First Morphological and Molecular Identification of Intestinal Helminths in Wild Turbot Scophthalmus maximus (Linnaeus, 1758) Along the Bulgarian Black Sea Coast
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analyses
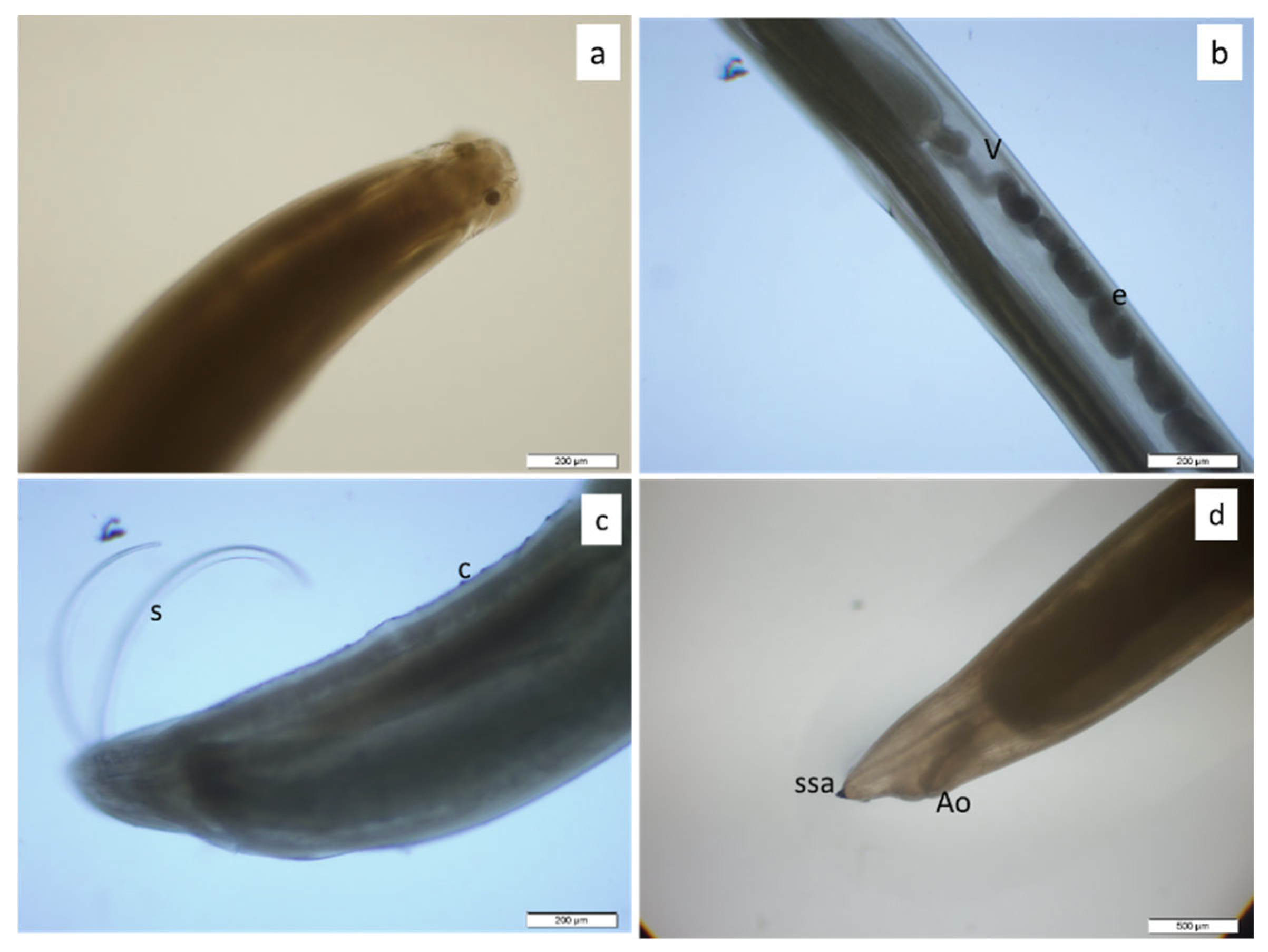
2.3. Parasitical Examination
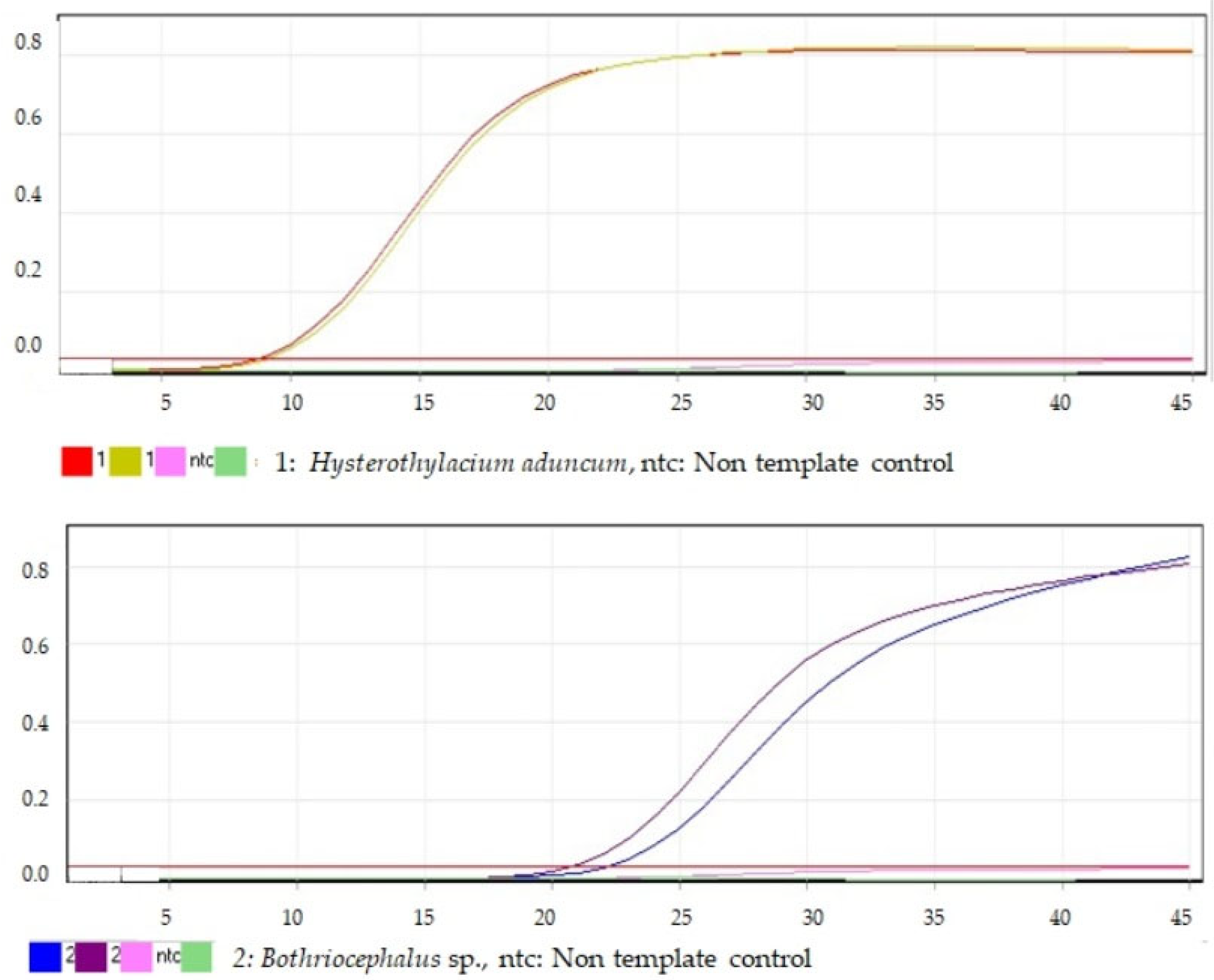
2.4. Real-Time PCR Analysis
2.4.1. DNA Isolation
2.4.2. Primer Optimization and Synthesis
2.4.3. PCR Analysis
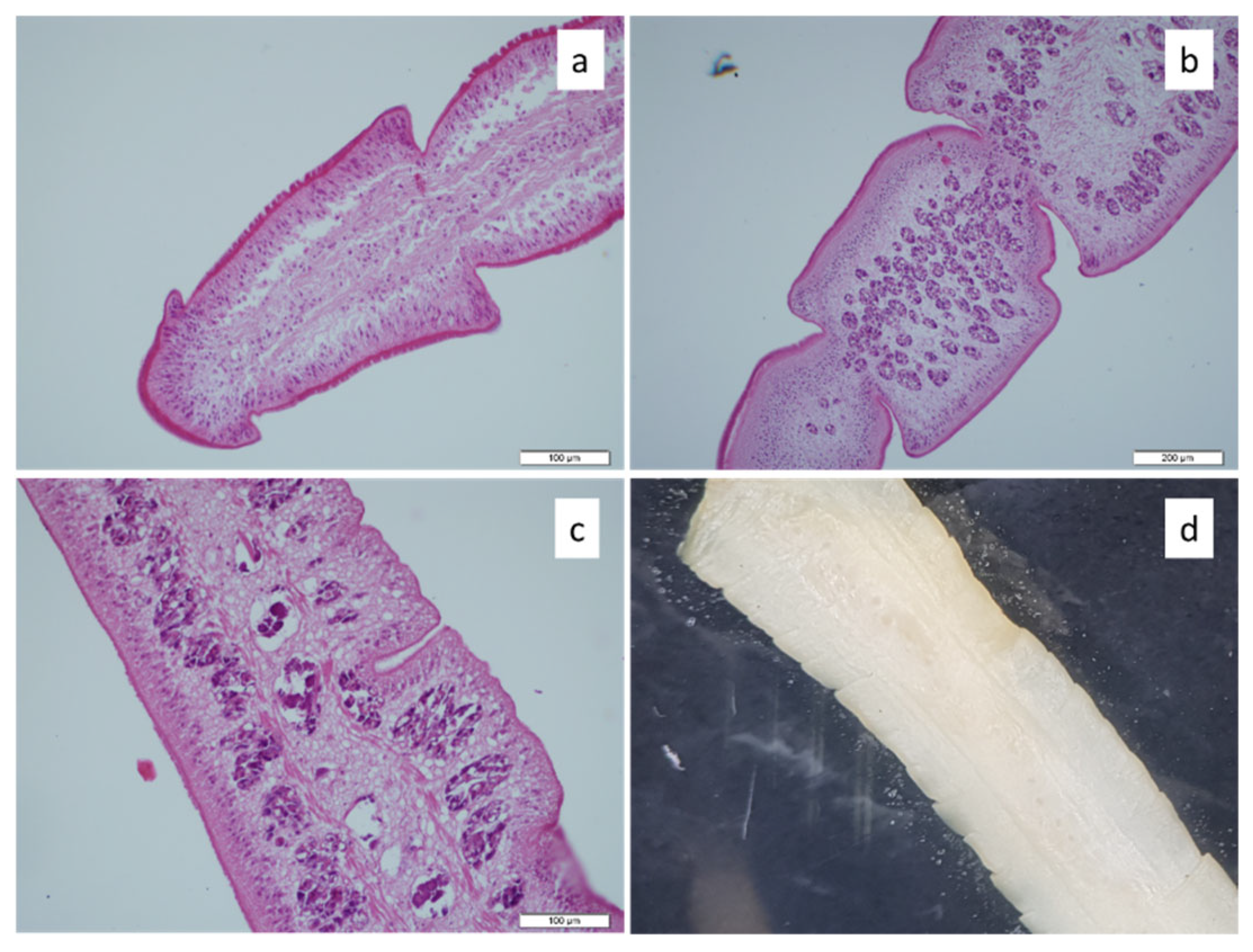
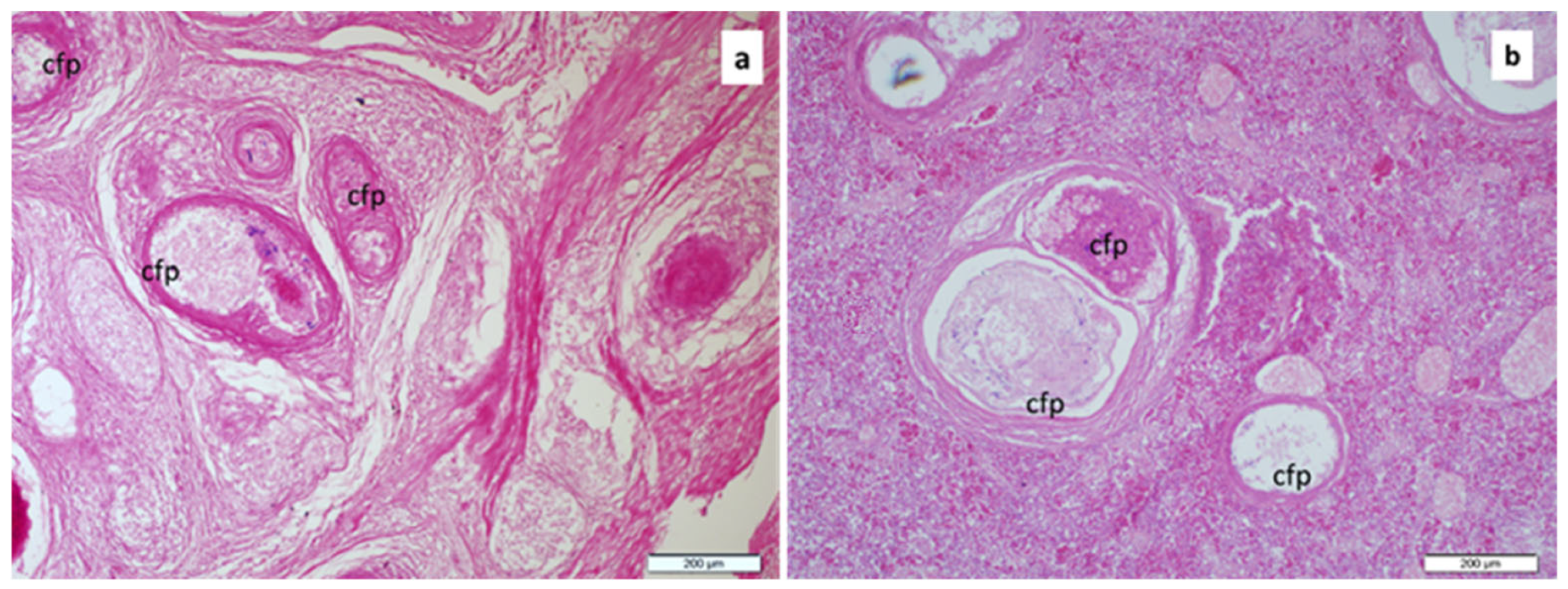
2.5. Histopathological Examination
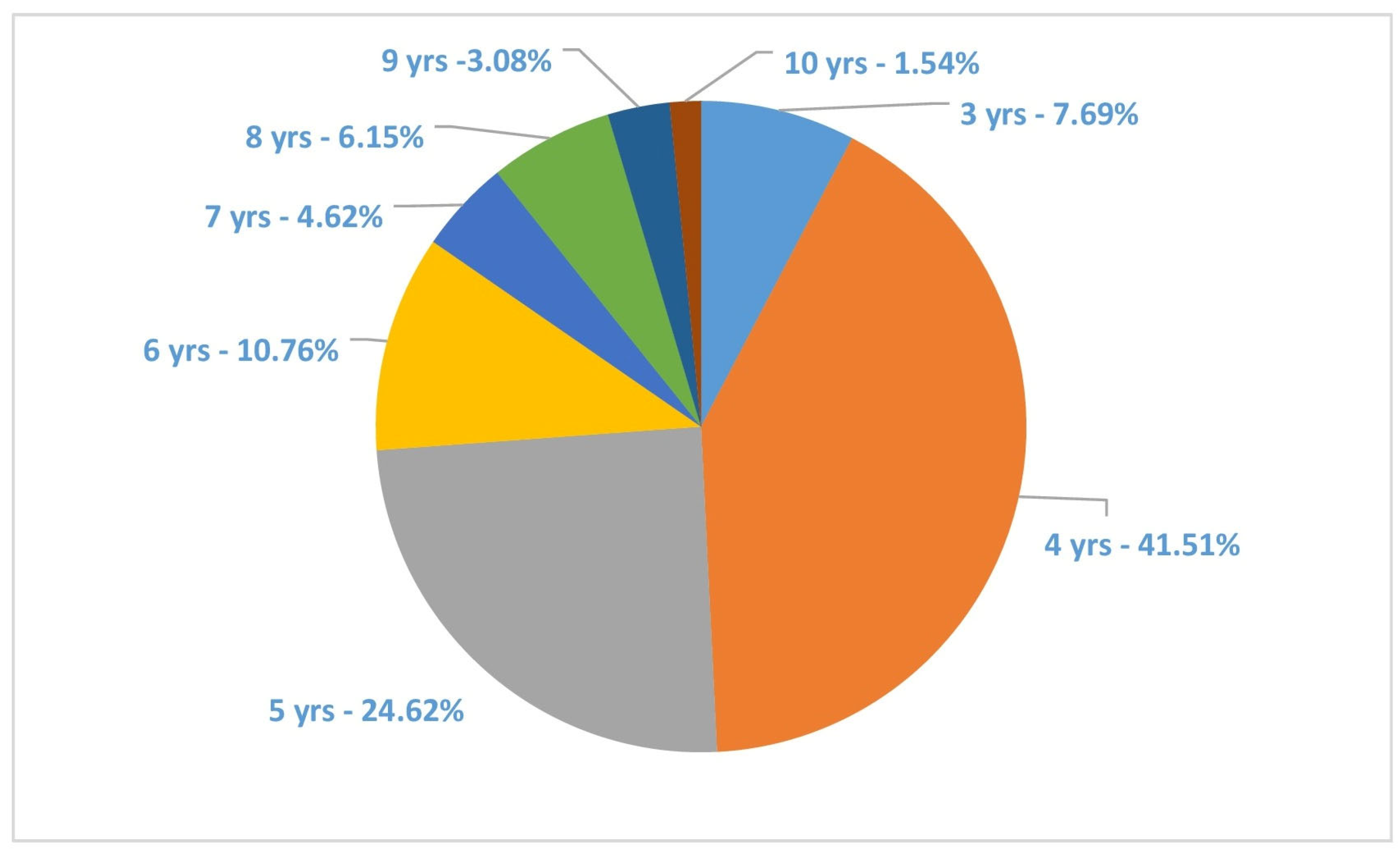
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikolov, V.; Ivanova, P.; Dzembekova, N.; Panayotova, M.; Raykov, V.; Dobrovolov, I. Application of allozyme markers for screening of turbot populations along Western Black Sea coast. ZooNotes 2015, 79, 1–15. [Google Scholar]
- Țoțoiu, A.; Patriche, N.; Niță, V.; Sîrbu, E.; Dima, F.M.; Nenciu, M.I.; Nistor, V. Epidemiology of Turbot (Scophthalmus maeoticus) bacterial contamination, a fishery limiting factor on the Romanian Black Sea. Fishes 2023, 8, 418. [Google Scholar] [CrossRef]
- Hulak, B.; Leonchyk, Y.; Maximov, V.; Tiganov, G.; Shlyakhov, V.; Pyatnitsky, M. The current state of the turbot, Scophthalmus maximus (Linnaeus, 1758), population in the northwestern part of the Black Sea. Fish. Aquat. Life 2021, 9, 164–175. [Google Scholar] [CrossRef]
