Morphomolecular Characterization of Rhadinorhynchus niloticus (Acanthocephala: Rhadinorhynchidae) from Nile Perch (Lates niloticus, Perciformes: Latidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
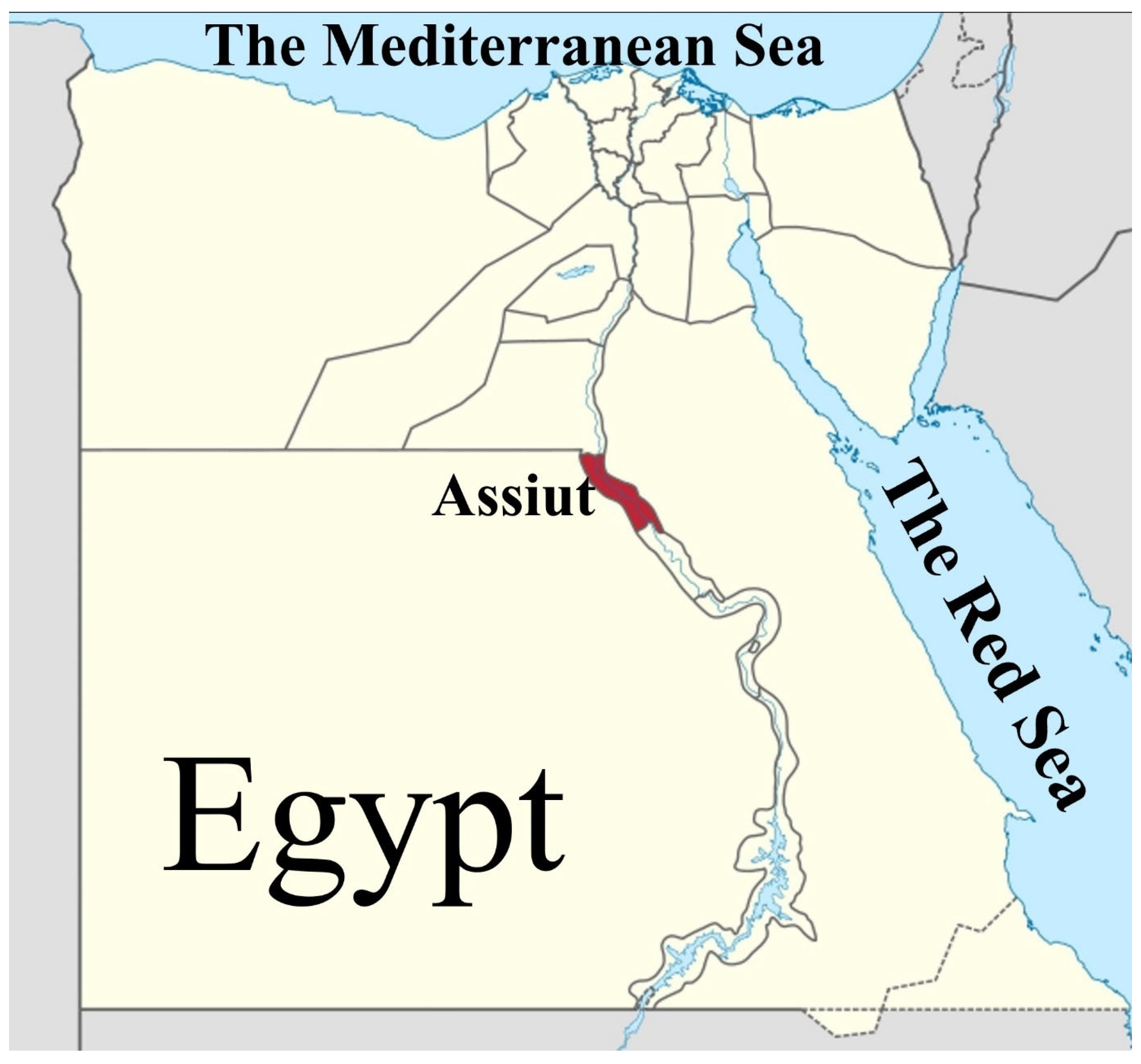
2.2. Study Area and Fish Sampling
2.3. Parasitological and Epidemiological Examinations
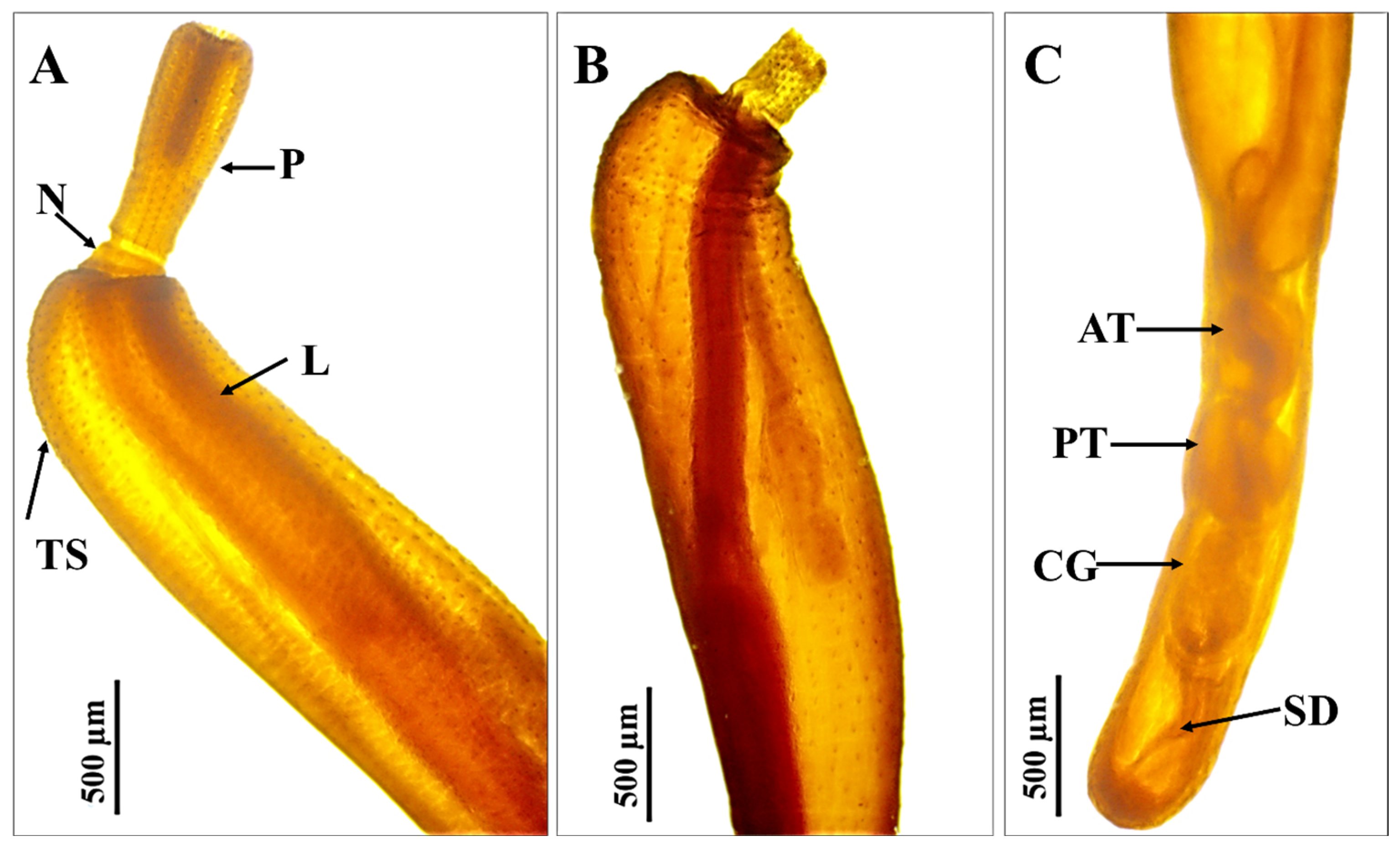
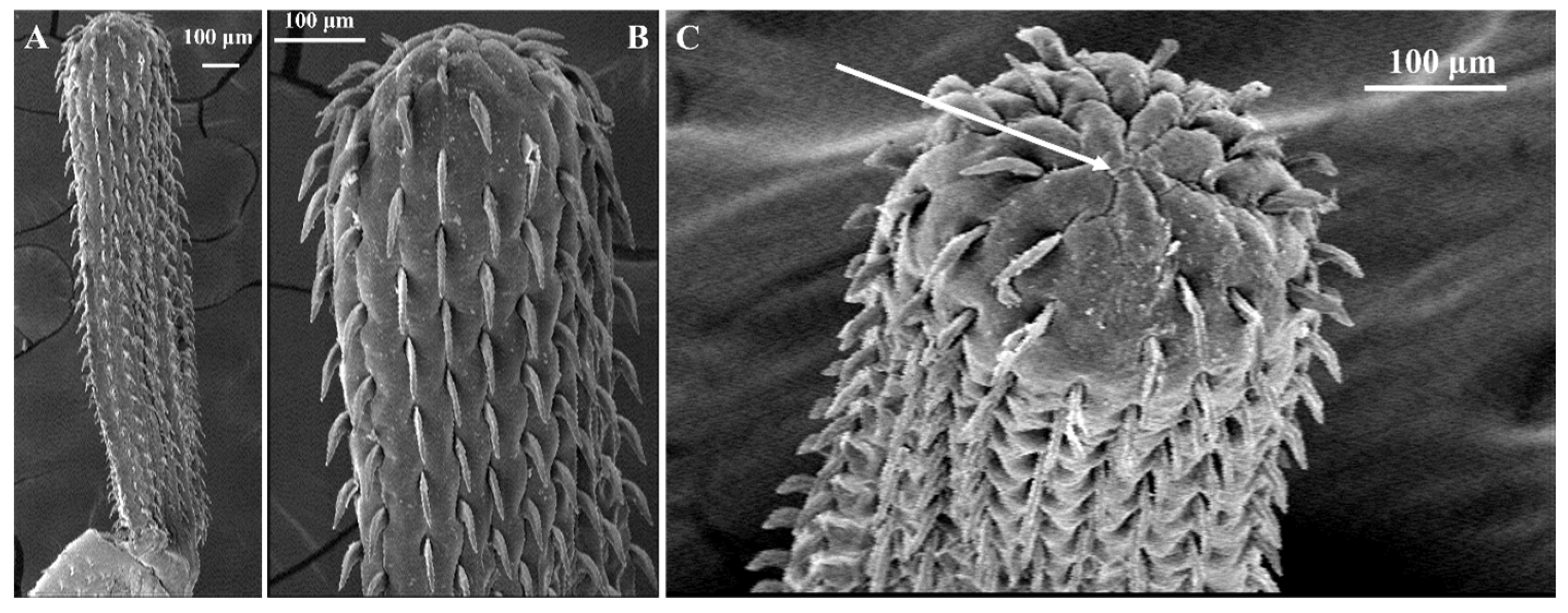
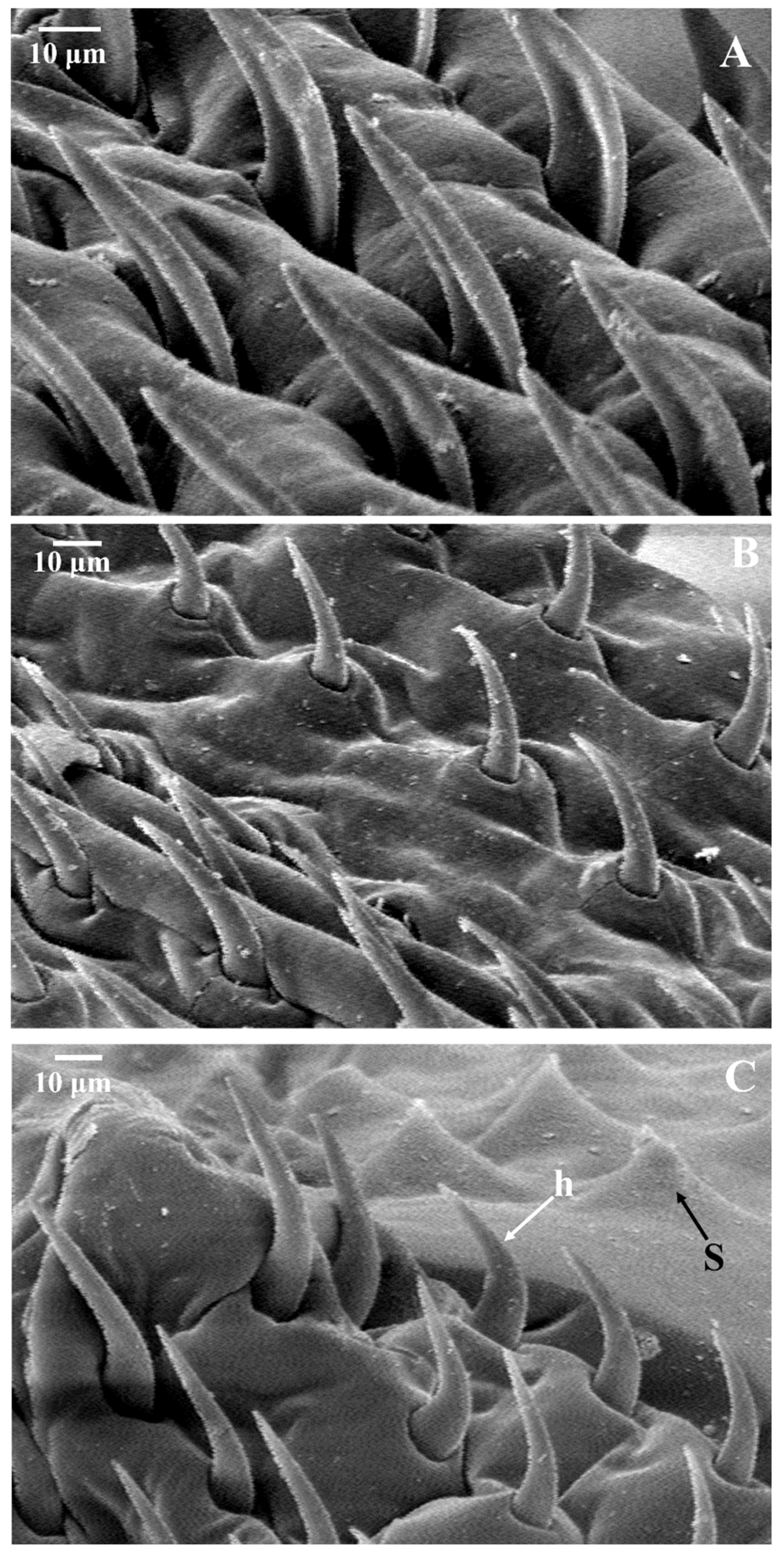
2.4. Morphological Examination
2.5. Molecular Characterization of the Parasite
| Parasite Name | Host | Country | GenBank Accession Numbers | Reference | |
|---|---|---|---|---|---|
| COI | 18S rRNA | ||||
| Rhadinorhynchidae Lühe, 1912 | |||||
| Rhadinorhynchusniloticus | Nile perch (Lates niloticus) | Egypt | PP859185 | MZ727194 | This study |
| Rhadinorhynchus gerberi (Lisitsyna, Kudlai, Cribb and Smit, 2019) | Trachinotus botla | South Africa | MN104897 | MN105739 | [28] |
| Rhadinorhynchus hiansi (Soota and Bhattacharya, 1981) | Sarda orientalis | Vietnam | MN203137 | MN203133 | [29] |
