yan, M.; Liu, D.; Tao, C.; Hu, B.; Sun, S.; Zheng, Y.; Wu, L. Research progress on the structural characterization, biological activity and product application of polysaccharides from Crataegus pinnatifida. Int. J. Biol. Macromol. 2023, 244, 125408. [Google Scholar] [CrossRef]
- Beaz-Hidalgo, R.M.; Figueras, M.J. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J. Fish Dis. 2013, 36, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; Chen, Z.; Wei, L.; Fan, Y.; Zhang, J.; Zhong, Q. Bactericidal efficacy comparisonof povidone iodineon Aeromonas hydrophila between detection methods. Fish. Sci. 2018, 37, 348–353. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Yang, S.; Feng, L.; Zhang, J.; Yan, C.; Zhang, C.; Huang, Y.; Li, Y. Effect of Purslane (Portulaca oleracea L.) on intestinal morphology, digestion activity and microbiome of Chinese Pond Turtle (Mauremys reevesii) during Aeromonas hydrophila Infection. Int. J. Mol. Sci. 2023, 24, 10260. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef]
- Hoon, K.B.; Pyo, H.S.; Hee, C.S.; Don, L.Y.; Hoon, L.C. Exploring rearing water temperature conditions for inducing body growth without water temperature stress in red-spotted grouper (Epinephelus akaara). Ocean Sci. J. 2023, 58, 25. [Google Scholar] [CrossRef]
- Zheng, X.; Chi, C.; Xu, C.; Liu, J.; Zhang, C.; Zhang, L.; Huang, Y.; He, C.; Jia, X.; Liu, W. Effects of dietary supplementation with icariin on growth performance, antioxidant capacity and non-specific immunity of Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 90, 264–273. [Google Scholar] [CrossRef]
- Li, Z.; Han, C.; Wang, Z.; Li, Z.; Ruan, L.; Lin, H.; Zhou, C. Black soldier fly pulp in the diet of golden pompano: Effect on growth performance, liver antioxidant and intestinal health. Fish Shellfish Immunol. 2023, 142, 109156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, D.; Wang, D.; Ye, B.; Wang, S.; Zhou, A.; Dong, Z.; Zou, J. Effect of dietary koumine on the immune and antioxidant status of carp (Cyprinus carpio) after Aeromonas hydrophila infection. Microb. Pathog. 2023, 186, 106464. [Google Scholar] [CrossRef]
- Feng, D.; Yu, Y.; Liu, K.; Su, Y.; Fan, T.; Guo, X.; Li, M. Effects of dietary leucine on growth, antioxidant capacity, immune response, and inflammation in juvenile yellow catfish Pelteobagrus fulvidraco. Front. Physiol. 2023, 14, 1247410. [Google Scholar] [CrossRef] [PubMed]
- El-Shenawy, N.S.; Mohammadden, A.; Al-Fahmie, Z.H. Using the enzymatic and non-enzymatic antioxidant defense system of the land snail Eobania vermiculata as biomarkers of terrestrial heavy metal pollution. Ecotoxicol. Environ. Saf. 2012, 84, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Ouyang, K.H.; Chen, H.; Yang, Z.; Hu, W.; Wang, N.; Liu, X.; Wang, W. Immunomodulatory effect of Cyclocarya paliurus polysaccharide in cyclophosphamide induced immunocompromised mice. Bioact. Carbohydr. Diet. Fibre 2020, 24, 100224. [Google Scholar] [CrossRef]