- GFCM. Working Group on the Black Sea (WGBS). In Proceedings of the Report of the 2nd Meeting of the Subregional Group on Stock Assessment in the Black Sea (SGSABS), Constanta, Romania, 10–12 November 2014; p. 237. [Google Scholar]
- Petrova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn 2022; Report under contract with the Executive Agency Fisheries and Aquacultures, program for data collection for fishing in 2021; Agricultural Academy Institute of Fish Resources: Varna, Bulgaria, 2022; p. 52. [Google Scholar]
- MacKenzie, K.; Abaunza, P. Parasites as biological tags for stock discrimination of marine fish: A guide to procedures and methods. Fish. Res. 1998, 38, 45–56. [Google Scholar] [CrossRef]
- Lester, R.J.G.; MacKenzie, K. The use and abuse of parasites as stock markers for fish. Fish. Res. 2009, 97, 1–2. [Google Scholar] [CrossRef]
- Güven, A.; Öztürk, T. Balık parazitlerinin biyoizlemdeki (Biyomonitoring) önemi. Süleyman Demirel Üniversitesi Eğirdir Su Ürünleri Fakültesi Derg. 2018, 14, 59–73. [Google Scholar] [CrossRef][Green Version]
- Skrzypczak, M.; Rolbiecki, L. Helmintofauna of turbot Scophthalmus maximus (Linnaeus, 1758) from the southern Baltic Sea including new data. Pol. J. Vet. Sci. 2015, 18, 599–605. [Google Scholar] [CrossRef]