| Rhadinorhynchus sp. | marine fish | Western Pacific coast of Mexico | - | AY062433 | [30] |
| HNA (Family Scianidae) | USA | DQ089712 | ˗ | [31] | |
| Rhadinorhynchus dorsoventrospinosus (Amin, Heckmann and Nguyen Van Ha 2011) | Decapteruskurroides | Vietnam | MN267179 | MH384435 | [32] |
| Rhadinorhynchus laterospinosus (Amin, Heckmann & Nguyen Van Ha 2011) | Auxisrockei | Vietnam | MK572743 | MK457183 | [33] |
| Rhadinorhynchus carangis (Yamaguti 1939) | Australian marine teleosts | Australia | MN705830 | [34] | |
| Rhadinorhynchus biformis (Smales 2014) | MN705829 | ||||
| Rhadinorhynchus johnstoni (Golvan 1969) | MN705827 | ||||
| Rhadinorhynchus pristis (Rudolphi 1802) | Nyctiphanes couchii (Bell) | Spain | JQ061132 | JQ061133 | [35] |
| Leptorhynchoididae Witenberg, 1932 | |||||
| Leptorhynchoides thecatus (Linton 1891, Kostylev 1924) | ˗ | USA | AY690577 | ˗ | [36] |
| Pomphorhynchidae Monticelli, 1905 | |||||