- Liu, W.; Zha, P.; Guo, L.; Chen, Y.; Zhou, Y. Effects of different levels of dietary chlorogenic acid supplementation on growth performance, intestinal integrity, and antioxidant status of broiler chickens at an early age. Anim. Feed Sci. Technol. 2023, 297, 115570. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, R.; Ge, L.; Yang, Q.; Lu, N.; Li, H.; Feng, Z. Effects of dietary n-6 polyunsaturated fatty acids (PUFA) composition on growth performances and non-specific immunity in pacific white shrimp (Litopenaeus vannamei). Aquacult. Rep. 2023, 28, 101436. [Google Scholar] [CrossRef]
- Singh, Z.; Karthigesu, I.P.; Singh, P.; Rupinder, K. Use of malondialdehyde as a biomarker for assessing oxidative stress in different disease pathologies: A review. Iran. J. Public Health 2014, 43, 7–16. [Google Scholar]
- Liang, S.; Luo, X.; You, W.; Luo, L.; Ke, C. The role of hybridization in improving the immune response and thermal tolerance of abalone. Fish Shellfish Immunol. 2014, 39, 69–77. [Google Scholar] [CrossRef]
- Pérez-Acosta, J.A.; Burgos-Hernandez, A.; Velázquez-Contreras, C.A.; Márquez-Ríos, E.; Torres-Arreola, W.; Arvizu-Flores, A.A.; Ezquerra-Brauer, J.M. An in vitro study of alkaline phosphatase sensitivity to mixture of aflatoxin B1 and fumonisin B1 in the hepatopancreas of coastal lagoon wild and farmed shrimp Litopenaeus vannamei. Mycotoxin Res. 2016, 32, 117–125. [Google Scholar] [CrossRef]
- Raja Rajeswari, P.; Velmurugan, S.; Michael Babu, M.; Albin Dhas, S.; Kesavan, K.; Citarasu, T. A study on the influence of selected Indian herbal active principles on enhancing the immune system in Fenneropenaeus indicus against Vibrio harveyi infection. Aquacult. Int. 2012, 20, 1009–1020. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Yang, Y.; Lu, Y.; Zhu, L.; Li, L.; Kong, X. Myo-inositol enhances the resistance and alleviates inflammation of Cyprinus carpio to Aeromonas hydrophila infection. Aquaculture 2024, 578, 740056. [Google Scholar] [CrossRef]
- Luo, Q.; Hu, Z.; Zhao, H.; Fan, Y.; Tu, X.; Wang, Y.; Liu, X. The role of TGF-β in the tumor microenvironment of pancreatic cancer. Genes Dis. 2022, 13, 788–799. [Google Scholar] [CrossRef]
- Abosalif, K.O.A.; Abdalla, A.E.; Junaid, K.; Eltayeb, L.B.; Ejaz, H. The interleukin-10 family: Major regulators of the immune response against Plasmodium falciparum infections. Saudi J. Biol. Sci. 2023, 30, 103805. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, X.; Zhang, S.; Liu, Y.; Xue, Q.; Luo, Y.; Yu, B.; Li, X.; Liu, Z. Dexamethasone inhibits IL-8 via glycolysis and mitochondria-related pathway to regulate inflammatory pain. BMC Anesthesiol. 2023, 23, 317. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Fu, C.; Zhao, H.; Wang, B.; Jia, S.; Chen, H.; Cai, H. Up-regulation of Core 1 Beta 1, 3-Galactosyltransferase suppresses osteosarcoma growth with induction of IFN-γ secretion and proliferation of CD8+ T Cells. Curr. Cancer Drug Targets 2023, 23, 265–277. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Wu, G. Role of NF-κB pathway in kidney renal clear cell carcinoma and its potential therapeutic implications. Aging 2023, 15, 11313. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Momeni, S.A.; Zarandi, P.K.; Chalbatani, G.M.; Dana, H.; Mirzaei, H.R.; Akbari, M.E.; Miri, S.R. The Role and Function of Ras-association domain family in Cancer: A Review. Genes Dis. 2019, 6, 378–384. [Google Scholar] [CrossRef]