- Türe, M. Molecular identification of Uronema marinum (Protozoa, Ciliophora, Scuticociliatia) in cultured turbot Psetta maxima larvae. Vet. Res. Forum 2021, 12, 121–124. [Google Scholar]
- Ürkü, C.; Atanasoff, A.; Petrova-Pavlova, E.; Tserkova, F. Morphological identification of intestinal parasites from turbot (Psetta maxima) along the Bulgarian Black sea coast. In Proceedings of the of 2nd Aquatic Biotechnology Symposium, Istanbul, Turkiye, 13–15 October 2021; p. 53. Available online: https://aquabiotech.istanbul.edu.tr/en/content/past-events/2021 (accessed on 2 August 2025).
- Pinkas, L.; Oliphant, M.S.; Iverson, I.L.K. Food habits study of albacore, bluefin tuna, and bonito in California waters. In Fish Bulletin; USA and California Department of Fish and Game: Sacramento, CA, USA, 1971; Volume 152, pp. 1–105. [Google Scholar]
- Cortes, E. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 1997, 54, 726–738. [Google Scholar] [CrossRef]
- Moravec, F. Parasitic Nematodes of Freshwater Fishes of Europe; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; p. 473. [Google Scholar]
- Yoshinaga, T.; Ogawa, K.; Wakabayashi, H. Experimental life cycle of Hysterothylacium aduncum (Nematoda: Anisakidae) in fresh water. Fish Pathol. 1987, 22, 243–251. [Google Scholar] [CrossRef]