| Tenuiproboscis sp. | Lutjanus argentimaculatus | India | JF694276 | ˗ | NPY |
| Longicollum pagrosomi (Yamaguti 1935) | Oplegnathusfasciatus | China | KY490048 | - | [37] |
| cultured red sea bream | Korea | - | KX641270 | NPY | |
| Echinorhynchidae Cobbold, 1879 | |||||
| Echinorhynchus sasakiae (Kita & Kajihara 2023) | Hexagrammos lagocephalus (Pallas) | Japan | LC757487 | LC757488 | [38] |
| Echinorhynchus gadi (Zoega in Müller 1776) | Salvelinus malma | Russia | KF156892 | - | [39] |
| Limnognathiamaerski | USA | - | AY218123 | [40] | |
| Neoechinorhynchidae Ward, 1917 | |||||
| Neoechinorhynchussalmonis | Salvelinus malma | Russia | KF156889 | - | [39] |
| Neoechinorhynchus sp. | Mugil cephalus | India | - | MN992025 | NPY |
| Tenuisentidae Van Cleave, 1936 | |||||
| Tenuisentis niloticus (Meyer 1932) | Heterotis niloticus (Cuvier) | Burkina Faso | KT970469 | KT970471 | [41] |
| Brachionidae | |||||
| Brachionus plicatilis (Müller 1786) | Arthrospira platensis | China | - | KY886363 | NPY |
| Scirpophagaincertulas | India | AY218090 | - | ||