- Wong, S.; Rawls, J.F. Intestinal microbiota composition in fishes is influenced by host ecology and environment. Mol. Ecol. 2012, 21, 3100–3102. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhu, H.; Li, Q.; Xu, G. The effects of different feeding regimes on body composition, gut microbial population, and susceptibility to pathogenic infection in largemouth bass. Microorganisms 2023, 11, 1356. [Google Scholar] [CrossRef]
- Luo, L.; Meng, X.; Wang, S.; Zhang, R.; Guo, K.; Zhao, Z.-G. Effects of dietary ginger (Zingiber officinale) polysaccharide on the growth, antioxidant, immunity response, intestinal microbiota, and disease resistance to Aeromonas hydrophila in crucian carp (Carassius auratus). Int. J. Biol. Macromol. 2024, 275, 133711. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Huang, J.; Jin, Z.; Guan, J.; Yu, H.; Zhang, M.; Yu, M.; Jiang, H.; Qiao, Z. Effects of Aeromonas hydrophila infection on the intestinal microbiota, transcriptome, and metabolomic of common carp (Cyprinus carpio). Fish Shellfish Immunol. 2023, 139, 108876. [Google Scholar] [CrossRef]
- Chang, S.; Wang, J.; Dong, C.; Jiang, Y. Intestinal microbiota signatures of common carp (Cyprinus carpio) after the infection of Aeromonas hydrophila. Aquacult. Rep. 2023, 30, 101585. [Google Scholar] [CrossRef]
- Liu, H.; Chen, G.; Li, L.; Lin, Z.; Tan, B.; Dong, X.; Zhou, X. Supplementing artemisinin positively influences growth, antioxidant capacity, immune response, gut health and disease resistance against Vibrio parahaemolyticus in Litopenaeus vannamei fed cottonseed protein concentrate meal diets. Fish Shellfish Immunol. 2022, 131, 105–118. [Google Scholar] [CrossRef]
- Jin, X.; Su, M.; Liang, Y.; Li, Y. Effects of chlorogenic acid on growth, metabolism, antioxidation, immunity, and intestinal flora of crucian carp (Carassius auratus). Front. Microbiol. 2023, 13, 1084500. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 63, 12073–12088. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ikegami, A.; Rajanna, C.; Kawsar, H.I.; Zhou, Y.; Li, M.; Sojar, H.T.; Genco, R.J.; Kuramitsu, H.K.; Deng, C.X. Identification and characterization of a novel adhesin unique to oral fusobacteria. J. Bacteriol. 2005, 187, 5330–5340. [Google Scholar] [CrossRef]
- McCormick, D.B. The vitamins: Fundamental aspects in nutrition and health. Am. J. Clin. Nutr. 1999, 70, 426–427. [Google Scholar] [CrossRef]
- Ofek, T.; Izhaki, I.; Halpern, M. Aeromonas hydrophila infection in tilapia triggers changes in the microbiota composition of fish internal organs. FEMS Microbiol. Ecol. 2023, 99, 137. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, J.; Guo, Z.; Zhong, H.; Luo, Y.; Wang, J.; Tang, Z.; Huang, T.; Li, M.; Zhu, J.; et al. Transcriptomics integrated with metabolomics reveals the effect of Lycium barbarum polysaccharide on apoptosis in Nile tilapia (Oreochromis niloticus). Genomics 2022, 114, 229–240. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Horman, T.; Fernandes, M.F.; Tache, M.C.; Hucik, B.; Mutch, D.M.; Leri, F. Dietary n-6/n-3 Ratio influences brain fatty acid composition in adult rats. Nutrients 2020, 12, 1847. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, W.; Arya, G.; Garcia, V. Long-Chain predominant lipid emulsions inhibit in vitro macrophage tumor necrosis factor production. JPEN. J. Parenter. Enter. Nutr. 1994, 18, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Huang, J.; Zhou, X.; Li, Y.; Qin, L. Relationship between serum phospholipid polyunsaturated fatty acid profile and systemic low-grade inflammation in patients with coronary heart disease. J. Trop. Med. 2019, 19, 181–184. [Google Scholar]
| Primers | Sequence (5′−3′) | |
|---|---|---|
| β-Actin | F | CAAGATGATGGTGTGCCAAGTG |
| R | TCTGTCTCCGGCACGAAGTA | |
| TNF-α | F | TTATGTCGGTGCGGCCTTC |
| R | AGGTCTTTCCGTTGTCGCTTT | |
| IFN-γ | F | AACAGTCGGGTGTCGCAAG |
| R | TCAGCAAACATACTCCCCAG | |
| IL-10 | F | GGAACGATGGGCAGATCAA |
| R | AACTGAAGGGGAAGGGGAAG | |
| NF-κB | F | AATGTGGTGCGTCTGTGCTT |
| R | TGTTGTCATAGATGGGGTTGGA | |
| IL-8 | F | CTGAGAGTCGACGCATTGGAA |
| R | TGGTGTCTTTACAGTGTGAGTTTGG | |
| TGF-β | F | GTTGGCGTAATAACCAGAAGG |
| R | AACAGAACAAGTTTGTACCGATAAG |
| Parameters | Group | ||||
|---|---|---|---|---|---|
| Control | 0.1% HP | 0.2% HP | 0.4% HP | 0.8% HP | |
| IBW (g) | 122.14 ± 8.73 | 121.09 ± 6.49 | 119.59 ± 9.71 | 113.18 ± 12.32 | 122.02 ± 14.90 |
| FBW (g) | 480.82 ± 118.31 | 399.92 ± 31.44 | 424.73 ± 71.66 | 478.60 ± 41.82 | 448.04 ± 28.08 |
| WGR (%) | 294.87 ± 25.59 ab | 231.74 ± 42.51 a | 253.46 ± 32.25 ab | 325.71 ± 54.09 b | 271.33 ± 56.84 ab |
| SGR (%) | 2.45 ± 0.12 ab | 2.13 ± 0.23 a | 2.25 ± 0.17 ab | 2.58 ± 0.23 b | 2.33 ± 0.27 ab |
| SR (%) | 100 | 100 | 100 | 100 | 100 |
| Group | |||
|---|---|---|---|
| CON | CG | HPAS | |
| chao | 977.60 ± 138.85 b | 79.18 ± 15.43 a | 71.16 ± 5.31 a |
| coverage | 0.99 ± 0.00 a | 1.00 ± 0.00 b | 1.00 ± 0.00 b |
| shannon | 3.83 ± 0.65 b | 1.43 ± 0.02 a | 1.77 ± 0.22 a |
| simpson | 0.08 ± 0.06 a | 0.34 ± 0.00 c | 0.26 ± 0.04 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Zhao, Z.; Wang, S.; Zhang, R.; Guo, K.; Zhao, C.; He, B.; Wang, W.; Wu, W. Hawthorn Polysaccharide Enhances Growth, Immunity, and Intestinal Health in Crucian Carp (Carassius auratus) Challenged with Aeromonas hydrophila. Fishes 2025, 10, 451. https://doi.org/10.3390/fishes10090451
Luo L, Zhao Z, Wang S, Zhang R, Guo K, Zhao C, He B, Wang W, Wu W. Hawthorn Polysaccharide Enhances Growth, Immunity, and Intestinal Health in Crucian Carp (Carassius auratus) Challenged with Aeromonas hydrophila. Fishes. 2025; 10(9):451. https://doi.org/10.3390/fishes10090451
Chicago/Turabian StyleLuo, Liang, Zhigang Zhao, Shihui Wang, Rui Zhang, Kun Guo, Cheng Zhao, Baoquan He, Wei Wang, and Wenhua Wu. 2025. "Hawthorn Polysaccharide Enhances Growth, Immunity, and Intestinal Health in Crucian Carp (Carassius auratus) Challenged with Aeromonas hydrophila" Fishes 10, no. 9: 451. https://doi.org/10.3390/fishes10090451
APA StyleLuo, L., Zhao, Z., Wang, S., Zhang, R., Guo, K., Zhao, C., He, B., Wang, W., & Wu, W. (2025). Hawthorn Polysaccharide Enhances Growth, Immunity, and Intestinal Health in Crucian Carp (Carassius auratus) Challenged with Aeromonas hydrophila. Fishes, 10(9), 451. https://doi.org/10.3390/fishes10090451