- Yamaguti, S. The cestodes of vertebrates. In Systema Helminthum; Interscience Publıshers: New York, NY, USA, 1959; Volume II, p. 271. [Google Scholar]
- Roberts, R.; Smail, D.; Munro, E. Laboratory methods. In Fish Pathology; WB Saunders: London, UK, 2001; pp. 380–412. [Google Scholar]
- Yavuzcan Yildiz, H.; Genç, E.; Kaya, D.; Yilmaz, B. Predictive model for the nematode (Hysterothylacium aduncum) in horse mackerel, Trachurus trachurus from the Black Sea. Mar. Life Sci. 2024, 6, 10–16. [Google Scholar] [CrossRef]
- Amarante, C.F.D.; Tassinari, W.D.S.; Luque, J.L.; Pereira, M.J.S. Factors associated with parasite aggregation levels in fishes from Brazil. Rev. Bras. Parasitol. Veterinária 2015, 24, 174–182. [Google Scholar] [CrossRef]
- Pekmezci, G.Z. Molecular characterization of Hysterothylacium aduncum (Nematoda: Raphidascarididae) larvae infecting Merlangius merlangus euxinus (Linnaeus, 1758) from the Turkish Black Sea coast based on mitochondrial small subunit ribosomal RNA gene analysis. Etlik Vet. Mikrobiyoloji Derg. 2019, 30, 64–69. [Google Scholar] [CrossRef]
- Aldık, R.; Cakır, F.; Tonguç-Yayintas, O.; Altinagac, U. Endoparacite investigation in some fish species with high economic value obtained from Canakkale, Turkey. J. Adv. VetBio Sci. Tech. 2021, 6, 202–210. [Google Scholar] [CrossRef]