3. Results
3.1. Parasitological and Epidemiological Examinations
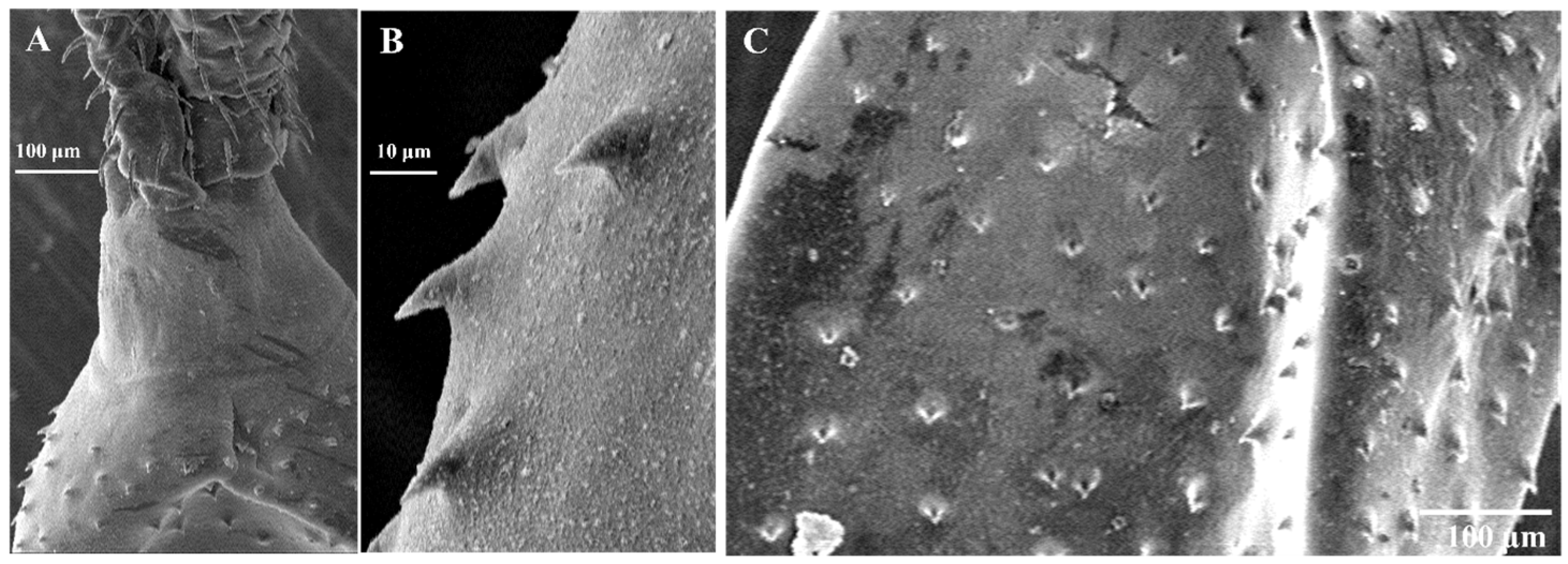
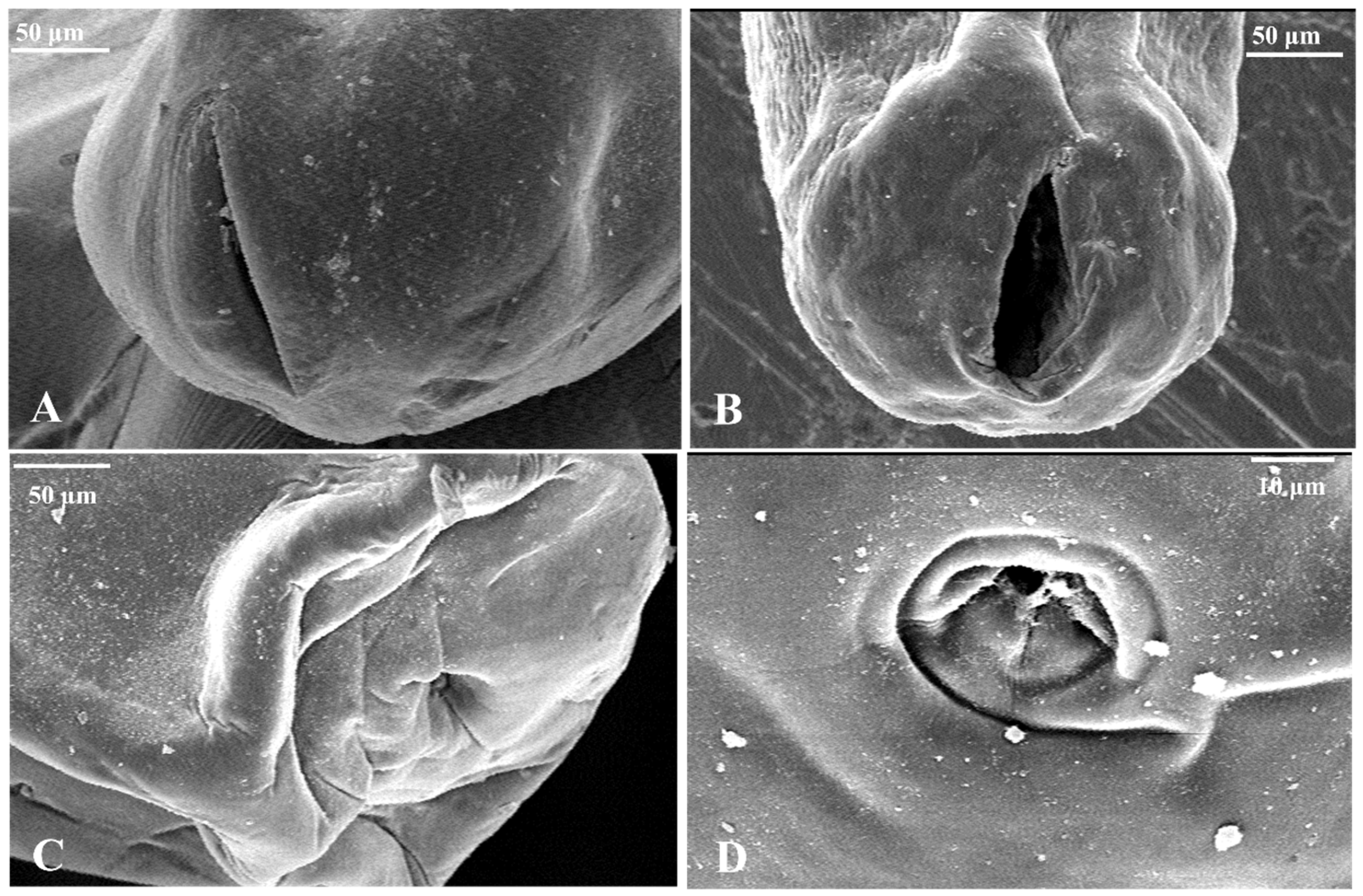
3.2. Morphological Examination
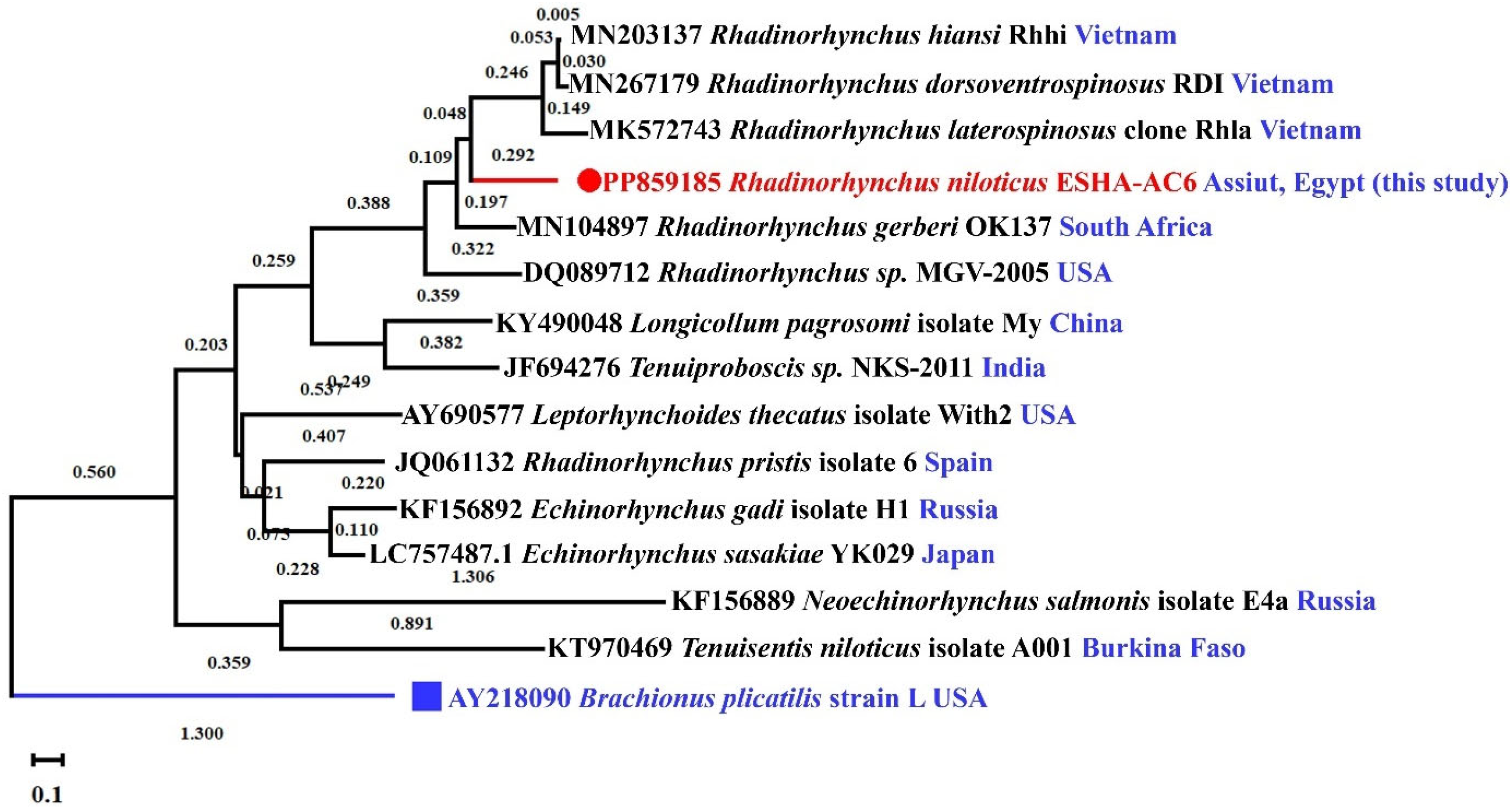
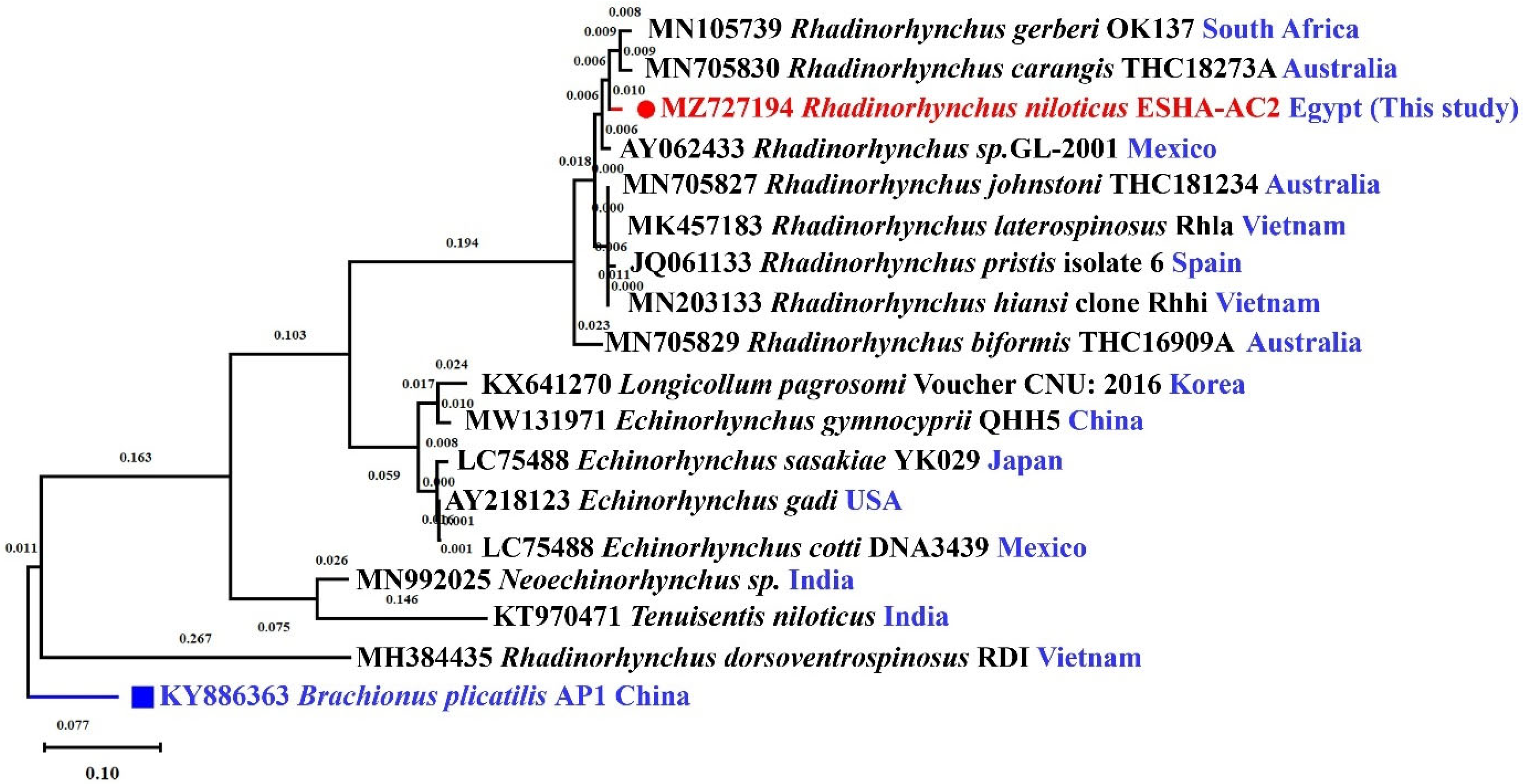
3.3. Molecular Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aloo, P.A.; Njiru, J.; Balirwa, J.S.; Nyamweya, C.S. Impacts of Nile Perch, Lates niloticus, introduction on the ecology, economy and conservation of Lake Victoria, East Africa. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2017, 22, 320–333. [Google Scholar] [CrossRef]
- Asnake, W. Nile Perch (Lates niloticus): The Promising White Meat of the World. J. Nutr. Food Sci. 2018, 8, 680–683. [Google Scholar] [CrossRef]
- Başusta, N.; Mutlu, E.; Deval, M.C. Parasitic isopods (Anilocra frontalis H. Milne Edwards, 1830 and Ceratothoa capri (Trilles, 1964)) from the Antalya Bay (Turkey) with new host records. Turk. J. Sci. Technol. 2017, 12, 11–15. [Google Scholar]
- Sebastião, F.d.A.; Braga de Oliveira, M.I.; Rocha, M.J.S.; Souza, D.C.d.M.; Ribeiro, P.; Majolo, C.; Crescêncio, R.; Chagas, E.C. Effect of a food additive in the control of the acanthocephalan Neoechinorhynchus buttnerae in Colossoma macropomum. Aquac. Res. 2021, 52, 635–642. [Google Scholar] [CrossRef]
- Roy, P.E. Nematode (Round Worm) Infection in Fish, 2nd ed.; IFAS: Gainesville, FL, USA, 2002. [Google Scholar]
- Paperna, I. Fish Disease and Disorders; CABI Publishing: Kelowna, BC, Canada, 2001; Volume 1. [Google Scholar]
- Ismen, A.; Bingel, F. Nematode infection in the whiting Marsangius euxinus off Turkish coast of the black sea. Fish. Res. 1999, 42, 183–189. [Google Scholar] [CrossRef]
- Amin, O.M. Classification of the acanthocephala. Folia Parasitol. 2013, 60, 273–305. [Google Scholar] [CrossRef]
- Gibson, D.; Wayland, M. World List of Marine Acanthocephala. Rhadinorhynchus Lühe, 1911. World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=20399 (accessed on 28 June 2025).
- Shvydka, S.; Sarabeev, V.; Estruch, V.D.; Cadarso-Suárez, C. Optimum Sample Size to Estimate Mean Parasite Abundance in Fish Parasite Surveys. Helminthologia 2018, 55, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists–A primer to quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Kildea, M.A.; Allan, G.L.; Kearney, R.E. Accumulation and clearance of the anaesthetics clove oil and AQUI-S™ from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture 2004, 232, 265–277. [Google Scholar] [CrossRef]
- Paugy, D.; Lévêque, C.; Teugels, G. The Fresh and Brackish Water Fishes of West Africa; Faune et Flore Tropicales: Paris, France, 2003; Volume 1. [Google Scholar]
- Eissa, A.E. Clinical and Laboratory Manual of Fish Diseases; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2016. [Google Scholar]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; Iowa State University Press: Ames, IA, USA, 2010. [Google Scholar]
- Abdallah, E.S.H.; Hamouda, A.H. Livoneca redmanii Leach, 1818 (Cymothoidae) a parasitic isopod infesting the gills of the European seabass, Dicentrarchus labrax (Linnaeus, 1758): Morphological and molecular characterization study. BMC Vet. Res. 2022, 18, 330. [Google Scholar] [CrossRef]
- Abdallah, E.S.H.; Hamouda, A.H. Morphological and molecular characterization of Lernanthropus kroyeri, a copepod infesting the gills of European seabass Dicentrarchus labrax. Egypt. J. Aquat. Res. 2023, 49, 49–55. [Google Scholar] [CrossRef]
- Hassan, E.S.; Mahmoud, M.M.; Metwally, A.M.; Moktar, D.M. Lamproglena monodi (Copepoda: Lernaeidae), infesting gills of Oreochromis niloticus and Tilapia zillii. Glob. J. Fish. Aquac. Res. 2013, 6, 1–16. [Google Scholar]
- Mahmoud, M.M.; Hassan, E.S.; Haridy, M.; Nour El Deen, E.A.; Kuraa, H.M.M.; Hanna, H.N.S. Parasitic infections of the gills of wild African Sharptooth Catfish (Clarias gariepinus). Assiut Vet. Med. J. 2018, 64, 31–39. [Google Scholar] [CrossRef]
- Thabit, H.; Abdallah, E.S.H. Morphological and molecular identification of third-stage Contracaecum larvae (Nematoda: Anisakidae) parasitizing Nile perch Lates niloticus in Egypt. Aquac. Res. 2022, 53, 4869–4881. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Abdallah, E.S.H.; Mahmoud, M.M.; Abdel-Rahim, I.R. Trichosporon jirovecii infection of red swamp crayfish (Procambarus clarkii). J. Fish. Dis. 2018, 41, 1719–1732. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochonrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Garey, J.R.; Near, T.J.; Nonnemacher, M.R.; Nadler, S.A. Molecular evidence for Acanthocephala as a subtaxon of Rotifera. J. Mol. Evol. 1996, 43, 287–292. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyna, I.O.; Kudlai, O.; Cribb, H.T.; Smit, J.N. Three new species of acanthocephalans (Palaeacanthocephala) from marine fishes collected off the East Coast of South Africa. Folia Parasitol. 2019, 66, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Amin, O.M.; Heckmann, R.A.; Dallarés, S.; Constenla, M.; Van Ha, N. Morphological and Molecular Description of Rhadinorhynchus hiansi Soota and Bhattacharya, 1981 (Acanthocephala: Rhadinorhynchidae) from Marine Fish off the Pacific Coast of Vietnam. J. Parasitol. 2020, 106, 56–70, 15. [Google Scholar] [CrossRef] [PubMed]
- García-Varela, M.; Cummings, M.P.; Pérez-Ponce de León, G.; Gardner, S.L.; Laclette, J.P. Phylogenetic analysis based on 18S ribosomal RNA gene sequences supports the existence of class polyacanthocephala (acanthocephala). Mol. Phylogenet Evol. 2002, 23, 288–292. [Google Scholar] [CrossRef]
- García-Varela, M.; Nadler, S.A. Phylogenetic relationships among Syndermata inferred from nuclear and mitochondrial gene sequences. Mol. Phylogenet. Evol. 2006, 40, 61–72. [Google Scholar] [CrossRef]
- Chaudhary, A.; Amin, O.M.; Heckmann, R.; Singh, H.S. The molecular profile of Rhadinorhynchus dorsoventrospinosus Amin, Heckmann, and ha 2011 (Acanthocephala: Rhadinorhynchidae) from Vietnam. J. Parasitol. 2020, 106, 418–427. [Google Scholar] [CrossRef]
- Amin, O.M.; Heckmann, R.A.; Dallarés, S.; Constenla, M.; Ha, N.V. Morphological and molecular description of Rhadinorhynchus laterospinosus Amin, Heckmann & Ha, 2011 (Acanthocephala, Rhadinorhynchidae) from marine fish off the Pacific coast of Vietnam. Parasite 2019, 26, 14. [Google Scholar] [CrossRef]
- Huston, D.C.; Cribb, T.H.; Smales, L.R. Molecular characterisation of acanthocephalans from Australian marine teleosts: Proposal of a new family, synonymy of another and transfer of taxa between orders. Syst. Parasitol. 2020, 97, 859–861. [Google Scholar] [CrossRef]
- Gregori, M.; Aznar, F.; Abollo, E.; Roura, A.; González, A.; Pascual, S. Nyctiphanes couchii as intermediate host for Rhadinorhynchus sp. (Acanthocephala, Echinorhynchidae) from NW Iberian Peninsula waters. Dis. Aquat. Org. 2013, 105, 9–20. [Google Scholar] [CrossRef]