- Özer, A.; Olguner, A.M. Metazoan parasites of some marine fish species collected at the Sinop coasts of the Black Sea. Ege J. Fish. Aquat. Sci. 2013, 30, 93–97. [Google Scholar] [CrossRef]
- Giragosov, V.E.; Khanaychenko, A.N. Macroscopic characteristics of the epizootic situation in the Black Sea turbot Scophthalmus maeoticus. In Contemporary Problems of Theoretical and Marine Parasitology; Bondarenko Publishing: Moscow, Russia, 2016; pp. 219–221. [Google Scholar]
- Totoiu, A.; Tiganov, G.; Galatchi, M.; Nenciu, M. Food array analysis in turbot (Psetta maeotica) at the Romanian Black Sea coast in 2013. Cercet. Mar. 2014, 44, 164–172. [Google Scholar]
- Maximov, V. Managementul Durabil al Calcanului la Litoralul Românesc; Editura Boldaş: Constanţa, Romania, 2012; p. 199. [Google Scholar]
- Stanev, E. Understanding Black Sea dynamics an overview of recent numerical modeling. Oceanography 2005, 18, 56–75. [Google Scholar] [CrossRef]
- Leduc, D.; Rowden, A.A.; Nodder, S.D.; Berkenbusch, K.; Probert, P.K.; Hadfield, M.G. Unusually high food availability in Kaikoura Canyon linked to distinct deep-sea nematode community. Deep Sea Res. Part II: Topical Stud. Oceanography 2014, 104, 310–318. [Google Scholar]
- Sapir, A. Why are nematodes so successful extremophiles? Commun. Integr. Biol. 2021, 14, 24–26. [Google Scholar] [CrossRef]
- Țoțoiu, A.; Radu, G.; Nenciu, M.I.; Patriche, N. Overview of health status of the main Romanian Black Sea coast fish. J. Environ. Prot. Ecol. 2018, 4, 1591–1602. [Google Scholar]
- Stoyanov, B.; Mutafchiev, Y.; Georgiev, B.B. Helminth parasites of the three-spined stickleback Gasterosteus aculeatus L., 1758 (Actinopterygii: Gasterosteidae) from a Black Sea coastal wetland, Bulgaria. Acta Zool. Bulg. 2023, 75, 285–300. [Google Scholar]
- Branson, E.; Riaza, A.; Alvarez-Pellitero, P. Myxosporean infection causing intestinal disease in farmed turbot, Scophthalmus maximus (L.), (Teleostei: Scophthalmidae). J. Fish Dis. 1999, 22, 395–399. [Google Scholar] [CrossRef]
- Britton, J.R.; Pegg, J.; Williams, C.F. Pathological and ecological host consequences of infection by an introduced fish parasite. PLoS ONE 2011, 6, e26365. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.T.; Peachey, J.E. Bothriocephalus scorpii [Cestoda: Pseudophyllidea] in turbot and brill from british coastal waters. J. Mar. Biol. Assoc. U.K. 1968, 48, 335–340. [Google Scholar] [CrossRef]
- Ahmad, F.; Fazili, K.M.; Sofi, O.M.; Sheikh, B.A.; Sofi, T.A. Distribution and pathologycaused by Bothriocephalus acheilognathi, Yamaguti 1934 (Cestoda: Bothriocephalidae): A review. Rev. Vet. 2018, 29, 142–149. [Google Scholar] [CrossRef]