- Steinauer, M.L.; Nickol, B.B.; Ortí, G. Cryptic speciation and patterns of phenotypic variation of a highly variable acanthocephalan parasite. Mol. Ecol. 2007, 16, 4097–4109. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.-X.; Amin, O.M.; Yang, Y. Morphological variability and molecular characterization of Pomphorhynchus zhoushanensis sp. nov. (Acanthocephala: Pomphorhynchidae), with comments on the systematic status of Pomphorhynchus Monticelli, 1905. Parasitol. Int. 2017, 66, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Kajihara, H. Morphological and molecular characterization of a new species of the genus Echinorhynchus Zoega in Müller, 1776 (Acanthocephala: Echinorhynchidae) parasitizing the rock greenling Hexagrammos lagocephalus (Pallas) (Scorpaeniformes: Hexagrammidae) from eastern Hokkaido, Japan. Syst. Parasitol. 2023, 100, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Malyarchuk, B.; Derenko, M.; Mikhailova, E.; Denisova, G. Phylogenetic relationships among Neoechinorhynchus species (Acanthocephala: Neoechinorhynchidae) from North-East Asia based on molecular data. Parasitol. Int. 2014, 63, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Giribet, G.; Sørensen, M.V.; Funch, P.; Kristensen, R.M.; Sterrer, W. Investigations into the phylogenetic position of Micrognathozoa using four molecular loci. Cladistics 2004, 20, 1–13. [Google Scholar] [CrossRef]
- Amin, O.M.; Evans, R.P.; Boungou, M.; Heckmann, R. Morphological and molecular description of Tenuisentis niloticus (Meyer, 1932) (Acanthocephala: Tenuisentidae) from Heterotis niloticus (Cuvier) (Actinopterygii: Arapaimidae), in Burkina Faso, with emendation of the family diagnosis and notes on new features, cryptic genetic diversity and histopathology. Syst. Parasitol. 2016, 93, 173–191. [Google Scholar] [CrossRef]
- Koblmüller, S.; Schöggl, C.A.; Lorber, C.J.; Van Steenberge, M.; Kmentová, N.; Vanhove, M.P.M.; Zangl, L. African lates perches (Teleostei, Latidae, Lates): Paraphyly of Nile perch and recent colonization of Lake Tanganyika. Mol. Phylogenet Evol. 2021, 160, 107141. [Google Scholar] [CrossRef]
- Elhawary, N.M.; Hamouda, A.H.; Bazh, E.K.; El-Bahy, N.M.; Sorour, S.S. Phenotypic and genomic characterization of Tylodelphys sp. metacercaria (Diesing 1850)(Trematoda: Diplostomidae) recovered from Lates niloticus (Linnaeus, 1758) Egypt. Vet. Med. Soc. Parasitol. J. EVMSPJ 2022, 18, 15–34. [Google Scholar]
- Thon, C.; Otachi, O.; Oldewage, A. Endo-helminths infestation in Nile perch, Lates niloticus, (L.,) and Nile tilapia, Oreochromis niloticus (L.,) in Winam Gulf of Lake Victoria, Kenya. Egerton J. Sci. Technol. 2019, 16, 1–139. [Google Scholar]
- Outa, J.O.; Dos Santos, Q.M.; Avenant-Oldewage, A.; Jirsa, F. Parasite diversity of introduced fish Lates niloticus, Oreochromis niloticus and endemic Haplochromis spp. of Lake Victoria, Kenya. Parasitol. Res. 2021, 120, 1583–1592. [Google Scholar] [CrossRef]
- Mazen, N.; Thabit, H. Light and scanning electron microscopy of three parasitic helminths from freshwater fishes in Assiut, Egypt. Assiut Vet. Med. J. 2005, 51, 1–12. [Google Scholar]
- El-Siefy, A.M.; Ibraheem, M.H.; Abd El-Kareem, S.G. Ovarian balls (Floating ovaries) of Rhadinorhynchus niloticus Mohamadain, 1989 from the Nile perch Lates niloticus Linnaeus, 1758; an electron microscope study. Helminthologia 2024, 61, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ebraheem, M.E. Studies on Some of the Parasites in Some of Nile Fishes in Sohag Governorate; Assiut University: Asyut, Egypt, 1992. [Google Scholar]
- El-Shahawy, I.; El-Seify, M.; Metwally, A.; Fwaz, M. Survey on endoparasitic fauna of some commercially important fishes of the River Nile, southern of Egypt (Egypt). Revue de Médecine Vétérinaire 2017, 168, 126–134. [Google Scholar]
- Smales, L.R. The genus Rhadinorhynchus (Acanthocephala: Rhadinorhynchidae) from marine fish in Australia with the description of four new species. Acta Parasitol. 2014, 59, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Travassos, L. Informações sobre a fauna helrninthologica de Matto Grosso (II Nota). Folha Medica 1923, 4, 12–16. [Google Scholar]
- Lühe, M. Acanthocephalen: Register der Acanthocephalen und Parasitischen Plattwürmer, Geordnet nach ihren Wirten; Brauer, A., Ed.; Gustav Fischer Verlag: Jena, Germany, 1911. (In German) [Google Scholar]
- Barton, D.P.; Smales, L.R. Acanthocephalan cystacanths from flatfish (Order pleuronectiformes) in Tropical Australian waters. J. Parasitol. 2015, 101, 429–435. [Google Scholar] [CrossRef]
- Hamouda, A.H.; Sorour, S.S.; El-Habashi, N.M.; Adam, E.-H.A. Parasitic infection with emphasis on Tylodelphys spp. as new host and locality records in Nile perch; Lates niloticus from Lake Nasser, Egypt. World’s Vet. J. 2018, 8, 19–33. [Google Scholar]