- Țoțoiu, A.; Stoica, E.; Ciucă, A.M.; Harcotă, G.E.; Nită, V.; Patriche, N. Parasites and microplastics in the gastrointestinal tract of Alosa immaculata from the Black Sea—Implications for health and condition. J. Mar. Sci. Eng. 2025, 13, 1316. [Google Scholar] [CrossRef]
- Audicana, M.T. Anisakis, something is moving inside the fish. Pathogens 2022, 11, 6. [Google Scholar] [CrossRef]
- Baron, L.; Branca, G.; Trombetta, C.; Punzo, E.; Quarto, F.; Speciale, G.; Barresi, V. Intestinal anisakidosis: Histopathological findings and differential diagnosis Pathology. Res. Pract. 2014, 210, 746–750. [Google Scholar] [CrossRef]


| Primers and Probe | Sequence 5′-3′ | Tm | Amp Self Comp | |
|---|---|---|---|---|
| Bothriocephalus sp. 18S-F | CGTTTTCCGACTCCGCTCTA | 59.83 | 145 | 0.00 |
| Bothriocephalus sp. 18S-R | CTCGGAAGCAGACACCACTT | 59.97 | 0.00 | |
| H. aduncum 18S-F | AAAGCGGGGACTGCTGTTTC | 61.17 | 70 | 1.00 |
| H. aduncum 18S-R | ACTGCGATTAAGGCGGTTTC | 58.92 | 0.00 | |
| Species of Parasites | 10–20 (n = 2) | 30–40 (n = 3) | 40–50 (n = 18) | 50–60 (n = 13) | 60–70 (n = 16) | 70–80 (n = 8) | 80–90 (n = 5) |
|---|---|---|---|---|---|---|---|
| Bothriocephalus sp. | 1 (50.00%) | 2 (66.67%) | 10 (55.56%) | 7 (53.84%) | 13 (81.25%) | 6 (75.00%) | 3 (60.0%) |
| Hysterothylacium aduncum | 1 (50.00%) | 2 (66.67%) | 11 (61.11%) | 11 (84.62%) | 13 (81.25%) | 7 (87.50%) | 3 (60.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasoff, A.; Urku, C.; Petrova-Pavlova, E.; Tserkova, F. First Morphological and Molecular Identification of Intestinal Helminths in Wild Turbot Scophthalmus maximus (Linnaeus, 1758) Along the Bulgarian Black Sea Coast. Fishes 2025, 10, 395. https://doi.org/10.3390/fishes10080395
Atanasoff A, Urku C, Petrova-Pavlova E, Tserkova F. First Morphological and Molecular Identification of Intestinal Helminths in Wild Turbot Scophthalmus maximus (Linnaeus, 1758) Along the Bulgarian Black Sea Coast. Fishes. 2025; 10(8):395. https://doi.org/10.3390/fishes10080395
Chicago/Turabian StyleAtanasoff, Alexander, Cigdem Urku, Elitsa Petrova-Pavlova, and Feriha Tserkova. 2025. "First Morphological and Molecular Identification of Intestinal Helminths in Wild Turbot Scophthalmus maximus (Linnaeus, 1758) Along the Bulgarian Black Sea Coast" Fishes 10, no. 8: 395. https://doi.org/10.3390/fishes10080395
APA StyleAtanasoff, A., Urku, C., Petrova-Pavlova, E., & Tserkova, F. (2025). First Morphological and Molecular Identification of Intestinal Helminths in Wild Turbot Scophthalmus maximus (Linnaeus, 1758) Along the Bulgarian Black Sea Coast. Fishes, 10(8), 395. https://doi.org/10.3390/fishes10080395