- Bazh, E.; Hamouda, A. Scanning morphology, prevalence and histopathology of some acanthocephalans infecting some River Nile fish. Bulg. J. Vet. Med. (Online First) 2019, 87, 239–250. [Google Scholar] [CrossRef]
- Mohamadain, H.S. Studies on Helminth Parasites of the Nile Fishes in Quena Province, A.R, Egypt; Faculty of Science Quena Branch, Assiut University: Qena, Egypt, 1989. [Google Scholar]
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-PP859185 Rhadinorhynchus niloticus | |||||||||||||||
| 2-MN104897 R. gerberi | 0.27 | ||||||||||||||
| 3-MN203137 R. hiansi | 0.29 | 0.27 | |||||||||||||
| 4-DQ089712 Rhadinorhynchus sp. | 0.30 | 0.31 | 0.30 | ||||||||||||
| 5-MN267179 R. dorsoventrospinosus | 0.31 | 0.28 | 0.03 | 0.30 | |||||||||||
| 6-MK572743 R. laterospinosus | 0.32 | 0.30 | 0.15 | 0.32 | 0.17 | ||||||||||
| 7-JQ061132 R. pristis | 0.44 | 0.42 | 0.41 | 0.43 | 0.44 | 0.45 | |||||||||
| 8-JF694276 Tenuiproboscis sp. | 0.45 | 0.44 | 0.47 | 0.42 | 0.44 | 0.51 | 0.47 | ||||||||
| 9-LC757487 Echinorhynchus sasakiae | 0.48 | 0.43 | 0.48 | 0.48 | 0.47 | 0.50 | 0.35 | 0.42 | |||||||
| 10-KF156892 E. gadi | 0.49 | 0.44 | 0.47 | 0.49 | 0.48 | 0.49 | 0.32 | 0.43 | 0.22 | ||||||
| 11-KY490048 Longicollum pagrosomi | 0.49 | 0.42 | 0.44 | 0.41 | 0.42 | 0.49 | 0.48 | 0.33 | 0.43 | 0.47 | |||||
| 12-AY690577 Leptorhynchoides thecatus | 0.49 | 0.39 | 0.46 | 0.49 | 0.47 | 0.49 | 0.39 | 0.45 | 0.36 | 0.38 | 0.47 | ||||
| 13-KF156889 Neoechinorhynchus salmonis | 0.55 | 0.57 | 0.44 | 0.58 | 0.60 | 0.60 | 0.50 | 0.55 | 0.56 | 0.53 | 0.63 | 0.60 | |||
| 14-KT970469 Tenuisentis niloticus | 0.60 | 0.60 | 0.62 | 0.59 | 0.65 | 0.65 | 0.49 | 0.55 | 0.49 | 0.49 | 0.63 | 0.53 | 0.53 | ||
| 15-AY218090 Brachionus plicatilis | 0.73 | 0.67 | 0.64 | 0.62 | 0.65 | 0.65 | 0.63 | 0.70 | 0.59 | 0.61 | 0.67 | 0.59 | 0.76 | 0.66 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-MZ727194 Rhadinorhynchus niloticus | ||||||||||||||||||
| 2-MN203133 R. hiansi | 0.01 | |||||||||||||||||
| 3-AY062433 Rhadinorhynchus sp. | 0.01 | 0.01 | ||||||||||||||||
| 4-MK457183 R. laterospinosus | 0.01 | 0.00 | 0.01 | |||||||||||||||
| 5-MN105739 R. gerberi | 0.02 | 0.02 | 0.02 | 0.02 | ||||||||||||||
| 6-MN705827 R. johnstoni | 0.02 | 0.00 | 0.02 | 0.00 | 0.02 | |||||||||||||
| 7-JQ061133 R. pristis | 0.02 | 0.00 | 0.02 | 0.00 | 0.02 | 0.00 | ||||||||||||
| 8-MN705830 R. carangis | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.03 | 0.02 | |||||||||||
| 9-MN705829 R. biformis | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.06 | ||||||||||
| 10-KX641270 Longicollum pagrosomi | 0.19 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.19 | |||||||||
| 11-LC757488 Echinorhynchus sasakiae | 0.20 | 0.21 | 0.21 | 0.21 | 0.20 | 0.21 | 0.21 | 0.21 | 0.21 | 0.04 | ||||||||
| 12-AY218123 E. gadi | 0.20 | 0.21 | 0.21 | 0.21 | 0.21 | 0.22 | 0.21 | 0.21 | 0.21 | 0.04 | 0.00 | |||||||
| 13-MW172280 E. cotti | 0.20 | 0.21 | 0.21 | 0.21 | 0.21 | 0.22 | 0.21 | 0.22 | 0.21 | 0.04 | 0.00 | 0.00 | ||||||
| 14-KT970471 Tenuisentis niloticus | 0.20 | 0.24 | 0.21 | 0.25 | 0.22 | 0.21 | 0.23 | 0.21 | 0.21 | 0.12 | 0.12 | 0.12 | 0.12 | |||||
| 15-MW131971 E. gymnocyprii | 0.21 | 0.21 | 0.22 | 0.21 | 0.21 | 0.22 | 0.21 | 0.22 | 0.22 | 0.02 | 0.04 | 0.04 | 0.04 | 0.11 | ||||
| 16-MH384435 R. dorsoventrospinosus | 0.34 | 0.29 | 0.34 | 0.29 | 0.32 | 0.34 | 0.28 | 0.35 | 0.35 | 0.26 | 0.30 | 0.30 | 0.30 | 0.22 | 0.31 | |||
| 17-MN992025 Neoechinorhynchus sp. | 0.34 | 0.35 | 0.34 | 0.35 | 0.35 | 0.35 | 0.36 | 0.35 | 0.33 | 0.26 | 0.27 | 0.27 | 0.27 | 0.07 | 0.26 | 0.21 | ||
| 18-KY886363 Brachionus plicatilis | 0.33 | 0.32 | 0.33 | 0.33 | 0.33 | 0.34 | 0.31 | 0.34 | 0.34 | 0.24 | 0.26 | 0.26 | 0.26 | 0.17 | 0.25 | 0.19 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, E.S.H.; Albano, M.; Thabit, H. Morphomolecular Characterization of Rhadinorhynchus niloticus (Acanthocephala: Rhadinorhynchidae) from Nile Perch (Lates niloticus, Perciformes: Latidae). Fishes 2025, 10, 397. https://doi.org/10.3390/fishes10080397
Abdallah ESH, Albano M, Thabit H. Morphomolecular Characterization of Rhadinorhynchus niloticus (Acanthocephala: Rhadinorhynchidae) from Nile Perch (Lates niloticus, Perciformes: Latidae). Fishes. 2025; 10(8):397. https://doi.org/10.3390/fishes10080397
Chicago/Turabian StyleAbdallah, Ebtsam Sayed Hassan, Marco Albano, and Hasnaa Thabit. 2025. "Morphomolecular Characterization of Rhadinorhynchus niloticus (Acanthocephala: Rhadinorhynchidae) from Nile Perch (Lates niloticus, Perciformes: Latidae)" Fishes 10, no. 8: 397. https://doi.org/10.3390/fishes10080397
APA StyleAbdallah, E. S. H., Albano, M., & Thabit, H. (2025). Morphomolecular Characterization of Rhadinorhynchus niloticus (Acanthocephala: Rhadinorhynchidae) from Nile Perch (Lates niloticus, Perciformes: Latidae). Fishes, 10(8), 397. https://doi.org/10.3390/fishes10080397







